Abstract
To elucidate the role of auxin in flower morphogenesis, its distribution patterns were studied during flower development in Arabidopsis thaliana (L.) Heynh. Expression of DR5::GUS was regarded to reflect sites of free auxin, while immunolocalization with auxin polyclonal antibodies visualized conjugated auxin distribution. The youngest flower bud was loaded with conjugated auxin. During development, the apparent concentration of free auxin increased in gradual patterns starting at the floral-organ tip. Anthers are major sites of high concentrations of free auxin that retard the development of neighboring floral organs in both the acropetal and basipetal directions. The IAA-producing anthers synchronize flower development by retarding petal development and nectary gland activity almost up to anthesis. Tapetum cells of young anthers contain free IAA which accumulates in pollen grains, suggesting that auxin promotes pollen-tube growth towards the ovules. High amounts of free auxin in the stigma induce a wide xylem fan immediately beneath it. After fertilization, the developing embryos and seeds show elevated concentrations of auxin, which establish their axial polarity. This developmental pattern of auxin production during floral-bud development suggests that young organs which produce high concentrations of free IAA inhibit or retard organ-primordium initiation and development at the shoot tip.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floral organs are initiated sequentially by the floral meristem. In Brassicaceae such as Arabidopsis, the latter produces four successive whorls, starting with the outermost whorl of sepals, and progressing inward with a second whorl of the petals, a third whorl of the stamens, and finally a fourth whorl of the carpels. The remaining apical meristem, enclosed by the carpels, ultimately forms a placenta on which the ovules develop (Sussex 1989). Unexpectedly, although the petal and stamen primordia in Arabidopsis appear simultaneously (early stage 5 of flower development), the stamens develop first (during stages 6 to 8), whereas the petal primordia do not grow until stage 9 (Bowman et al. 1989); a phenomenon which has wide occurrence among flowering plants (Endress 1994). However, the mechanism which controls this surprising developmental pattern is unknown.
The old opinion that floral organs are homologous to foliage leaves (see Smyth 2005) has been confirmed by genetic studies showing that the floral organs are modified leaves. In the Arabidopsis ABC triple mutant, apetala2, pistillata and agamous1 (Bowman et al. 1991; Weigel and Meyerowitz 1994) and in the quadruple mutant ap1, ap2, ap3/pi and ag (Goto et al. 2001), the absence of organ identifying activities causes all floral organs to develop as leaves.
The auxin indole-3-acetic acid (IAA) produced in young leaves is the major controlling signal in vascular pattern formation (Aloni 1987, 2004; Turner and Sieburth 2002). The leaf-venation hypothesis (Aloni 2001) explains how free auxin induces and controls vascular differentiation and venation pattern formation in foliage leaves of dicotyledonous plants. As the floral organs are modified leaves, the above concept can also be applied to predict the sites and timing of free-auxin production during morphogenesis of floral organs. However, additional specific modifications are required for the different floral organs, as proposed below. In foliage leaves, hydathodes, the water-secreting glands, are the primary sites of free-auxin production. The production of free IAA in a leaf primordium begins at the tip, during leaf ontogeny, gradually progressing basipetally along the leaf margins, and finally appearing also in the lamina (Aloni 2001). This developmental pattern of free-IAA production was experimentally and analytically confirmed in rosette leaves of Arabidopsis (Müller et al. 2002; Aloni et al. 2003) and is in agreement with the developmental patterns of auxin-conjugate hydrolases in the leaf margins and lamina (Rampey et al. 2004).
Genetic evidence shows that in Arabidopsis the polar transport of auxin controls flower formation and differentiation (Okada et al. 1991; Bennett et al. 1995; Oka et al. 1999; Reinhardt et al. 2003). Genes regulating floral organ development and gynoecium vascularization were discovered (Sessions and Zambryski 1995; Roe et al. 1997; Sessions et al. 1997; Bowman et al. 1999; Eshed et al. 1999; Alvarez and Smyth 1999, 2002), indicating probable auxin involvement in flower development. Furthermore, auxin controls vascular polarity during gynoecium morphogenesis (Nemhauser et al. 1998, 2000; Christensen et al. 2000). Although auxin was detected in the youngest flower bud at the inflorescence tip and its concentrations remarkably increased after pollination (Müller et al. 2002), there is no physiological understanding so far as to where and when free IAA accumulates during flower development, and the hormonal mechanisms that regulate floral organ growth, e.g., why the stamens develop before the petals, and venation pattern formation are still a mystery.
To clarify the role of auxin in flower development, we assume that, as in foliage leaves (Aloni 2001; Aloni et al. 2003), also in each floral organ the free-auxin production starts at the tip. Accordingly, vascular tissues would develop basipetally. The two innermost whorls, those of stamens and carpels, form the most modified floral organs that need further explanation. We suggest that these reproductive organs need extensive production of phytohormones for their biological functions. Consequently, the development of these two innermost whorls would trigger successive cascades of phytohormone production during reproductive organ morphogenesis and, as indicated above, auxin produced in growing flowers (Müller et al. 2002) would be a major regulatory signal in flower morphogenesis. According to the above assumptions, we suggest the following concept, with specifications for each floral organ:
-
1.
For the first whorl, the sepals, leaflike green organs most similar to foliage leaves, their free-auxin production and vascular pattern formation are already described (Aloni 2001).
-
2.
In the second whorl, the petals are characterized by a brief phase of rapid growth during a late stage of flower development (Bowman et al. 1989). Therefore, the petals are assumed to produce very low free IAA and hence to induce relatively simple vascular patterns restricted to the procambium configuration induced in their early primordial stage.
-
3.
In the third whorl, the stamens, IAA is synthesized in the anthers. High concentration of free IAA would be needed to promote the growth of pollen tubes to the ovules within the gynoecium.
-
4.
The fourth whorl, the gynoecium, produces free auxin in the stigma. Ovules produce low IAA concentrations. After fertilization, free auxin will boost in the developing embryos.
The present study was designed to test our concept pertaining to the role of auxin in Arabidopsis flower morphogenesis. The localization of free auxin can be visualized in leaf primordia (Aloni et al. 2003) by the expression of GUS fused to the highly active synthetic auxin-response element, referred to as DR5 (Ulmasov et al. 1997). The DR5::GUS expression pattern was demonstrated to reflect free-auxin concentrations in shoots and roots of Arabidopsis transformants between 10−8 and 10−4 M (Ulmasov et al. 1997; Sabatini et al. 1999). The comparison of quantified GUS activity (Sabatini et al. 1999) with auxin concentration gradients measured by mass spectrometry (Marchant et al. 2002) in Arabidopsis roots clearly showed a positive correlation of free auxin and GUS expression in DR5::GUS transformed Arabidopsis. Therefore, analysis of DR5::GUS gene expression in transformed Arabidopsis visualizes the sites of intense free-IAA concentrations (Sabatini et al. 1999; Friml et al. 2002; Marchant et al. 2002; Avsian-Kretchmer et al. 2002; Aloni et al. 2003; Mattsson et al. 2003; Zgurski et al. 2005) during flower and seed development. Accordingly, this study analyzes the role of free-auxin accumulation in controlling flower and silique (fruit) development in Arabidopsis. The distribution of conjugated IAA was elucidated here in Arabidopsis flowers by immunolocalization with specific polyclonal antibodies, as recently with polyclonal and monoclonal antibodies in Arabidopsis leaves (Aloni et al. 2003).
Materials and methods
Plant material
DR5::GUS transformants of Arabidopsis thaliana (L.) Heynh. ecotype Columbia, kindly provided by T. J. Guilfoyle (Columbia, MO, USA), were grown on soil (Avi Saddeh mix; Pecka Hipper Gan, Rehovot, Israel), in 5-cm pots. Plants were grown in a growth chamber under long-day conditions (16-h-light/8-h-dark cycles) at 23°C. The light intensity was 100 μE m−2 s−1 at the plant level.
The transformant DR5::GUS comprises seven-copy tandem direct repeats of 11 bp that include the auxin-responsive TGTCTC element, which is placed upstream of a minimal −46CaMV35S promoter-it GUS reporter gene, as described in detail by Ulmasov et al. (1997). DR5 shows stronger auxin responsiveness than does the natural composite AuxRE (D1-4(8x)AuxRE) or the GH3 transformant (Ulmasov et al. 1997). In order to elucidate the developmental changes in auxin patterns during flower and seed development, we serially analyzed all flowers and siliques along each of the studied plants. More than 2,000 Arabidopsis flowers were analyzed during this study.
Immunolocalization of auxin
Inflorescences of 5-week-old plants were fixed at 22°C for 4 h with 4% (w/v) paraformaldehyde in 0.1x phosphate-buffered saline (PBS) containing 0.1% (v/v) Triton X-100 (0.1× PBS: 13.7 mM NaCl, 0.15 mM KH2PO4, 0.79 mM Na2HPO4, 0.27 mM KCl, pH 7). Comparative tissue fixation with 4% (w/v) N-(3-dimethylethylaminopropyl)-N′-ethylcarbodiimide-HCl (EDC), as previously used (Aloni et al. 2003), resulted in no different auxin-antibody labeling pattern or intensity as obtained with paraformaldehyde, indicating that the IAA antibodies that were used here recognized auxin conjugates independent of the fixation method. This suggests that the low concentration of 1–5% of free auxin, which was reported to be linked via the auxin-carboxyl group to proteins only upon EDC and not paraformaldehyde fixation and hence is recognizable by IAA-HSA (human serum albumin) raised antibodies, is completely hidden by the high concentration pool of conjugated auxin compounds. Therefore, the antibody-labeled auxins in our study have to be regarded as endogenous pre-conjugated auxin compounds.
After dehydration with a graded series of ethanol at 22°C, the tissue was imbedded overnight in Steedman’s wax, a polyester with a low melting point (PEG 400 distearate in 1-hexadecanol 9:1, w/w). Longitudinal sections (12 μm thick) were prepared with a cryomicrotome (Cryocut CM 3050, Leica, Bensheim, Germany) and collected on poly-L-lysine coated slides. The sections were dewaxed in decreasing ethanol concentrations, rinsed with 0.1× PBS, incubated with buffer for 30 min and with 100% methanol for 10 min at −20°C. For immunolabeling, the sections were incubated overnight with rabbit polyclonal antibodies, raised against IAA-HSA conjugate, at a dilution of 1:100 (kindly provided by S. Veselov, University of Ufa, Russia). The cross-reactivity of these antibodies had a high degree of specificity for IAA over structurally and physiologically related compounds (Veselov et al. 1992). These primary antibodies were labeled with the green fluorescent Alexa conjugate (488 goat anti-rabbit IgG, H+L, Molecular Probes, Eugene, OR, USA) as secondary antibody, diluted 1:200 with 1× PBS and applied for 2 h at 22°C. After immunolabeling, the sections were mounted on slides in 1,4-diazabicyclo(2.2.2)octane (DABCO)-containing buffer. Control sections were incubated by following the same protocol as described above, but with 1% (w/v) BSA in 0.1× PBS without primary IAA antibodies or with inactivated primary antibodies. Generally these control sections showed weak green or yellow autofluorescence (ESM a-f, viewed by epifluorescence microscopy, Aristoplan, Leica, filter block I3: excitation BP 450–490 nm, emission LP 515 nm; for further controls see ESMs in Aloni et al. 2003 and Veselov et al. 2003). About 500 sections were viewed with a confocal laser scanning microscope (TCS SP-MP, DM IBRE inverted microscope; Leica). The fluorescence was excited with 488-nm light (emission 515–525 nm) using a 50-mW krypton/argon laser. Further details were described recently (Langhans et al. 2001; Aloni et al. 2003; Veselov et al. 2003).
β-Glucuronidase analyses
For histochemical staining of GUS activity, inflorescences were vacuum infiltrated for 30 min with the staining solution containing 1 mM 5-bromo-4-chloro-indolyl-β-D-glucuronide at pH 7.0 (X-Gluc; Molecular Probes, Eugene, OR, USA) and 0.1% Tween 20, and were incubated for 48 h at 37°C according to Jefferson (1987). Then the plants were cleared with a solution of 100% chloral hydrate: 90% lactic acid (2:1, v/v) and were kept at room temperature for at least 24 h. Samples were viewed in 90% lactic acid (light microscope model BH2, Olympus, Japan). Micrographs were reproduced from color slides taken with an OM-2 camera (Olympus) on Fujichrome 64T film (64 ASA Fuji Photo Film, Japan).
Selective removal of floral organs
Very young floral buds were gently opened under a SMZ800 stereoscopic zoom microscope (Nikon, Japan) under magnifications ranging from ×10 to ×40. In each treated flower, only one type of floral organ was removed from the whole plant. After treatment, the floral bud was gently closed again. Usually, five flowers were treated per inflorescence, leaving intact control flowers between the treated flower buds. In about half of the inflorescences all flowers received the same treatment, namely, the same floral organ was removed. In the remaining half, each flower in the inflorescence was treated differently, either by removing one or two sepals, or three petals, or the upper part of 3–4 stamens, or the upper part of the gynoecium. In each experiment all the flowers were harvested together between 2 and 7 d after organ removal. Because of variability in the results, stemming from the major difficulty of removing the organs in extremely small flower buds, more than 100 flowers were used for each treatment. All experiments were repeated three times.
Results
Effect of exogenous auxin application on GUS expression in flowers
The effect of exogenous free-auxin concentrations on DR5::GUS expression during flower morphogenesis was studied on excised inflorescences from the same developmental stage of 4-week-old Arabidopsis plants which were incubated with 0.1–100 μM external α-NAA. The pattern confirmed that auxin-dependent DR5::GUS expression reflects auxin responsiveness not only due to an increase in free-auxin concentration but also due to different tissue sensitivity, e.g., low response of petals in comparison to strong response of sepals, anthers, style and stigma (Fig. 1a–e). Such different tissue response is assumed to result from pre-existing different endogenous auxin concentrations (Nakamura et al. 2003) and accordingly in our study petals with low versus anthers with high endogenous auxin concentrations show different auxin responsiveness. The major sites of high endogenous auxin concentration in developing floral buds are the young stamens (Fig. 1g) and stigma (Fig. 2f). Shortly after stamen removal, the otherwise unstained petals showed elevated GUS expression (Fig. 3h), and removal of the stigma caused detectable GUS expression in the otherwise unstained distal cells of the remaining gynoecium (Fig. 3c), indicating an increase in free-IAA concentration in the petals and in the apical gynoecium, and not a change in tissue sensitivity. Therefore, IAA-dependent DR5::GUS responsiveness is termed here as “production”, standing for free-auxin concentration, resulting from de novo synthesis, accumulation and hydrolysis of auxin conjugates.
Auxin-dependent DR5::GUS gene expression in transformed Arabidopsis during early stages of flower morphogenesis. a–e Effect of exogenously applied α-NAA on GUS expression in flowers; 0 μM (a), 0.1 μ M (b), 1 μ M (c), 10 μ M (d), and 100 μ M (e). (f) Inflorescence with very weak (arrow) or without any GUS activity in the youngest flower buds (insert); larger buds with transient GUS expression in the petals and style and persistent in the stamens. (g) Young flower with strong GUS expression only in the stamens, in anthers and filaments (large arrow), before development of petals and of the vascular tissues in the gynoecium (arrowhead); GUS activity in the tip of sepals (small arrow). (h) Close-up of a very young anther with high GUS activity in the tapetum cells (arrow). (i) GUS expression in parenchyma cells (arrow) of the pollen sacs, without GUS expression in the filament. (j) Strong GUS expression at the tip of the filament (arrow), after dispersal of the pollen grains. The tip of the stamen’s vascular bundle is marked by small arrow. (k) Young nectary (arrow) without GUS activity. Bars = 25 μm (h), 100 μm (i,j), 200 μm (g,k), 500 μm (a–f)
IAA-dependent DR5::GUS gene expression in transformed Arabidopsis in (a–c) nectaries, (d,e) petals, (f,g) stigma and (g–j) during pollen germination. (a) Incipient low GUS expression at the tip (arrow) of a nectary before anthesis. (b) Early strong GUS expression (arrow) in a nectary of a younger flower promoted by four-stamen removal. Both flowers shown in (a) and (b) are from the same inflorescence. (c) GUS activity in nectaries (arrows) continues after pollination, and is detectable below a developing silique with differentiating seeds with strongest GUS expression where the embryonic root develops (arrowheads), while unfertilized ovules (small arrows) lack GUS expression. (d) Confined sites of low GUS expression in the tip of a petal and at the tip of a vascular bundle (arrows).(e) Close-up of a petal with GUS activity at the tip and the upper side of a vascular bundle loop (arrows). (f) Early stage of GUS expression in the stigma (arrowhead) inducing the central vascular bundle of the gynoecium and anthers with lower GUS activity (arrow). (g) Mature gynoecium with germinating pollen grains (arrow). Well-developed wide stylar xylem (white arrow). A few pollen grains (arrowhead) with high GUS expression are still in the anthers (arrowhead). (h) Strong GUS expression in germinating pollen grains (arrows) and (i) in a pollen tube (arrow) and an empty pollen grain (arrowhead) from which most of the cytoplasm has moved into the tip of the elongating pollen tube. (j) High GUS activity in a germinating pollen grain whose pollen tube (arrow) penetrates the cuticle of one of the stigmatic papillae. Bars = 20 μm (j), 50 μm (h, i), 100 μm (a, b, e), 200 μm (c, d, f, g)
IAA-dependent DR5::GUS gene expression in transformed Arabidopsis after (a,b) pollen germination, (d) in a fertilized embryo sac, (e,f) developing siliques, (g–i) flowers from which some floral organs had been experimentally removed and (c) following the removal of the upper part of the gynoecium. (a) Stigmatic papillae through which pollen tubes have grown lack GUS activity (arrow), in contrast to GUS expression in intact papillae (upper left side). (b) Collapse of stigmatic papillae after pollination results in strong GUS activity in the parenchyma cells located immediately beneath the stigma (arrowhead) with well-developed stylar xylem (white arrow). (c) GUS expression at the cut surface (arrowhead), where the upper portion of the gynoecium was removed. Note degenerated ovules. (d–f) Polar patterns of GUS expression after fertilization. (d) Longitudinal view of an embryo sac one day after fertilization, showing an early stage of polarity in which GUS expression (arrow) is concentrated at the basal end (located at the upper side), where the embryonic root will develop. (e) GUS expression at the base of developing seeds and in their funiculus (this stage is more advanced than the stage of developing seeds shown in Fig. 2c). (f) In a more advanced developmental stage, GUS activity is mainly in the funiculus. (g–i) Effect of selective removal of floral organs. (g) GUS expression induced at the tip (arrow) of a remaining stamen filament after excision of anthers and upper filament tissues. (h) Elevated GUS expression in the petals (black arrow) induced by the removal of three anthers (white arrow). (i) GUS expression throughout a very young gynoecium and stamen induced by sepal removal at an early developmental stage. At this stage the gynoecia of intact flowers do not show GUS expression (see Fig. 1g). Bars = 20 μm (d), 100 μm (a–c, g), 200 μm (e, f, h, i)
Concerning the specificity of the IAA-dependent DR5::GUS expression, it was recently reported that not only auxin but also brassinolide (BL) are effective independently of each other (Nakamura et al. 2003). According to these findings, a transient activation of the auxin-response element, as found in our study, is characteristic for an effect of IAA but not of BL. Furthermore, the obviously higher IAA than BL concentration required for activation of the AuxRE (Nakamura et al. 2003), analytically found during flower and silique development in various organs of Arabidopsis (Müller et al. 2002), as well as the present detection of high auxin-conjugate concentrations by immunolocalization (Fig. 4a–i), also indicate that IAA and not BL are likely to be responsible for the observed DR5::GUS expression here.
Distribution of conjugated auxin detected by immunolocalization with rabbit polyclonal antibodies (green Alexa Fluor 488 fluorescence) in DR5::GUS-transformed Arabidopsis, viewed by CLSM. (a) Auxin distribution in three developmental stages of flower primordia. The youngest flower bud (arrow) before any visible organ initiation is already loaded with conjugated auxin, while the most developed flower (before petal growth) shows differential auxin patterns with highest auxin concentrations mainly in the anthers and gynoecium. (b) Close-up, all cells of the youngest flower (arrow), including the upper layer of tunica, with high auxin concentrations. In the older flower primordium, the auxin is concentrated in the promeristem (arrowhead) and sepals (s), with a basipetally decreasing auxin concentration. (c) Very young stamen primordia (arrow) with the highest auxin concentrations, higher than in the promeristem (arrowhead) and sepals which already protect the stamens. (d) Elevated auxin in the stigma, developing ovules in the gynoecium (g) and the differentiating pollen sacs, before petal initiation. (e) Early stage of petal growth characterized by a short phase of high auxin concentration (arrowhead). The gynoecium and anthers (arrow) continue to maintain high auxin concentrations during later developmental stages. (f) Auxin labeling in nectaries (arrow). (g) Auxin accumulation in pollen grains (arrows). (h) High auxin concentrations in the stigma (arrowhead) and ovules (arrow), and lower in the sepals (s) and petals (p). (i) Close-up with auxin in the stigmatic papillae (arrowhead) and ovules (arrow). Bars = 40 μm (b, c, g), 80 μm (a, d–f, h, i)
Gradual shifts in the sites of free-auxin production during flower development
In very young flower buds (stage 8 according to Bowman et al. 1989), free-auxin-dependent DR5::GUS expression appears in the tip of the sepals (Figs. 1f insert, g, small arrows), and is followed by a conspicuous but transient GUS expression in young petals and style (Fig. 1f), longer persisting in young stamens (Fig. 1g). When the basipetal flow of IAA from the anthers diminishes, GUS expression becomes evident in the nectary glands (Fig. 2a) and re-appears as small spots at the tip of elongating petals (Fig. 2d,e). Parallel to the remarkable IAA accumulation in developing and mature pollen grains, high GUS expression re-appears in the stigma (Fig. 2f). After fertilization, low GUS expression in the young embryo (Fig. 3d) is followed by increasing expression in the developing embryos and seeds (Figs. 2c, 3e).
Young anthers produce extremely high concentrations of free auxin
Very young flowers, younger than stage 7, with strong accumulation of conjugated auxin (Fig. 4a–e), show only very weak or no GUS expression (Fig. 1f, arrow and insert). At stage 8, in young growing stamens, very high GUS expression is apparent in the anthers and along their filaments (Fig. 1f,g). High magnifications of young anthers reveal that the tapetum cells show very high free-auxin-dependent GUS expression (Fig. 1h), before it can be detected in the developing pollen grains. Other parenchyma cells in the anthers, in the walls of the pollen sacs, may also display GUS expression (Fig. 1i,k). In later developmental stages (stages 9 and 10), when free-IAA production decreases, GUS expression is detected only in the anthers (Fig. 1i,k) and is lacking in the stamen filament. After anther dehiscence and the release of pollen grains, the distal cells of the filament begin to show high GUS expression, (Fig. 1j) which is absent during pollen grain development, when high GUS expression appears in the anther cells (Fig. 1i,k). Only after the release of the pollen grains, which apparently repress free-auxin production in the filament tissues below them, the upper cells of the filament can start to show GUS expression (Fig. 1j).
Pollen grains accumulate high IAA concentrations
In very young anthers (stages 8 and 9), the tapetum cells (Fig. 1h) display strong GUS expression, which is lacking in very young pollen grains. Gradients of GUS activity were detected in the walls of very young pollen sacs, with the highest GUS activity in the upper tapetum cells (data not shown). In maturing pollen grains GUS staining is enhanced in the grains (Fig. 2g, arrowhead, inside an open anther).
Nectary glands at the base of the stamens produce free auxin
While young nectaries (up to stage 9) do not show GUS expression (Fig. 1k), it starts just before anthesis (Fig. 2a), increases gradually during pollination, and continues for a relatively long period, up to embryo maturation (Fig. 2c).
Petals produce low free-auxin concentrations
Although the petals are induced before or with the stamens, petal development is retarded during the early stages of continuous stamen development (see Fig. 4a–e). Intact petals show transient (Fig. 1f) and later very low GUS expression only in their tip and in distal sites of their veins (Fig. 2d,e).
Distinct free-auxin production in the stigma
Stigmatic papillae of a young gynoecium show initial transient GUS activity (Fig. 1f) and are soon free from detectable GUS expression (Fig. 1g). However, shortly before pollen maturation, these stigmatic papillae start to display considerable GUS expression giving the stigma a sky blue color, which is always lighter than the dark blue staining of the pollen grains in mature anthers (Fig. 2f,g). Stigmatic papillae that are not pollinated show high GUS expression (Fig. 3a) in contrast to the pollinated papillae (Fig. 3a, arrow).
Upon heavy pollination the stigma stops GUS expression after pollen grain germination on almost all stigmatic papillae. Only then, the parenchyma cells located immediately beneath the stigma start to display high GUS expression (Fig. 3b), indicating that the stigma papillae, while active, inhibit free-IAA production in those parenchyma cells.
Vascular differentiation in the gynoecium
The high apparent concentration of free auxin by the stigmatic papillae (Figs. 2f–j, 3a) induces a wide stylar xylem (Figs. 2g, 3b) immediately beneath them, which supplies water for pollen-grain hydration. The gradual development of the gynoecium midvein and its stylar xylem can easily be followed, step by step, during flower morphogenesis (Figs. 1g, 2f,g).
In the funiculus, the stalk by which the ovule is attached to the placenta, the tiny xylem strand originating from the ovule does not extend into the longitudinal bundle of the gynoecium (Figs. 2g, 3c) to facilitate the seed abscission after fertilization.
Very high concentrations of free IAA in germinating pollen grains and growing pollen tubes
During hydration and germination, the pollen grains and the tubes show elevated IAA-dependent GUS expression (Fig. 2g–j), higher than before hydration. The tips of the pollen tubes penetrate the papillar cuticle (Fig. 2j) and continue to grow intrusively along the stigmatic papillae to the center of the style into the transmitting tract toward the embryo sacs. The cytoplasm of a germinating pollen grain, detected by its high GUS expression, is concentrated at its growing tip (Fig. 2h–j). Early germinating pollen grains loose their cytoplasm and hence GUS activity (Fig. 2i, arrowhead), while the latest germinating pollen grains are also the last with the dark blue GUS staining (Fig. 2h,i, arrows).
Free auxin induces axial polarity in developing embryos and seeds
About a day after fertilization, incipient low IAA-dependent GUS expression appears as small spots on the basal side (upper end) of the embryo sac (Fig. 3d). The ovules do not show any GUS activity, neither before fertilization (Fig. 2f, g), nor even later in a growing silique, as long as an ovule is not fertilized (Fig. 2c, the two ovules on the left side). Embryo and seed development is polar, and high free-auxin-dependent GUS expression is evident at the root side (Figs. 2c, 3e, f). The orientation of the micrographs in all figures is correct, emphasizing that the embryos usually develop anatropically with their roots upward, towards the stigma (Figs. 2c, 3e, f). Maturing seeds stop GUS activity in their late developmental stages. However, they continue, for a short duration, to show GUS expression in the embryonic root side (Fig. 3f), which concentrates for a while in the funiculus.
Selective removal of floral organs alters flower development and free-auxin production
In order to ascertain that free-auxin production regulates relationships among the floral organs, various modes of selective floral-organ removal were tested on young flower buds at stages 8 and 9 of flower development.
Since young stamens show extremely high GUS expression, their possible regulatory role was investigated first. Careful removal of four stamens out of six at stage 8 (anthers and the upper portion of the filament) accelerated GUS expression in the nectary glands (Fig. 2b). This also promoted early petal elongation. Two days after stamen removal the length of the petals extended to 252±16.8 μm while in intact control flowers the petal length was only 22.5 ± 0.6 μm (mean values ± SE, n=10); this difference was highly significant (p < 0.01; by t test). Hence, although the petals may be induced before the stamens, petal elongation was suppressed by the young stamens during early stages of floral bud development (up to stage 9). Shortly after stamen removal, the petals tended to show elevated GUS expression (Fig. 3h), indicating that young growing stamens retard free-IAA production in petals. Anther removal also caused GUS expression in the distal cells of the remaining stamen filament (Fig. 3g), and extended the duration of detectable GUS expression in the sepals. Interestingly, stamen removal promoted growth of the gynoecium and stigmatic papilla elongation (data not shown).
Removal of one or two sepals reduced the size of the petals, stamens and gynoecium (probably owing to loss of sepal protection), but increased GUS expression in the petals (data not shown) and even in the gynoecium (Fig. 3i), thus evincing induced modifications of floral organ development in the acropetal direction.
Partial removal of the gynoecium causes detectable GUS expression in the distal cells of the remaining gynoecium (Fig. 3c), while most of the ovules degenerate, indicating that free-IAA production in the stigma is required for gynoecium and ovule development. Furthermore, after removal of the distal gynoecium part, the petals stop elongation and remain inside the sepals. When the gynoecium was removed from five or more flowers of an inflorescence, apical dominance was released in that inflorescence, resulting in early and rapid growth of secondary inflorescences. This infers that free auxin from growing flowers and developing seeds regulates inflorescence architecture.
Petal removal (done at stage 9) exerts no detectable effect on other organs, suggesting that their free-IAA production is too low to affect the development of the neighboring floral organs.
Auxin immunolocalization
Analysis of the earliest stages of flower primordia with IAA antibodies revealed high concentrations of auxin conjugates during early stages of flower development (Fig. 4a–d). At the beginning, the smallest flower bud before sepal differentiation is already loaded with conjugated auxin (Fig. 4a, b), even the uppermost tunica layer of the promeristem (Fig. 4b). Gradients of decreasing concentrations of auxin conjugates from the promeristem tip (Fig. 4a, b), in developing anthers (Fig. 4c, d), differentiating pollen grains (Fig. 4d, e, g), young nectaries (Fig. 4f), developing ovules (Fig. 4d, e, g) and growing stigmatic papillae are obvious (Fig. 4h, i).
Discussion
Genetic investigations provided evidence that the polar transport of auxin regulates flower formation, gynoecium morphogenesis and vascularization (Okada et al. 1991; Bennett et al. 1995; Nemhauser et al. 1998, 2000; Oka et al. 1999; Christensen et al. 2000; Reinhardt et al. 2000). Our study with specific IAA antibodies visualizes that very young flowers accumulate high concentrations of conjugated auxin, from which free-auxin may be later released, while DR5::GUS expression shows where and when free auxin is produced or accumulates during Arabidopsis flower and seed morphogenesis (Fig. 5). The justification of the interpretation of DR5::GUS expression as “IAA production” throughout this analysis is given in the first chapter of “Results”. The present findings support our assumption that the tip of each floral organ is a primary site of free-auxin production, which controls floral organ development and induces its vascular tissues. The study also demonstrates that auxin may play two opposing roles in flower development: free IAA produced by a floral organ promotes its own development and differentiation, but can repress the growth or activity of a neighboring organ. Experimental modifications of flower development by selective removal of young floral organs provide new insights into the interactions between floral organs and the role of free auxin in controlling synchronized flower development.
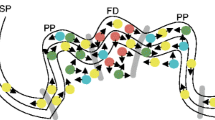
Schematic diagrams showing the gradual changes in sites (blue spot locations) and concentrations (blue symbol sizes) of free-IAA production (detected by DR5::GUS expression) during Arabidopsis flower and early fruit development. Arrows mark sites of auxin production starting at the tip of floral organs during their development (a–e) and at the ovules and developing seeds in the gynoecium (d,e). The ontogeny of the gynoecium midvein, characterized by its wide fan xylem induced by free IAA descending from the stigma (d,e), and the short xylem veinlets induced by developing seeds are illustrated by red lines (e). (a) Young floral bud with incipient free-IAA production at the tip of the sepals (the bud is loaded with conjugated auxin). (b) Free-IAA production at the sepal tips and massive auxin production in the stamens, demonstrating stamen dominance characterized by complete petal suppression. (c) Decreased auxin production in the stamens (DR5::GUS activity limited to the anthers) is followed by incipient auxin production in the growing petals and stigma. (d) High free-IAA production in the stigma; low auxin production in the ovules, the nectaries, the petal tips and stamen-filament tips. (e) Residual free-IAA production beneath the stigma, elevated auxin production in developing seeds, and continuous production in nectaries
Recently, a family of auxin-conjugate hydrolases was found to be expressed in leaves, flowers and siliques; they are expressed in the anthers, IAR3 also at the tips of the sepals and stigma, and ILL1 and 3 in the seeds (Rampey et al. 2004). In the present study we found that from a very early developmental stage the flower primordia contain high concentrations of conjugated auxin (Fig. 4a–i) from which free auxin may be later released. The pattern of auxin-conjugate hydrolases (Rampey et al. 2004) can explain the apparent free-auxin patterns found in the present study, while the ILL2::GUS expression pattern explains the developmental patterns of free-auxin production found in leaves (Aloni et al. 2003). The local free-IAA production sites which induce the delicate framework of the high-order veins inside the leaf lamina (Aloni et al. 2003) cannot be explained by an acropetal IAA movement (Benková et al. 2003; Reinhardt et al. 2003), but only by local hydrolysis of conjugated auxin (Rampey et al. 2004) during the advanced stages of leaf-primordium development (Aloni 2001; Aloni et al. 2003).
Role of free IAA in shoot and flower organ development
The present results of our selective floral organ removal illustrate how a floral organ, by producing considerable free-auxin concentrations, can inhibit or retard the development of other floral organs in both the acropetal and basipetal directions. The best example found here is the suppression of petal elongation and nectary gland activity by the auxin-producing stamens (Fig. 5b). These results are in line with early investigations on apical dominance that decapitation of a shoot resulted in rapid growth of one or more axillary buds below the cut, suggesting that the polar flow of IAA produced in the apical bud and young leaves inhibits the outgrowth of axillary buds (Thimann and Skoog 1933, 1934; Thimann et al. 1971). In tissue cultures, elevated auxin concentrations inhibit shoot-organ development and promote root formation (Skoog and Miller 1965; Taiz and Zeiger 2002).
Inhibition of polar auxin export blocks leaf formation at the shoot apical meristem of tomato. After three to five weeks of in vitro culture with the auxin efflux inhibitors NPA or TIBA and repeated removal of the basal stem portion, the tomato shoot tip becomes pin shaped (Reinhardt et al., 2000). The NPA is known to induce intracellular accumulation and not a decrease of IAA (Petrášek et al. 2003), as was already suggested by Nemhauser et al. (2000). Therefore, over-optimal inhibitory concentration and not lack of IAA will induce leaf primordium-deficient pin-shaped shoot apices. Remarkably, three days after transfer to medium without NPA, the pins spontaneously initiated new primordia (Reinhardt et al. 2000), demonstrating that these NPA-suppressed shoot primordia are pre-patterned and that their development was suppressed by the auxin transport inhibitor. Near to Arabidopsis shoot tips, the stipules are the closest sites of strong free-auxin production (Fig. 1a in Aloni et al. 2003). These leaf appendages do not induce vascular tissues, suggesting that their free auxin is transported acropetally to the shoot tip. This acropetal auxin transport likely induces the polar pattern of PIN1 in the acropetal membranes of the differentiating epidermal cells (Benková et al. 2003; Reinhardt et al. 2003). By RT-PCR analysis of mRNA expression of the key enzymes of auxin biosynthesis (IGS and NIT), we found that auxin is synthesized in the vegetative apical meristem of Arabidopsis (Fig. 4a in Aloni et al. 2003). Moreover, auxin immunolocalization with monoclonal antibodies demonstrated that the vegetative shoot tip and youngest leaf primordia are loaded with high concentrations of conjugated auxin (Fig. 3c,d in Aloni et al. 2003) before they show detectable free auxin by DR5::GUS expression. The present findings in the Arabidopsis inflorescence show that the youngest flower at the tip, including its tunica and the differentiating epidermal cells, is already loaded with very high concentrations of conjugated auxin (Fig. 4a,b), indicating that the acropetal free-auxin transport through the epidermal cells (Benková et al. 2003; Reinhardt et al. 2003) may have a limited effect on organ development at the shoot tip. It was also suggested that during the early stage of leaf-primordium development, auxin could move acropetally along the midvein from the primordium base to its tip (Avsian-Kretchmer et al. 2002), which is opposite to the flow model suggested by the PIN1 patterns (Benková et al. 2003; Reinhardt et al. 2003). Here we show that when the auxin concentration decreased in the sepals, the developing stamens and gynoecium produce auxin (large flower in Fig. 4a) and that their auxin is unlikely to arrive from neighboring organs via the epidermis. The evidence that the stamens produce high free-auxin concentrations that inhibit petal growth (Fig. 1g) during early flower development, supported by our results from selective removal of stamens, confirm the negative regulatory role of auxin on neighboring shoot-organ development. In flowers the free-auxin producing stamens (Fig. 1g) suppress growth of genetically designed organs (petals) (Bowman et al. 1991; Weigel and Meyerowitz 1994), and auxin only influences the time of their appearance and the rate of their elongation. In conclusion, our findings suggest that at the shoot tip, auxin (originating from the continuous spiral pattern of stipules surrounding the shoot tip, two stipules per leaf primordium) regulates adjacent shoot-organ development (Benková et al. 2003) or affects phyllotaxis (Reinhardt et al. 2003) by inhibition or retardation and not by promoting neighboring shoot-organ initiation or development.
The simultaneous appearance of the primordia of petals and stamens is defined as the start of stage 5 of Arabidopsis flowers (Bowman et al. 1989). The stamens develop during stages 6 to 8, while the petal primordia do not grow until stage 9 of flower development (Bowman et al. 1989) because of petal primordia suppression by the high free-IAA concentrations produced in the anthers. Petals in intact flowers produce very low free-IAA concentrations (Figs. 2d,e, 4h) and therefore probably cannot compete with the high free-auxin-producing stamens. When some of the anthers are removed experimentally, the petals are able to produce higher amounts of free auxin (Fig. 3h). Naturally, the petals reach maximum size and the nectaries start to produce free auxin just before anthesis (a known phenomenon widely occurring among flowering plants; see Endress 1994), at the time when the polar auxin flow from the anthers diminishes. These findings indicate that free-IAA production in the anthers synchronizes the development of both the petals and the nectaries, so that they will start to function shortly before pollination.
In Arabidopsis, the nectary glands which develop at the base of the stamens are ABC-independent floral structures. They arise from cells previously expressing the B class genes, and their proper development requires the down-regulation of B class gene activity (Baum et al. 2001). Our findings show that the nectary glands in Arabidopsis start to produce free auxin just before pollen release. However, even after fertilization, the nectaries continue to produce free and conjugated auxin for a relatively long period, up to seed maturation (Figs. 2c, 4f), probably because the hormonal repression by the stamens has ceased.
Further evidence for the retarding role of free auxin in flower development is the two naturally occurring post-pollination phenomena detected in the reproductive organs. In both cases, when the distal cells (in an anther or a stigma) stop producing free auxin, the parenchyma cells immediately below them take over and start to produce IAA. Evidently, the anthers retard the production of free IAA in the stamen filament until pollen dispersal. Only then distinct IAA-dependent GUS activity begins in the upper cells of the filament (Figs. 1j, 3g). A corresponding process occurs in the gynoecium: when the stigmatic papillae on which pollen grains have germinated stop free-IAA production, the parenchyma cells located immediately beneath the stigma start to produce free auxin (Fig. 3b). Moreover, after experimental removal of the upper portion of the gynoecium, the distal parenchyma cells of the remaining lower part of the gynoecium begin to produce free auxin (Fig. 3c).
In addition to such IAA retardation phenomena, a naturally occurring inhibition was detected in the gynoecium, more specifically in the funiculus, where the xylem of the short vascular bundle induced by the ovule does not continue into the longitudinal bundle descending from the stigma (Figs. 2g, 3c). This finding suggests that the continuous concentrated polar IAA flow originating in the stigma inhibits the low-level IAA fluxes from the ovules and thereby prevents a vascular connection of the ovules to the longitudinal bundles of the gynoecium. Similarly, Sachs (1969) demonstrated experimentally that a bundle, which is well supplied with auxin, inhibits the differentiation of nearby additional vascular elements.
Role of free IAA in gametophyte development and embryogenesis
The present study shows that maturing pollen grains apparently accumulate extremely high concentrations of free auxin. During early stages of anther development, the tapetum cells produce high free-IAA concentrations, and they probably supply the hormone to the developing pollen grains. Generally, most of the auxin in plant cells, up to 99%, is stored as auxin conjugates (Ljung et al. 2002; Taiz and Zeiger 2002). Accordingly, also mature pollen grains are loaded with auxin conjugates, thus, forming a pool of bound auxin from which free IAA can be released by hydrolysis (Ljung et al. 2002) during pollen hydration and pollen-tube growth. GUS expression of pollen grains in the anthers and during germination on the stigma indicates that during hydration free-IAA concentration sharply increases in the grains (Fig. 2g–i). IAA homeostasis is dependent on the combined effects of auxin biosynthesis, conjugation, degradation and transport (Ljung et al. 2002). The changes in the intensity of GUS expression during pollen development in the anther and later during germination on the stigma indicate a shift from an early stage of an IAA-pool build-up to the germinating stage wherein free-IAA is released by hydrolysis.
In plant embryogenesis the establishment of the apical-basal axis is a critical event. Polarity is evident in the embryo sac, the zygote and the developing embryo (Souter and Lindsey 2000; Jürgens 2001; Berleth and Chatfield 2002; Friml et al. 2003). The present study shows clear polar distribution of free IAA in the developing embryo (shortly after fertilization, Fig. 3d). The embryo root is induced at the basal side where free-auxin is accumulated, whereas the shoot develops from the apical end at a low free-IAA concentration. This primary axial polarity determines the polarity of the vascular tissues, which are induced by free-auxin release in the tip of developing cotyledons and leaf primordia (Aloni 2001; Aloni et al. 2003; Mattsson et al. 2003).
In conclusion, auxin is a major controlling signal that synchronizes flower development in Arabidopsis. A floral organ that produces high concentrations of free auxin inhibits or retards the development of neighboring organs. The best example for such organ regulation is the release of high concentrations of free IAA from young anthers, which retard petal growth and nectary gland activity, almost up to flower maturation.
The main function of flowers is sexual reproduction. IAA is likely involved in the mechanism which controls the growth of the male gametophyte to the egg cell in the ovule. Our findings suggest that free auxin is involved, because developing pollen grains accumulate high concentrations of IAA, which probably promote pollen germination on the stigma and trigger the rapid intrusive growth of pollen tubes to the embryo sac. Immediately after fertilization, elevated concentrations of free auxin are produced in developing embryos and seeds, thereby determining their axial polarity and their polar development.
Abbreviations
- DR5::GUS :
-
Auxin response element fused to β-glucuronidase
- IAA:
-
Indole-3-acetic acid
- NAA:
-
α-naphthaleneacetic acid
- ESM:
-
Electronic supplementary material
References
Aloni R (1987) Differentiation of vascular tissues. Annu Rev Plant Physiol 38:179–204
Aloni R (2001) Foliar and axial aspects of vascular differentiation: hypotheses and evidence. J Plant Growth Regul 20:22–34
Aloni R (2004) The induction of vascular tissues by auxin. In: Davies PJ (ed) Plant hormones: biosynthesis, signal transduction, action! Kluwer, Dordrecht, pp 471–492
Aloni R, Schwalm K, Langhans K, Ullrich CI (2003) Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis. Planta 216:841–853
Alvarez J, Smyth DR (1999) CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMUS. Development 126:2377–2386
Alvarez J, Smyth DR (2002) CRABS CL A W and SPATULA genes regulate growth and pattern formation during gynoecium development in Arabidopsis thaliana. J Plant Sci 16:17–41
Avsian-Kretchmer O, Cheng J-C, Chen L, Moctezuma E, Sung ZR (2002) IAA distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny. Plant Physiol 130:199–209
Baum SF, Eshed Y, Bowman JL (2001) The Arabidopsis nectary is an ABC-independent floral structure. Development 128:4657–4667
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutant of Arabidopsis thaliana. Plant J 8:505–520
Berleth T, Chatfield S (2002) Embryogenesis: pattern formation from a single cell. In: Somerville C, Meyerowitz E (eds) The Arabidopsis book. American Society of Plant Biologists. http://www.aspb.org/publications/arabidopsis/toc.cfm
Bowman JL, Smyth DR, Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell 1:37–52
Bowman JL, Smyth DR, Meyerowitz EM (1991) Genetic interactions among floral homeotic genes of Arabidopsis. Development 112:1–20
Bowman JL, Baum SF, Eshed Y, Putterill J, and Alvarez J (1999) Molecular genetics of gynoecium development in Arabidopsis. Curr Top Develop Biol 45:155–205
Christensen SK, Dagenalis N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100:469–478
Endress PK (1994) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambridge
Eshed Y, Baum SF, Bowman JL (1999) Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99:199–209
Friml J, Wisniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415:806–809
Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153
Goto K, Kyozuka J, Bowman JL (2001) Turning floral organs into leaves, leaves into floral organs. Curr Opin Genetics Develop 11:449–456
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Jürgens G (2001) Apical-basal pattern formation in Arabidopsis embryogenesis. EMBO J 20:3609–3616
Langhans M, Ratajczak R, Lützelschwab M, Michalke W, Wächter R, Fischer-Schliebs E, Ullrich CI (2001) Immunolocalization of plasma-membrane H+ -ATPase and tonoplast-type pyrophosphatase in the plasma membrane of the sieve element-companion cell complex in the stem of Ricinus communis L. Planta 213:11–19
Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G (2002) Biosynthesis, conjugation, catabolism and homeostasis of indol-3-acetic acid in Arabidopsis. Plant Mol Biol 50:309–332
Marchant A, Bhalerao O, Casimiro I, Eklof J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14:589–597
Mattsson J, Ckurshumova W, Berleth T (2003) Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol 131:1327–1339
Müller A, Düchting P, Weiler EW (2002) A multiple GC-MS/MS technique for the sensitive and qualitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana. Planta 216:44–56
Nakamura A, Higuchi K, Goda H, Fujiwara T, Sawa S, Koshiba T, Shimada Y, Yoshida S (2003) Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in Arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol 133:1843–1853
Nemhauser JL, Zambryski PC, Roe JL (1998) Auxin signaling in Arabidopsis flower development?. Curr Opin Plant Biol 1:531–535
Nemhauser JL, Feldman LJ, Zambryski PC (2000) Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127:3877–3888
Oka M, Miyamoto K, Okada K, Ueda J (1999) Auxin polar transport and flower formation in Arabidopsis thaliana transformed with indoleacetamide hydrolase (iaaH) gene. Plant Cell Physiol 40:231–237
Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3:677–684
Petrášek J, Černá A, Schwarzerová, K, Elčkner M, Morris DA, Zažímalova E (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic?. Plant Physiol 131:254–263
Rampey RA, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B (2004) A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiol 135:978–988
Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12:507–518
Reinhardt D, Pesce E-R, Stieger P, Mandel T, Baltensperger, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426:255–260
Roe JL, Nemhauser JL, Zambryski PC (1997) TOUSLEND participates in apical tissue formation during gynoecium development in Arabidopsis. Plant Cell 9:335–353
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in Arabidopsis root. Cell 99:463–472
Sachs T (1969) Polarity and the induction of organized vascular tissues. Ann Bot 33:263–275
Sessions RA, Zambryski PC (1995) Arabidopsis gynoecium structure in the wild type and ettin mutants. Development 121:1519–1532
Sessions A, Nemhauser JL, McColl A, Roe JL, Feldman KA, Zambryski PC (1997) ETTIN patterns in the Arabidopsis floral meristem and reproductive organ. Development 124:4481–4491
Skoog F, Miller CO (1965) Chemical regulation of growth and organ formation in plant tissue cultured in vitro. In: Bell E (ed) Molecular and Cellular Aspects of Development. Harper and Row, New York, pp 481–494
Smyth DR (2005) Morphogenesis of flowers – our evolving view. Plant Cell 17:330–341
Souter M, Lindsey K (2000) Polarity and signalling in plant embryogenesis. J Exp Bot 51:971–983
Sussex I (1989) Developmental programing of shoot meristem. Cell 56:225–229
Taiz L, Zeiger E (2002) Plant physiology, 3rd ed. Sinauer, Sunderland, Mass, USA
Thimann KV, Skoog F (1933) Studies on the growth hormone of plants III The inhibiting action of growth substance on plant development. Proc Nat Acad Sci USA 19:712–716
Thimann KV, Skoog F (1934) On the inhibition of development and other functions of growth substances in Vicia faba. Proc Royal Soc London B 114:317–339
Thimann KV, Sachs T, Mathur KN (1971) The mechanism of apical dominance in Coleus. Physiol Plant 24:68–72
Turner S, Sieburth LE (2002) Vascular patterning. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book, American Society of Plant Biologists. Rockville, MD, http://www.aspb.org/publications/arabidopsis/toc.cfm
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Veselov SY, Kudoyarova GR, Egutkin NL, Gyuli-Zade VZ, Mustafina AR, Kof EM (1992) Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole-3-acetic acid. Physiol Plant 86:93–96
Veselov D, Langhans M, Hartung W, Aloni R, Feussner I, Götz C, Veselova S, Schlomski S, Dickler C, Bächmann K, Ullrich CI (2003) Development of Agrobacterium tumefaciens C58-induced plant tumors and impact on host shoots are controlled by a cascade of jasmonic acid, auxin, cytokinin, ethylene and abscisic acid. Planta 216:512–522
Weigel D, Meyerowitz EM (1994) The ABCs of floral homeotic genes. Cell 78:203–209
Zgurski JM, Shama R, Bolokoski DA, Schultz EA (2005) Asymmetric auxin response precedes asymmetric growth and differentiation of asymmetric leaf1 and asymmetric leaf2 Arabidopsis leaves. Plant Cell 17:77–91
Acknowledgements
We thank Profs. Tom J. Guilfoyle (University of Missouri, Columbia Mo, USA) for the kind gift of the DR5::GUS transformed seeds of Arabidopsis thaliana, Stanislav Veselov (University of Ufa, Russia) for the kind gift of polyclonal auxin antibodies, Rivka Dulberger (Tel Aviv University, Israel) for insightful advice, Dr. Martha Schwartz and Varda Wexler (Tel Aviv University, Israel) for helpful assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Orna Aloni for continuous support and management over many years.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Aloni, R., Aloni, E., Langhans, M. et al. Role of auxin in regulating Arabidopsis flower development. Planta 223, 315–328 (2006). https://doi.org/10.1007/s00425-005-0088-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0088-9