Abstract
Flax (Linum usitatissimum L.) phloem fibers elongate considerably during their development and intrude between existing cells. We questioned whether fiber elongation is caused by cell tip growth or intercalary growth. Cells with tip growth are characterized by having two specific zones of cytoplasm in the cell tip, one with vesicles and no large organelles at the very tip and one with various organelles amongst others longitudinally arranged cortical microtubules in the subapex. Such zones were not observed in elongating flax fibers. Instead, organelles moved into the very tip region, and cortical microtubules showed transversal and helical configurations as known for cells growing in intercalary way. In addition, pulse-chase experiments with Calcofluor White resulted in a spotted fluorescence in the cell wall all over the length of the fiber. Therefore, it is concluded that fiber elongation is not achieved by tip growth but by intercalary growth. The intrusively growing fiber is a coenocytic cell that has no plasmodesmata, making the fibers a symplastically isolated domain within the stem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant cells elongate in two ways, by tip growth or by intercalary, also called diffuse growth (Wasteneys and Galway 2003). In case of diffuse growth, in general all cells in a tissue elongate to the same extent in a coordinated way. However, phloem fibers have been suggested to intrude between other cells, called intrusive growth (Esau 1977; Fahn 1990). In coordinated growth, the conjoint cells increase their surface synchronously and some cells become longer than the neighboring ones only by earlier cessation of division. In case of intrusive growth, elongation of the cell occurs at a rate exceeding that of the conjoint cells. Intrusively growing cells penetrate between other cells and have to force their way in between walls of other cells, surpassing, in case of bast fibers, thousands of neighboring cells and reaching the length of several centimeters (McDougall et al. 1993). This asynchronous elongation with the surrounding cells must have a specific mechanism and regulation, which both still are largely unknown. Two possible mechanisms of intrusive growth are cell elongation by growth of the very cell tip—cell tip growth—, and cell elongation by growth along the surface of the entire cell—diffuse growth. Tip growing cells are characterized by specific cytoplasmic zones at the very tip (Geitmann and Emons 2000), and longitudinally aligned microfilaments (Ketelaar et al. 2002, 2003) and longitudinally aligned microtubules (Sieberer et al. 2002) in the subapical region, whereas cells with diffuse growth do not show such organization. The study on the mechanism of intrusive growth is hampered by the inability to reproduce it in vitro or to isolate the cells at this stage of development in sufficient amounts for biochemical or molecular-biological study.
Intrusive growth is a characteristic of several plant tissues (Esau 1977; Fahn 1990; Lev-Yadun 2001) with sclerenchyma fibers being the best-known example. The cells of this type are present in various plant organs and are especially developed in crops like flax and hemp. To answer the question whether tip growth or diffuse growth causes the intrusive growth in flax fibers, we have applied various approaches of microscopy and show that they elongate by diffuse growth.
Materials and methods
Flax seeds (Linum usitatissimum L. cv. Belinka) were obtained from the Russian Flax Research Institute (Torzhok, Russia), grown in a phytotron at 20/18°C with 16-h light period. Plants of 35–45 day old (fast growth period) were used for experiments. Elongation of the stem was analyzed by marking stems with ink at 1 cm intervals and daily measurement over a period of 16 days.
For anatomical observations, stem sections of 1.0-cm length were fixed in 4% paraformaldehyde (PFA), dehydrated and embedded in glycol methacrylate (Technovit 7100, Kulzer Germany). Sections were stained with 1% toluidine blue in water. Longitudinal and transverse sections were observed with a Nikon Optiphot microscope and images were recorded with a Panasonic wv-E 550 3-CCD camera.
For the detection of fiber cell elongation, life fibers were pulse labeled with 0.001% Calcofluor White M2R (CFW, Sigma) and observed immediately (control), or after 24 h, 48 h or 72 h. To this end plants were put in horizontal position, leaves were dissected over a length of several cm and epidermis and cortex cells of the stem were partly removed in that area with a razor blade. This part of the stem was then laid on top of a microscope slide. Calcofluor White was applied and the site was covered with a cover slide. After rinsing in water, the plants were kept in position until monitoring with a Nikon Labophot epifluorescence microscope using UV illumination and photography with a Sony DXC-950P 3CCD color video camera.
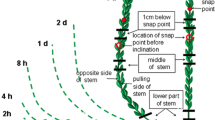
For the observation of cytoplasmic streaming, thick (0.3 mm) longitudinal sections of 3–4 cm long stem segments were made in the region where fibers still elongate, that is above snap point (SP, Gorshkova et al. 2003a). Snap point is defined as the place where the stem does not easily break any more upon a pulling force. Sections were placed on a microscope slide in 1/2 MS culture medium and examined with a Nikon Optiphot microscope. Time-lapse imaging was done using a Sony DXC-950P 3CCD color video camera with 2–3 min intervals lasting up to several hours. Movement of mitochondria in living fibers was visualized in longitudinal thick sections of the stem, also taken above SP, and after pulse staining the sections with 1 μg/ml DiOC6 (Molecular Probes) in water for 5 min, and three washings in water, 5 min each. Cytoplasmic streaming was recorded with a Nikon Labophot equipped with the Sony video camera and recorded on tape.
Fiber length was determined by light microscopy in successive sections of the stem. To this end, stem sections of 3-cm length were macerated in 10% CrO3 and 10% HNO3 for 1–5 h depending on the distance from the stem tip. Only the lengths of fibers with thickened cell walls were measured by this technique.
Microtubules (MTs) were visualized by immunofluorescence microscopy on whole mount preparations. Stem segments of 2-cm length were cut into two halves and prefixed in 2% paraformaldehyde (PFA) + 0.05% glutaraldehyde (GA) and then in 4% PFA + 0.1% GA, both in microtubule stabilizing buffer (MSB) containing 50 mM Pipes, 5 mM EGTA, 5 mM MgSO4·7H2O (pH 6.9) for 1 h. After rinsing, the samples were incubated in 1% Macerozyme in 50 mM Mes buffer (pH 6.3) for 30 min at room temperature (RT) for samples above SP; or in 1% Macerozyme + 0.5% cellulase Onozuka R10 (Serva, Germany) in the same buffer for 2–3 h at RT for samples below SP. After washing in Mes buffer, samples were transferred to phosphate buffered saline (PBS) for two 5 min washes. Free aldehydes were reduced by treatment with 0.1 M hydroxyltetra-ammoniumchloride (HAH) in PBS for 10 min. Samples were incubated in 0.1% acetylated BSA (blocking agent, Aurion, Wageningen, NL) in PBS for 15 min for blocking negatively charged sites and ions. Then, they were incubated in primary antibody [anti-α-tubulin monoclonal antibody, clone DM 1a-mouse IgG (Sigma)] diluted 1:300 in blocking agent overnight at 4°C. Following three 10-min washes in blocking agent, samples were incubated in secondary antibody (Goat anti Mouse conjugated with Alexa 488, Molecular Probes) with blocking agent for 2 h at RT. After rinsing in blocking agent and PBS, samples were embedded in the antifading solution Citifluor AF3 (Canterbury, UK). All rinsing solutions and the blocking agent contained 0.05% Triton X-100. In addition, several samples were stained with DAPI (4′-6-diamidino-2-phenylindole, Molecular Probes) as a DNA stain in a concentration of 1 μg/ml. Samples were analyzed for microtubular configurations using a Biorad MRC 600 confocal laser scanning microscope (CLSM) at 488 nM wave length. Optical sections were obtained at 1.0-μm steps and z-series were projected by Confocal assistant 2.04.
Stem pieces were also physically fixed by propane plunging, substituted in the FreasySub (JEOL Europe, Schiphol, The Netherlands) in pure methanol containing 0.05% glutaraldehyde, and after warming to room temperature, rehydrated and labeled in the same way as the chemically fixed samples. Overall microtubule alignment was the same as in the chemically fixed samples.
For the analysis of the fiber ultrastructure, 2-cm segments from the apical and subapical part of the flax stem were prefixed for 1 h in 2.5% glutaraldehyde and then cut into pieces of 0.3-cm length and fixed according to a standard procedure in 2.5% (v/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 4 h, rinsed and post fixed for 2 h in 1% (w/v) OsO4. After dehydratation in an ethanol series, acetone and propylene oxide, the samples were embedded in Epon. Serial cross sections were made at 4–6 μm intervals. The ultrathin sections were obtained with an LKB 8800 ultramicrotome and stained with saturated aqueous uranyl acetate followed by staining with Reynolds’ lead citrate. Observations were made with a Hitachi-125 transmission electron microscope.
Results
Localization and morphology of phloem fiber cells during their development
Flax stems elongate in the first 3-cm tip region but this growth is mostly concentrated in the upper 1 cm (Fig. 1a). The growth rate of the stem was on average 5.5 mm per day (Fig. 1a). Within the first 1 cm, the stem elongation was analyzed by measuring leaf to leaf distances. It appeared that stem elongation is most intensive from 4 mm to 9 mm (Fig. 1b). From the number of leaf (primordia) per region and an average growth rate of 5.5 mm per day, it can be deduced that the first 1-mm zone takes about 20 h to be formed, the first 2 mm about 28 h and the first 11 mm 2 days. The distance between leaves increases about 20-fold in the top region. In longitudinal sections of the top portion of the flax stem, strands with procambium cells are distinguished within 100 μm from the apex. Young fibers could be recognized at a distance of 0.4–0.5 mm from the stem tip in the region of the fourth to fifth leaf primordium seen in longitudinal sections (Fig. 2a, b). The fiber cells were located at a distance of 5–6 cell rows from the epidermis, elongated in coordination with the neighboring cells and reached a length of 100–200 μm at this stage (Fig. 2a). The characteristic features of the fibers at this stage of development (coordinated growth stage) are the longitudinal shape, the round-shaped ends, the light color (due to the large vacuole and thin layer of cytoplasm), the elongate shape of the nucleus, and the absence of cell divisions. They have a diameter of 4–7 μm.
Fig. 1 Graphic representation of the elongation of 1 cm stem parts of flax during a time period of 16 days (a). Note that the elongation is restricted to the first 3 cm. The growth rate can be deduced from the increase in length of region 1, being 5.5 mm per day. The inset shows the upper part of the flax stem with marking of the stem regions. b Schematic representation of the growing flax stem tip with 10 leaf primordia (o). Distances between successive leaves on section are depicted in the table in this panel and show the length increase in micrometers (µm). Note that the distance between leaves increases 20-fold. Fig. 2 Longitudinal sections of a flax stem showing an area with coordinated growth (a) and an area where intrusive growth has just started (c). Arrows indicate tips of fibers. b Overview of a longitudinal section of the stem. The regions from which the Figs. a and c are enlargements, are indicated with rectangulars. d Isolated fiber at the intrusive growth stage, showing spindle shaped ends at two sides. The white arrow-heads point to the “knees” from where the spindle shaped ends developed. Bars a and c 20 µm; b 1000 µm; d 10 µm. Fig. 3 Time-lapse imaging of a young fiber showing chloroplast movement through the very tip of the cell indicating that the very tip does not have the characteristics of tip-growing cells. Arrow-heads point to one and the same chloroplast. Images are taken with 2–3 min intervals except for the last image. Bar 10 µm
The fiber shape changed significantly at a distance of 1–2 mm from the stem tip. Both ends of the cell formed a characteristic “knee” (Fig. 2c), that is a curve in the cell shape at the very site of the outgrowth because the extension of the cell had a smaller diameter than the cell itself. Such “knees” were clearly seen on both sides of fibers when the fibers were isolated by partial enzymatic digestion at this stage of development (Fig. 2d). The formation of the “knees” is the starting point of the intrusive growth. The curve in the cell shape often disappeared within the next several millimeters of the stem and the shape of the fiber became spindle-like. The diameter of the intruding part of the fiber was often far less than that of the original part (e.g., * in Fig. 7b, h, 8b) and the latter increased during the intrusive growth from the initial 4–7 μm at 0.5–1 mm from the tip, to about 15 μm at 5 mm from the tip, and reached 20–25 μm at 5–30 cm from the tip. Incidentally, thicker fibers were observed with a maximum of 50 μm.
The mode of intrusive elongation
Time-lapse photography with 2–3 min time intervals, of a young fiber in semi thick sections of about 0.7 mm revealed the movement of chloroplasts throughout the fiber cytoplasm. Such chloroplasts also reached the outmost tip of the fiber at the stage of intrusive growth (Fig. 3). The movement of mitochondria was visualized with time lapse photography after staining with DiOC6. They moved throughout the cytoplasm and were also observed in the very tip of the young fibers. The rate of plastid movement, calculated from scaled micrographs was approximately 2 μm per minute. Electronic supplementary material is available (ESM S1). A zone without large organelles but with accumulation of Golgi vesicles is characteristic for cells with cell tip growth. Our data indicate that there is no such zone and thus, cell tip growth is unlikely to happen in flax fibers.
Fiber elongation was further studied with Calcofluor White (CFW) which reveals the presence of cellulose microfibrils in the cell wall. Staining with CFW and immediate observation after rinsing resulted in an evenly stained cell wall (control Fig. 4a). When CFW was washed away and the sample was maintained for 24–72 h under the microscope, the cell wall of the fibers showed a patchy CFW fluorescence all over the length of the fiber (Fig. 4b, c). This patchy appearance, which is intriguing in itself, nevertheless indicates elongation of the fiber cell wall all over its length, i.e., intercalary growth.
Fig. 4 a–c Flax fibers pulse-stained with Calcofluor White and observed with UV microscopy at time 0 h (a, control) and 24 h after pulse treatment (b, c). Note the even staining in the cell wall of the control (a, arrow) and the patchy appearance of fluorescence after 24 h in the middle part (b) and tip region (c) of the fiber (arrow-heads). Bar a–c 50 µm. Fig. 5 Immunocytochemical localization of microtubules in whole mount preparations of flax fibers, depicted as Z-projections. a Flax fiber in coordinated growth stage with cortical microtubules in an orientation transversal to the long cell axis. See also electronic supplementary material (ESM) S2. b Flax fiber in coordinated growth stage with cortical microtubules with an orientation markedly deviating from transversal. See also ESM S3. c Flax fiber in intrusive growth stage with tapered ends, one of which is shown in the micrograph. Cortical microtubules have transverse orientation in the tapered end region (arrow) and helical orientation in the central zone (arrow-head). See also ESM S4. d Two flax fibers in the intrusive growth stage showing helical cortical microtubules with an orientation greatly deviating from transversal. See ESM S5. e Flax fiber in the region below snap point with microtubules parallel to the long axis of the cell. See also ESM S6. f–g Two images of the same part of a fiber below snap point showing the cortical microtubules parallel to the long axis of the fiber (f) and the thickened cell wall at dim bright field (g). SCW secondary cell wall; C cytoplasm. Bar a–d, f–g 10 µm; e 25 µm. For 3-D impressions see ESM, S2–S6. Fig. 6a Longitudinal section of a multinucleate flax fiber at the coordinated growth stage showing synchronous nuclear divisions within that fiber. b–e Almost synchronous division of nuclei within one fiber observed during the intrusive growth stage. Note the vacuole in e (*) between the just divided nuclei. f–g Microtubules during successive stages of cell division in flax fibers showing a preprophase band of microtubules (f) and an oblique spindle (g). see also ESM S7, S8. h Multinucleate flax fiber showing the distribution of nuclei stained with DAPI. F fiber, P parenchyma cell. Bar a and h 20 µm; b–g 10 µm
As it cannot be excluded that the fibers in the culture condition as described above, do change their behavior and might stop cell tip growth, it was decided to fix cells and to visualize the microtubular cytoskeleton in both the tip region and other parts of elongating fibers. Microtubules in the tip zone will show either an organization characteristic for tip growing cells or a pattern known for diffuse growing cells. To this end MT configurations were visualized by immunocytochemistry in whole mount samples, and the mode of fiber elongation was deduced from the MT configuration. At the coordinated growth stage, cortical MTs had a transverse orientation with respect to the length axis of the fiber (Fig. 5a). During the start of the intrusive elongation, fibers exhibited helical cortical microtubules (Fig. 5b). The middle part of the cell often had a helical ordering of MTs, while the tip region showed a transversal orientation of MTs (Fig. 5c). Later in development, most elongating fibers exhibited helical MTs with varying angles (Fig. 5d). When fiber elongation had already completed, the orientation of the MTs both in the middle and tip part of the cell was longitudinal (Fig. 5e). Such cells had thickened secondary cell walls (Fig. 5f, g). An overview of the major cortical MT configurations in successive regions of the stem is given in Table 1. Electronic supplementary material (ESM) is available (see legend Fig. 5).
Symplasmic isolation of the fibers at the stage of intrusive growth
Plasmodesmata connections of phloem fibers with neighboring cells were counted on electron micrographs at the stages of coordinated and intrusive growth. At the coordinated growth stage, plasmodesmata were observed in cell walls between fibers and parenchyma cells and in cell walls between two fibers. The frequency of plasmodesmata per micrometer of fiber cell wall length was rather low at the coordinated growth stage (Table 2) being in fiber–parenchyma twice as high as in fiber–fiber surfaces. At the stage of intrusive growth, plasmodesmata were, however, never observed. This is a signal of symplasmic isolation.
Karyokinesis is disconnected from cytokinesis and differs at the stages of coordinated and intrusive growth
Although fibers did not exhibit cell division, their nuclei divided. Fibers became multinucleate at a very early stage of development, i.e., in the region 1.5 mm from the apex. Nuclear divisions within one fiber occurred synchronously or almost synchronously during the coordinated growth stage (Fig. 6a). At the intrusive growth stage, the number of nuclei within a fiber increased further but the total number of nuclei per cell became irregular and not equal to 2n, e.g., 14, 22, 23, 26 and 28 nuclei were counted in individual isolated fibers after DAPI staining. Nuclei were dispersed unevenly along the cell, sometimes forming clusters of 2–3 (Fig. 6h).
Nuclear division in the fibers was normal (Fig. 6b–e), but cytokinesis did not occur; Preprophase bands were observed at the onset of karyokinesis (Fig. 6f); Mitotic spindles had a normal appearance but their orientation was often oblique to the long axis of the fiber (Fig. 6g). However, phragmoplasts and cell plates were not formed. Instead, vacuoles were present between the dividing nuclei (Fig. 6e).
Fiber cell wall thickening
There is progressive cell wall thickening starting from about 5 cm from the tip onward, i.e., from snap point region, and lasting for weeks until almost all available space within the fiber is occupied (Fig. 7). Cell wall thickening starts in the fibers located at the outside of the vascular bundle. Cell walls in the fibers more to the center of the stem started to thicken later (Fig. 7), similar as shown by Gorshkova et al. (2003a). Thus, at one level of the stem at or below snap point, some fibers within a bundle do have thickened cell walls whereas others have not (see Fig. 7e , 8c).
Fig. 7 Cross sections of a flax stem at increasing distances from the stem tip showing the process of cell wall thickening from 3 to 70 cm from the tip. a Overview of stem section in which the epidermis (Ep), the cortex (Co), the endodermis (En) and the fiber region (FR) are labeled. b–h Details of the developing phloem fibers at 3 cm (b), at 5.5 cm (c), at 7 cm (d), at 8 cm (e), at 15 cm (f), at 25 cm (g) and 70 cm (h). All images are in the same orientation as (a). Note that the cell walls of different fibers within the bundle depicted in (e) have different thicknesses because of different stage of development, the ones with the thickest cell walls being positioned at the outer side of the bundle. Note that fibers within a section vary in diameter because of intrusive growth; see e.g. * in (b) and (h). Bars a–h 50 µm. Fig. 8 a Representation of flax fiber length measurements in successive stem regions running from 0 to 550 mm below the tip. Lengths of fibers are given in milli meter and range from 1 to 25 mm. The three lines indicate maximal values, average values, and minimal values found for each sample. The fibers measured, are those that already have formed thickened cell walls. Note that longer fibers are found in lower parts of the stem. b–c Isolated flax fibers after maceration in CrO3, HNO3 and lactic acid overnight, and observed with differential interference contrast (DIC) microscopy. b Fiber from the region 5–10 cm below the tip with long tapered end which is the result of intrusive growth. c Fibers from the region 15–20 cm below the tip. Note that the fiber cell walls still vary in thickness amongst fibers but have a certain thickness per fiber. Arrow points to thin-walled fiber. Bars b–c 200 µm
Fiber lengths were measured at various heights in the stem. Fibers were first isolated by maceration (Fig. 8b, c), hence, only fibers with relatively thick cell walls were properly preserved and measured. It appeared that longer fibers were found in lower regions of the stem with a maximum length of 25 mm in the lower part, more than 21 cm from the stem tip (Fig. 8a).
Discussion
Flax phloem fibers are primary fibers which originate from the protophloem close to the apical meristem (Esau 1943).
The mode of the intrusive elongation of flax fibers has long been considered to be tip growth (Esau 1943, 1977; Fahn 1990). However, the data on autoradiography of labeled fibers (Gorshkova et al. 2003a) are indicative for diffuse elongation (that is along the whole surface of the cell). To obtain further insight in this process, the tip of the growing fiber was analyzed by tubulin immunolabeling, by electron microscopy and by light microscopy.
Cells elongating by tip growth are known to contain two specific zones of cytoplasm in the tip (Review: Geitmann and Emons 2000), a zone with vesicles at the very tip, and a zone with longitudinal ER, Golgi bodies, mitochondria, plastids and other small organelles in the subapex with the nucleus at the base of the subapex (Miller et al. 2000). However, in flax, it was found that organelles such as plastids and mitochondria moved to the very tip of the fiber (Fig. 3 and ESM1) indicating that a tip growth machinery is absent.
The subapical area of a tip growing cell contains longitudinally aligned actin filaments (Ketelaar et al. 2002; 2003), and longitudinal microtubules (Medicago: Sieberer et al. 2002; Arabidopsis: Van Bruaene et al. 2004). In the intrusively elongating flax fiber, the microtubules in the subapex, on the contrary, were either aligned transversally to the long axis of the cell, or they had a helical orientation. Such orientations are typical for diffusely elongating cells (Tiwari and Wilkins 1995; Wasteneys and Galway 2003).
Microtubules are found in transversal, helical and parallel arrays in the fibers. These arrays were found in a position dependent way (Table 1). As microtubules are oriented perpendicularly or obliquely with respect to the growth axis of the cell in cells undergoing diffuse growth, the growth state of a cell can be inferred from the orientation of its microtubules (Lloyd 1999; Wasteneys 2000; Bichet et al. 2001). Indeed, phloem fibers in the coordinated growth stage exclusively exhibited transversal arrays of MTs and only elongated; fibers at the intrusive growth stage had helical patterns with varying pitch, and both elongated and widened; and fibers with parallel arrays were found from snap point onward. They had their final length and width and had started secondary cell wall formation.
The fibers that exhibited “knee-like” structures and tapered ends are thought to be the ones starting intrusive growth. Interestingly the formation of the “knees” often coincided with the presence of two types of MT configurations within one fiber. The regions distal from the “knees” had microtubules in transversal position whereas helical patterns were often found between the “knees”. This points to differences in diffuse growth rates over the length of the fiber. Initially, the tips might elongate more and widen less than the middle region of the fiber and indeed fibers often had very slender and long distal ends at two sides.
Further evidence for diffuse growth of fibers was obtained by the Calcofluor White pulse-chase label experiments. Whereas CWF-stained fibers showed a homogenous staining pattern in their cell walls directly after staining, the staining became patchy after a chase for 24 h. The patchy appearance of fluorescence indicates that there is probably deposition of cell wall material all over the length of the fiber, now recognized as unstained cell wall material. The patchy appearance, however, also shows that there may not be a pure diffuse incorporation of new cell wall material. The reason of such patterning has not been revealed but might be related with localized deposition of cellulose. Plasmodesmata were no longer found in fiber cell walls after the coordinated growth stage. This might be the result of diffuse growth of individual fibers and it makes the fibers a symplasmically isolated domain. Interestingly, the fibers had well-developed chloroplasts which likely function in the supply of carbohydrates.
Flax fibers have long been known to be multinucleate. In the first discussions on the mode of fiber development this fact was the argument for fiber formation by fusion of a row of parenchyma cells (cf. Anderson 1927). During fiber elongation the cell, however, becomes a syncytium, because karyokinesis is not followed by cytokinesis. Although there is no cell plate formation, preprophase bands are being formed. They may determine the position of the nucleus. Well-known examples of multinucleate plant cells are syncytial endosperm (van Lammeren 1986; Fahn 1990) and feeding cells induced by nematodes (de Almeida Engler et al. 2004). According to our data, the nuclear divisions in a fiber occur only during the elongation of the cell. Multinuclearity is, however, not a prerequisite for a cell to reach a significant length. The cotton seed hairs, being not fibers, but trichomes from botanical point of view, reach similar lengths as flax fibers, though they have a single nucleus (Basra 1999).
Fiber elongation starts at the very stem tip and continues until snap point (Gorshkova, 2003a). Cell wall thickening of fibers starts before snap point and continues for many weeks throughout the stem (see also Gorshkova et al. 2003b). In the maceration experiment described above we found shorter fibers above snap point and longer ones below (Fig. 8a). How to explain this when fibers do not elongate any more below snap point? The fibers measured at snap point, are the ones which withstood maceration. Thin walled fibers were not measured. It is to be expected that the fibers which will become the longest ones in a later phase of development, are not yet thick walled at snap point and are therefore, not measured after maceration. The first fibers that differentiate are the ones which lay at the outer side of the fiber bundle and those that are formed at last are found at the inner side of a fiber bundle. Thus, it can be deduced that within a bundle of fibers, the shorter ones are positioned at the outer side and the longer ones more inwardly.
Based on the data obtained we conclude that flax fibers initially have a coordinated growth stage with characteristic cell properties during a limited period of time (see Table 3 for an overview). This phase is followed by an intrusive growth stage lasting longer and again with characteristic cell properties (Table 3). Fiber elongation started in almost the very tip of the stem, ended at snap point, and is characterized by coenocyte formation and symplastic isolation. All data concerning CFW staining, organelle movement, and patterning of the microtubular cytoskeleton in the subapical region of the elongating fiber point to a diffuse mode of cell elongation.
Abbreviations
- CFW :
-
Calcofluor White
- CLSM :
-
Confocal laser scanning microscope
- GA :
-
Gluraraldehyde
- ESM :
-
Electronic supplementary material
- HAH :
-
Hydroxyltetra-ammoniumchloride
- Mes :
-
2-(N-morpholino)ethanesulphonic acid
- MSB :
-
Microtubule stabilizing buffer
- MT(s) :
-
Microtubule(s)
- PBS :
-
Phosphate buffered saline
- PFA :
-
Paraformaldehyde
- Pipes :
-
Piperazine-N,N′-bis(2-ethanesulfonic acid)
- RT :
-
Room temperature
- SP :
-
Snap point
References
de Almeida Engler J, Van Poucke K, Karimi M, De Groodt R, Gheysen G, Engler G, Gheysen G (2004) Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode-infected roots. Plant J 38:12–26 http://dx.doi.org/10.1111/j.1365-313X.2004.02019.x
Anderson DB (1927) A microchemical study of the structure and development of flax fibers. Amer J Bot 14 (1):187–211
Basra AS (ed) (1999) Cotton fibers: developmental biology, quality improvement, and textile processing. Food Products Press, Binghamton, p 387
Bichet A, Desnos T, Turner S, Grandjean O, Höfte H (2001) BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J 25:137–148 http://dx.doi.org/10.1046/j.1365-313x.2001.00946.x
Esau K (1943) Vascular differentiation in the vegetative shoot of Linum. III. The origin of bast fibers. Amer J Bot 30:579–586
Esau K (1977) Anatomy of seed plants. Wiley, New York
Fahn A (1990) Plant anatomy. Pergamon Press, Oxford, p 587
Geitmann A, Emons AMC (2000) The cytoskeleton in plant and fungal cell tip growth. J Microsc 198:218–245 http://dx.doi.org/10.1046/j.1365-2818.2000.00702.x
Gorshkova TA, Ageeva MV, Sal’nikov VV, Pavlencheva NV, Snegireva AV, Chernova TE, Chemikosova SB (2003b) Stages of bast fiber formation in Linum usitatissimum L. Bot J (Russian) 12:1–11
Gorshkova TA, Sal’nikov VV, Chemikosova SB, Ageeva MV, Pavlencheva NV, van Dam JEG (2003a) The snap point: a transition point in Linum usitatissimum bast fiber development. Ind Crops and Prod 18:213–221 http://dx.doi.org/10.1016/S0926-6690(03)00043-8
Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NCA, Grierson CS, Dogterom M, Emons AMC (2002) Positioning of nuclei in Arabidopsis root hairs: an actin regulated process of tip growth. Plant Cell 14:2941–2955 http://dx.doi.org/10.1105/tpc.005892
Ketelaar T, de Ruijter NCA, Emons AMC (2003) Unstable F-actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell 15:285–292 (Published online December 19, 2002; http://dx.doi.org/10.1105/tpc.007039
Lev-Yadun S (2001) Intrusive growth—the plant analog of dendrite and axon growth in animals. New Phytologist. 150: 508–512 http://dx.doi.org/10.1046/j.1469-8137.2001.00143.x
Lloyd C (1999) How I learned to love carrots: the role of the cytoskeleton in shaping plant cells. Bioessays, 21: 1061–1068 http://dx.doi.org/10.1002/(SICI)1521-1878(199912)22:1<1061::AID-BIES12>3.0.CO;2-2
McDougall GJ, Morrison IM, Steward D, Weyers JDB, Hillman JR (1993) Plant fibers: botany, chemistry and processing for industrial use. J Sci Food Agric 62:1–20
Miller DD, Leferink -ten Klooster HB, Emons AE (2000) Lipochito-oligosaccharide nodulation factors stimulate cytoplasmic polarity with longitudinal endoplasmic reticulum and vesicles at the tip in vetch root hais. MPMI 13:1385–1390
Sieberer BJ, Timmers ACJ, Lhuissier FGP, Emons AMC (2002) Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs. Plant Physiology 130:977–988 http://dx.doi.org/10.1104/pp.004267
Tiwari SC, Wilkins TA (1995) Cotton (Gossipium hirsutum) seed trichomes expand via diffuse growing mechanism. Can J Bot 73:746–757
Van Bruaene N, Joss G, van Oostveldt P (2004) Reorganization and in vivo dynamics of microtubules during Arabidopsis root hair development. Plant Physiol 136:3905–3919 http://dx.doi.org/10.1104/pp.103.031591
Van Lammeren AAM (1986) Structure and function of the microtubular cytoskeleton during endosperm development in wheat: an immunofluorescence study. Protoplasma 146:18–27
Wasteneys G O (2000) The cytoskeleton and growth polarity. Curr Opin Plant Biol 3:503–511 http://dx.doi.org/10.1016/S1369-5266(00)00120-5
Wasteneys GO, Galway ME (2003) Remodeling the cytoskeleton for growth and form: an overview with some new views. Ann Rev Plant Biol 54:691–722 http://dx.doi.org/10.1146/annurev.arplant.54.031902.134818
Acknowledgements
This work was supported by the program Molecular and Cellular Biology of the Russian Academy of Sciences (MCB RAS), by grants 03-04-96223 and 03-3.10-232 of the Russian Foundation of Basic Research (RFBR) -Tatarstan scientific program, and by fellowships of The Dutch Ministry of Agriculture, Nature Management and Fisheries to AMV and PB. We thank Dr. L.E. Muravnik (Botanical Institute, St. Petersburg) for the help in statistical evaluation of electron microscopy data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ageeva, M.V., Petrovská, B., Kieft, H. et al. Intrusive growth of flax phloem fibers is of intercalary type. Planta 222, 565–574 (2005). https://doi.org/10.1007/s00425-005-1536-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-1536-2