Abstract
Leaf senescence is characterized by nitrogen remobilization to developing seeds of annual plants, or surviving organs of perennial species. It has been demonstrated that high carbohydrate levels (carbon “feast”) are associated with the onset of the senescence process. Therefore, the development of model systems allowing the manipulation of leaf carbohydrates constitutes a logical first step in the investigation of processes important during early phases of senescence, such as plastidial protein degradation. In this study, sugar accumulation was induced either by the incubation of excised, mature barley (Hordeum vulgare L.) leaves under relatively strong light, or by the interruption of sieve tubes at the base of the leaf lamina by “steam-girdling”. Accelerated chlorophyll degradation and net proteolysis confirmed successful senescence induction in both model systems, but suggested that girdled leaves are more useful than excised leaves to study proteolysis. Activities or transcript levels of several proteolytic enzymes, including plastidial (aminopeptidases, Clp protease), cytosolic (proteasome) and vacuolar (thiol proteases, an aspartic protease and a serine carboxypeptidase) proteases were clearly induced under these conditions; some of these genes also reacted to other stimuli such as leaf excision. The most interesting finding was the specific induction of a carboxypeptidase gene (cp-mIII) in girdled leaves accumulating high carbohydrate levels. As a previous study from our laboratory, using a genetic approach, has indicated that one or several carboxypeptidases are involved in leaf N remobilization, the detailed characterization of cp-mIII (and, possibly, closely related genes) may considerably improve our understanding of whole-plant N recycling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Senescence is a genetically controlled, highly regulated developmental process aimed at salvaging mobile nutrients prior to cell death. The reallocation of nitrogen from senescing vegetative organs, especially leaves and stems, to maturing seeds is particularly important. In fact, 70% or more of the leaf nitrogen is exported during seed development in most annual crop plants (Peoples and Dalling 1988); therefore, it is almost impossible to overestimate the importance of this process for nitrogen (protein) yield formation. Within photosynthetically active leaf cells, up to 75% of the reduced nitrogen is located in the chloroplasts (Hörtensteiner and Feller 2002). Stromal enzymes, mainly ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco, in C3 plants), represent the major fraction of chloroplast nitrogen (Peoples and Dalling 1988; Hörtensteiner and Feller 2002). Since proteins must be degraded prior to the retranslocation of resulting amino acids to developing seeds, a functional understanding of the processes involved in plastidial and, to a lesser extent, cytosolic protein degradation appears crucial for our overall knowledge of higher plant nitrogen metabolism. While experimental evidence indicates that the degradation of photosynthetic proteins such as Rubisco is initiated by plastidial proteases, the contribution of these enzymes and of other proteases (especially those present in lytic vacuoles) to the complete degradation of plastidial proteins in vivo is unclear (see Hörtensteiner and Feller 2002 for a recent review). A genetic approach (using quantitative trait locus mapping) to this problem has indicated a role for one or several carboxypeptidases to whole-plant N recycling; as such enzymes have previously been located in vacuolar compartments, these results appear intriguing (Yang et al. 2004).
Leaf senescence can be induced or influenced by a range of exogenous (e.g., illumination, temperature, nutrient availability) and endogenous factors (e.g., hormonal balance, source/sink relationships for nutrients and assimilates; Feller and Fischer 1994). Several lines of work indicate that the senescence of mature (source) leaves can be induced by darkness (potentially leading to sugar starvation), or by the accumulation of carbohydrates in different species (Feller and Fischer 1994; Yoshida 2003; Pourtau et al. 2004). The terms “carbon feast” and “carbon famine” were coined by Koch (1996) for these physiological conditions. Senescence was accelerated, and Rubisco and other photosynthetic proteins were degraded when detached tobacco source leaves were incubated under strong light (leading to the accumulation of sugars from photosynthesis), or directly supplied with glucose (Krapp et al. 1991). Interruption of phloem export from wheat leaves by girdling led to sugar accumulation and accelerated chlorophyll and protein degradation (Feller and Fischer 1994). In an interesting whole-plant study comparing leaves along the main axis of tobacco plants, Masclaux et al. (2000) found peak carbohydrate levels in leaves at the onset of senescence, when certain protease activities started to increase. These results should be considered in context with recent data obtained by Jongebloed et al. (2004), indicating that sieve tube occlusion and carbohydrate accumulation preceded chlorophyll degradation at the onset of castor bean leaf senescence. Transgenic plants or mutants with enhanced hexokinase protein contents showed premature leaf senescence, while the onset of senescence was delayed in plants with lowered hexokinase (Dai et al. 1999; Yoshida 2003). Since hexokinase has emerged as a sensor for the carbohydrate status of higher plant cells (Moore et al. 2003), these results confirm that high sugar levels are one of the signals inducing natural leaf senescence in higher plants. However, it should be mentioned that the physiological reaction of assimilate sinks (e.g., root tips) to carbohydrate status may be different from that of source tissues. It has been demonstrated that senescence in such tissues can be induced by carbon famine (Brouquisse et al. 2001).
Based on these findings, the induction of leaf senescence by carbon feast and/or enhanced sugar signaling appears to be an interesting experimental system for the characterization of senescence-associated processes, such as protein degradation and N remobilization. In this context, several authors (Fröhlich and Feller 1992; Fischer et al. 1998; Masclaux et al. 2000) have demonstrated that proteases are induced in source tissues accumulating carbohydrates, and Feller and Fischer (1994) have shown accelerated net proteolysis in girdled wheat leaves. However, no molecular analysis of the induced enzymes (such as identification of candidate genes) has been reported so far.
The physiology, biochemistry and molecular biology of leaf senescence have been investigated using a number of species and approaches. Isolated chloroplasts, leaf disks and segments, cut leaves, girdled leaves with blocked phloem export and whole plants (Crafts-Brandner et al. 1984a, b; Mitsuhashi et al. 1992) have been utilized to address specific problems. While the final goal of such studies is a comprehensive understanding of whole-plant processes, specific questions often require the use of simpler systems. Excised leaves have frequently been utilized, as large numbers can be treated under standardized environmental conditions. Incubation of such leaves under illumination leads to carbohydrate accumulation from photosynthesis (interrupted phloem export; Krapp et al. 1991); accordingly, this experimental approach may represent a useful model system to study the influence of carbon feast conditions on senescence-associated protein degradation. Assimilate export from mature leaves can also be interrupted by selective inactivation of phloem vessels at the leaf base, e.g. by the application of heat (“steam-girdling”; Feller and Fischer 1994). Considering the fact that Jongebloed et al. (2004) have recently found that sieve tube occlusion and carbohydrate accumulation preceded chlorophyll degradation at the onset of castor bean leaf senescence, girdling experiments may be especially appropriate to mimic conditions at the onset of natural leaf senescence, while allowing the coordination and enhancement of the signal in large quantities of plant material.
Research presented in this manuscript is aimed at the identification of protease genes induced under carbon feast conditions, and potentially involved in the early stages of photosynthetic protein degradation in naturally senescing leaves. To achieve this goal, results from two experimental systems (excised leaves, and leaves with blocked phloem export) are compared. Since dark incubation (potentially inducing carbon famine) has frequently been used in the past in senescence studies (Feller and Fischer 1994), this treatment is also included.
Materials and methods
Plant materials, growth conditions and treatments
For leaf excision experiments, barley seeds (Hordeum vulgare L., cv. “Harrington”) were germinated and grown on potting soil in a greenhouse of the Montana State University (Bozeman, MT) Plant Growth Center for 14 days, with a 22/18°C day/night temperature cycle. When necessary, days were extended to a 16-hour photoperiod, using Son-Agro 430 W high-pressure sodium lamps (Philips, Somerset, NJ). Seedlings were fertilized twice per week with Peter’s Professional General Purpose fertilizer (4 g l−1 ; Scotts-Sierra Horticultural Products Company, Marysville, OH). After 14 days, secondary (fully mature) leaves were excised, immediately re-cut under water using a razor blade, and incubated with their cut bases in a beaker containing 60 mL deionized water (10 leaves/beaker). Leaves were incubated at 21±1°C and illuminated under strong light (250–300 μE m−2 s−1) or weak light (15–20 μE m−2 s−1) in a daily cycle of 16 h light/8 h darkness, or in permanent darkness. Artificial light from wide-spectrum fluorescent tubes (F40PL/AQ; General Electric, Cleveland, OH) was used, either unfiltered for strong-light incubations, or after filtering through several layers of a white tissue to obtain the weak light intensity. The spectral distribution of light between the strong- and weak-light intensity incubations was compared, and found to be unchanged by the tissue used for filtering. Leaves were harvested at 0 days (immediately after cutting), and after 2 days, 4 days, 6 days and 8 days incubation under the different light regimes. They were quickly blotted dry on paper towels, immediately frozen in liquid nitrogen, and stored at −80°C until extraction and analysis.
For attached leaf treatments, barley seeds (H. vulgare L., cv. “Harrington”) were germinated and grown as above for 7 days without fertilizer application. After this time, plants were transferred to a growth chamber (Conviron, Winnipeg, Manitoba, Canada) with a 22/16°C day/night temperature cycle and a 16 h photoperiod with a light intensity of 200 μE m−2 s−1. Secondary (fully mature) leaves of some plants were treated for 5 s with a steam-heated (95°C) hypodermic needle at the base of the leaf to interrupt the phloem. Untreated control leaves were compared to leaves where the phloem was completely interrupted or where two opposing phloem interruptions, each spanning half the lamina, were separated by ∼1 cm (Feller and Fischer 1994). Leaves were harvested at 0 days (immediately after girdling), and 4 days, 8 days and 12 days after girdling. They were immediately frozen in liquid nitrogen, and stored at −80°C until extraction and analysis.
All data points shown in this manuscript represent means from three independently incubated samples, with each sample consisting of at least five leaves. Samples mixed from three independent extractions were used for SDS and native PAGE (protease activity) gels, and typical results from 2 to 3 replications are shown for Northern analyses.
Carbohydrate, amino acid and chlorophyll analysis
Leaf material was ground to a fine powder in liquid nitrogen, using mortar and pestle. Soluble sugars were extracted from 40-mg samples with 400 μl 100 mM MOPS buffer pH 7.2, containing 5 mM EDTA in 1.5-ml tubes, using a motor unit and pestles fitting the conical bottom of the tubes. Extracts were centrifuged (20,000g, 5 min, 4°C), pellets were re-extracted with 200 μl buffer, and soluble sugars were assayed with an anthron reagent in the pooled supernatants as described by Smidansky et al. (2003). Pellets were washed twice with 500 μl 80% ethanol prior to hydrolysis of insoluble sugars with 1.6 N perchloric acid and analysis, again as described by Smidansky et al. (2003).
Chlorophylls were extracted with 80% acetone from liquid nitrogen powder and spectrophotometrically assayed at 649 and 665 nm, as described by Feller and Fischer (1994).
Amino acids (soluble α-amino nitrogen) were assayed with a TNBS reagent (Fischer et al. 1998), using the same extraction methods as for proteases (below), but omitting the desalting step. As for enzyme activity assays, all steps were performed at 0–4°C to minimize in vitro cleavage of proteins (potentially leading to increased free amino acid levels).
Denaturing and native electrophoresis
Total protein was extracted from liquid nitrogen powder (1:4, w/v) in 25 mM Tris/HCl pH 7.5 containing 1% (w/v) insoluble polyvinylpolypyrrolidone and 0.1% (v/v) β-mercaptoethanol using mortar and pestle. Samples were centrifuged (10 min, 20,000g, 4°C), and supernatants were directly used for protein separation by denaturing (SDS-) and native polyacrylamide gel electrophoresis. All extraction steps were performed at 0–4°C. For SDS-PAGE, samples were mixed with 2× sample buffer (Laemmli 1970), boiled for 5 min, and separated on 1.5 mm, 13% minigels, using the Laemmli (1970) buffer system. Samples equal to the proteins extracted from 3% of a leaf (∼30 μg protein at 0 days) were loaded in each lane. Proteins were visualized by staining with Coomassie Brilliant Blue R-250. For native PAGE, samples were mixed with glycerol (final concentration: 10% v/v) and bromophenol blue (final concentration: 0.007% w/v). Proteins were separated using 1.5 mm, 9% minigels using the Laemmli (1970) buffer system, except that SDS was omitted, and that the running gel and running buffer contained 0.02% (w/v) casein. Samples equal to the proteins extracted from 5% of a leaf (∼50 μg protein at 0 days) were loaded in each lane. For the detection of protease activity bands, gels were incubated for 3 h at 37°C in 200 mM Na acetate buffer pH 5.4 containing 0.2% (v/v) β-mercaptoethanol, or in 200 mM Tris/HCl pH 7.5 (without mercaptoethanol) prior to staining with Coomassie Brilliant Blue R-250 (Fischer et al. 1998). This method visualized proteases as clear bands on a dark blue background.
Protease activity assays
For the analysis of endo- and exoproteolytic activities, protein extracts prepared as described above were desalted by centrifugation through Sephadex G-25 columns equilibrated with 25 mM Tris/HCl pH 7.5, containing 0.1% (v/v) β-mercaptoethanol (Feller et al. 1977). Again, all steps were performed at 0–4°C. Aminopeptidase activities were assayed using 2 mM L-leu-p-nitroanilide in 100 mM Na phosphate buffer pH 7.0 with 1% (v/v) DMSO as substrate. The assays were performed kinetically, at room temperature, during 10 min at 405 nm, using a SPECTRAmax PLUS384 spectrophotometer (Molecular Devices, Sunnyvale, CA.). Carboxypeptidase assays were performed using 2 mM N-carbobenzoxy-L-phe-L-ala in 100 mM Na acetate pH 5.0, containing 2% (v/v) DMSO as substrate. Substrate was omitted for blanks. Enzyme assays (in wells of microtitration plates) were incubated for 1 h at 37°C, and liberated α-amino groups were assayed with a TNBS reagent as described by Fischer et al. (1998). The method was calibrated with 0–50 nmol gly. Endopeptidases were quantified using 1% (w/v) azocasein as substrate, either in 0.2 M Na acetate buffer pH 5.4 containing 0.1% (v/v) β-mercaptoethanol, or in 0.2 M Tris/HCl buffer, pH 7.5. After 3 h of incubation at 37°C, undigested azocasein and large fragments were precipitated with cold TCA (final concentration: 5%, w/v), and small fragments resulting from proteolytic digestion were assayed in microplates as described by Fischer et al. (1998).
RNA extraction and Northern blot analysis
Probes for proteases and photosynthetic genes were obtained from the Clemson University Genomics Institute (CUGI) barley EST project (Clemson, SC http://www.genome.clemson.edu/projects/barley). Candidate genes were selected from all four catalytic classes of proteases, based on EST database annotations. The cDNAs utilized were between 500 and 900 bp long gene fragments; the presence of inserts in the pBluescript SK(-) plasmids (Stratagene, La Jolla, CA) obtained was verified by PCR using T3 and T7 primers. Additionally, the identity of all probes used was confirmed by DNA sequencing, using fluorescent chain-terminating dideoxynucleotides (Prober et al. 1987). Clone HV_Cea0017L04f (CUGI designation) was found to be 100% identical in a 162 amino acid overlap to GenBank accession number P05698 (H. vulgare Rubisco large subunit; gene designation: rbcL); clone HV_Cea0006E13f was 66.2% identical in a 139 amino acid overlap to GenBank accession number P10690 (Spinacia oleracea photosystem II 10 kDA polypeptide; gene designation: psbR); clone HVSMEf0005P20f was 91.4% identical in a 105 amino acid overlap to GenBank accession number Q10716 (Zea mays cysteine proteinase 1 precursor ccp1; gene designation: Hvcp1); clone HVSMEc0017P13f was 87.4% identical in a 254 amino acid overlap to GenBank accession number P42211 (Oryza sativa aspartic proteinase precursor; gene designation: HvAP); clone HVSMEg0003O18f was 95.4% identical in a 281 amino acid overlap to GenBank accession number P21529 (H. vulgare serine carboxypeptidase III precursor; gene designation: cp-mIII); clone HV_Ceb0002C20f was 87.6% identical in a 186 amino acid overlap to GenBank accession number P31542 (Lycopersicon esculentum ATP-dependent Clp protease ATP-binding subunit clpA homologue; gene designation: clpC Footnote 1.); clone HVSMEa0002G11f was 84.5% identical in a 207 amino acid overlap to GenBank accession number U92540 (Oryza sativa proteasome α subunit; gene designation proteasome α); and clone HVSMEc0007C19f was 95.3% identical in a 254 amino acid overlap to GenBank accession number P05167 (H. vulgare thiol protease aleurain precursor). Probe labeling was accomplished by the random prime method according to instructions from the supplier (Gibco BRL, Rockville, MD), using PCR-amplified, agarose gel-purified templates. Unincorporated nucleotides were removed by spinning through Bio-Gel P-30 microcolumns (Biorad, Hercules, CA).
The RNA extraction protocol was modified from that of McCarty (1986) to use Trizol reagent (Gibco). Liquid nitrogen powder (∼200 mg) was extracted with 0.5 ml extraction buffer (50 mM Tris/HCl pH 9.0, 200 mM NaCl, 1% (w/v) sarcosyl, 20 mM EDTA, and 5 mM DTT). Phenol/chloroform/isoamyl alcohol (25:24:1; 0.5 ml) was then added, and samples were vortexed. Tubes were spun for 5 min at 20,000g at 4°C, and 0.5 ml of upper aqueous phase was removed to a fresh tube. To the aqueous solution, 1 ml of Trizol reagent (Gibco) and 0.2 ml of chloroform were added, and samples were vortexed after each addition. Tubes were again centrifuged for 5 min at 20,000g at 4°C; aqueous phases were transferred to fresh tubes, extracted with chloroform, and precipitated and washed with ethanol. Pellets were resuspended in TE buffer, and RNA concentrations were determined spectrophotometrically.
RNA samples (10 μg per lane) were separated on 1.2% formaldehyde agarose gels and blotted onto positively charged nylon membrane (Smidansky et al. 2003). Hybridization was performed over night at 65°C in 0.5 M Na phosphate pH 7.2, containing 7% (w/v) SDS. Hybridized membranes were washed twice in 2× SSPE (3.6 M NaCl/0.2 M Na phosphate/0.02 M EDTA, pH 7.3) and 0.1% (w/v) SDS at 65°C (15 min/wash) and twice in 0.2× SSPE at 65°C (20 min/wash) and exposed to X-ray film with an intensifying screen at −80°C.
Density analysis of 1-D gels
Density of bands from protein gels and Northern blots were analyzed using the gel analyzer package in Image J, a free, Java-based image processing application available from the National Institutes of Health (http://rsb.info.nih.gov/ij/index.html).
Results
Influence of light intensity and phloem disruption on carbohydrate accumulation and leaf senescence
Total soluble (Fig. 1a, b) and insoluble (Fig. 1c, d) carbohydrates were analyzed in excised leaves incubated under strong light (250–300 μE m−2 s−1), weak light (15–20 μE m−2 s−1) and in darkness, and in attached leaves that were girdled, shift-girdled or untreated (control) to confirm that the methods used are adequate to study physiological processes associated with major differences in leaf carbohydrate levels. Soluble sugars accumulated 24-fold and 28-fold, respectively, during the first 4–6 days of incubation of excised leaves under strong light, followed by a small decrease. Girdled leaves showed a similar pattern, but with an even higher level of soluble sugar accumulation. In contrast, only a minor accumulation was observed in excised leaves after 4–8 days under weak light, and start levels were maintained in leaves incubated in darkness (Fig. 1a). Both control and shift-girdled leaves showed little increase, and there were no differences between the two treatments (Fig. 1b). Insoluble carbohydrates also accumulated in excised leaves incubated under strong light (Fig. 1c) and in girdled leaves (Fig. 1d), but their accumulation was more transient than the increase of soluble sugars, and overall levels were much lower. Essentially no changes in insoluble sugars were observed in dark-incubated leaves, in leaves incubated under weak light (Fig. 1c), and little difference was observed between control and shift-girdled leaves (Fig. 1d). It is noteworthy that barley leaves can accumulate fructans besides sucrose and hexoses under conditions limiting carbohydrate export from source leaves (Koroleva et al. 1998). Methods used in this manuscript do not differentiate between these carbohydrates, but short-chain fructans are quantified with the soluble carbohydrate fraction, and long(er)-chain fructans with the insoluble fraction.
Changes in carbohydrate, amino acid and chlorophyll contents during senescence of excised or attached barley leaves. Soluble sugars (a, b), insoluble sugars (c, d), amino acids (e, f) and chlorophylls (g, h) were assayed in excised leaves incubated under strong light, weak light and in darkness or in attached leaves, which were untreated (control), girdled, or shift-girdled. Means and standard deviations of three independent replications are shown
Amino acid levels increased in all treatments after leaf excision (Fig. 1e). While no major differences between strong light and weak light-incubated material were observed from 0 to 6 days, somewhat higher levels were observed in dark-incubated plant material. The decrease visible in strong light and dark-incubated leaves between 6 and 8 days may be associated with a loss of membrane integrity and metabolite leakage in advanced stages of senescence. In contrast, in attached leaves (Fig. 1f), amino acids only increased in girdled material and remained at start levels in shift-girdled and untreated controls. Interestingly, amino acid levels only changed after a lag phase of ∼4 days, in contrast to carbohydrates, which accumulated immediately.
Carbohydrate and amino acid accumulation was accompanied by a rapid degradation of chlorophylls in excised plant material incubated under strong light (Fig. 1g and 2a). Over 70% were degraded after 4 days in strong light; while chlorophylls were also degraded under weak light and in darkness, they were considerably more stable under these conditions than under strong light, leading to well-visible differences after 4 days (Fig. 2a). Excised, light-incubated leaves senesced from the tip, comparably to naturally senescing cereal leaves, while leaf bases senesced first in dark-incubated leaves (Fig 2a). Chlorophylls were also rapidly degraded in girdled leaves (Figs. 1h, 2b); however, it should be noted that degradation was only initiated after a lag phase of ∼4 days, comparably to the lag observed prior to amino acid accumulation. No chlorophyll degradation was observed in control (attached) plant material from 0 to 12 days, and only a minor decrease occurred in shift-girdled leaves.
Senescence of excised (a) and attached (b) barley leaves. Excised leaves are shown after 4 days in strong light, weak light and darkness. Attached leaves, which were untreated (control), girdled or shift-girdled are shown after 12 days. Red arrows indicate girdling positions. Leaves in (b) were excised immediately prior to documentation
Total proteins, analyzed by SDS-PAGE, were quite stable during the first 4 days in excised leaves under both weak and strong light (Fig. 3a), while considerable net protein degradation was observed in darkness during this period. After 6 days, both subunits of Rubisco and other soluble leaf proteins strongly decreased under all incubation conditions examined; however, protein levels remained conspicuously higher in leaves incubated under weak light, indicating a lower rate of net proteolysis. Total proteins were stable for the first 8 days for attached leaves under all treatments, but decreased appreciably in girdled samples between 8 and 12 days (Fig. 3b).
Changes of protein profiles during senescence of excised (a) or attached (b) barley leaves. a Leaves were incubated under strong light (SL), weak light (WL) and in darkness (D). b Leaves were either untreated (C), girdled (G), or shift-girdled (SG). Each lane was loaded with proteins equivalent to 3% of one leaf (∼30 μg at 0 days). The positions of the large (LSU) and small (SSU) subunits of Rubisco are indicated on the right, and the size of marker proteins (M) is indicated on the left. For Rubisco large subunits, numbers in each lane represent the density of the band as a percentage of the densest band (at day 0)
Protease activities
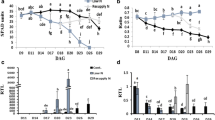
A combined biochemical and molecular approach was utilized to identify proteases upregulated under conditions leading to carbohydrate accumulation and accelerated leaf senescence. A 15–20-fold increase of aminopeptidase activity was observed under conditions leading to carbohydrate accumulation in both experimental systems used. Essentially no changes were observed in excised leaves incubated under weak light or in darkness (Fig. 4a), or in control and shift-girdled leaves (Fig. 4b). Carboxypeptidase activities were only slightly enhanced in strong light-incubated (excised) plant material during the first 4 days, but these enzyme(s) were increased after extended (8 days) incubation in darkness (Fig. 4c). In contrast, carboxypeptidase(s) were strongly induced under carbon feast conditions in attached leaves, with no increases detected in shift-girdled or untreated controls (Fig. 4d). Major endopeptidases were assayed at both acidic and slightly alkaline pH values, using azocasein as a substrate (Fig. 4e–h). At both pH values, a considerable increase in total endoproteolytic activity was measured in excised and attached leaves accumulating carbohydrates. A moderate increase in endopeptidase activity at pH 5.4 was also observed in excised leaves incubated under weak light or in darkness (Fig. 4e). At pH 7.5, endoproteolytic activity measured in leaves under weak light was almost unchanged during the first 4 days, leading to important differences between the two treatments at 4 days. In contrast, substantial increases in overall endoproteolytic activity were observed for pH 7.5 during extended incubation in weak light and darkness, and values were comparable for all three treatments after 8 days. Interestingly, while activities increased during 4–6 days in excised leaves incubated in strong light before starting to decrease, the induction observed in girdled leaves was both more dramatic and more sustained. Additionally, while a substantial increase of endoproteolytic activities was observed after 6–8 days in excised leaves incubated under weak light (Fig. 4e, g), activities in shift-girdled leaves increased more weakly and remained exactly at the same levels as untreated controls (Fig. 4f, h).
Changes in protease activities during senescence of excised and attached barley leaves. Aminopeptidase (a, b), carboxypeptidase (c, d), endopeptidase activities at pH 5.4 (e, f) and 7.5 (g, h) were assayed in excised leaves incubated under strong light, weak light and in darkness, and in attached girdled, shift-girdled and untreated (control) leaves. Means and standard deviations of three independent replications are shown
To investigate if one (major) or several endopeptidases contributed to the activities shown in Figs. 4e–h, enzymes were separated by native PAGE, followed by development of the casein-containing gels at pH 5.4 (in presence of β-mercaptoethanol) and 7.5 (Fig. 5a, b). Two major protease bands (labeled 1 and 2) were identified in excised leaf material (Fig. 5a). One of these (1) was only weakly detected throughout the experiment in attached leaves, but a third band with a lower molecular weight and/or higher net charge was found to be present in this plant material (Fig. 5b). Proteases 2 and 3 (in attached leaves only) were clearly induced under conditions leading to carbohydrate accumulation, and remained at lower levels in excised leaves incubated in weak light and darkness, and in attached control and shift-girdled leaves. Based on results obtained with excised leaves (Fig. 5a), protease 1 is regulated differently. This enzyme was strongly upregulated after 6–8 days of dark incubation, but was only weakly detectable in all other treatments. Additionally, it should be noted that, while all enzyme bands were visible at both pH values tested, activities were generally higher at the lower pH, suggesting that some or all of the enzymes are located in compartments with a low pH value, such as lytic vacuoles.
Changes in endopeptidase activity profiles during senescence of excised (a) or attached (b) barley leaves. a Leaves were incubated under strong light (SL), weak light (WL) and in darkness (D). b Leaves were either untreated (C), girdled (G), or shift-girdled (SG). Each lane was loaded with proteins equivalent to 5% of one leaf (∼50 μg at 0 days). Proteins were separated by native PAGE, and protease activity bands were visualized at pH 5.4 and 7.5. Arrows with numbers indicate the positions of protease activity bands
Expression of photosynthetic and protease genes
A series of Northern blots was utilized to identify protease genes upregulated under conditions leading to carbohydrate accumulation (Fig. 6a, b). As it has previously been demonstrated that photosynthetic genes are no longer expressed in source tissues under these conditions (Koch 1996), protease gene expression was compared to mRNA levels of the large subunit of Rubisco and of the 10 kD subunit of photosystem II. Both rbcL and psbR continued to be expressed at high levels during the first 2 days of incubation in strong or weak light, but psbR was no longer expressed in darkness (Fig. 6a). Expression levels decreased after 4 days in both strong and weak light; however, the signals were considerably higher under weak light and remained detectable even after extended incubation (8 days) under these conditions. No expression of rbcL or psbR was detected after 4–8 days in darkness. Similarly, high expression levels of these genes were maintained in attached control and shift-girdled leaves, but transcripts became low to undetectable 8–12 days after girdling.
Photosynthetic and protease gene expression in excised (a) and attached (b) barley leaves. Transcript levels of Rubisco large subunit (rbcL), photosystem II 10 kDa polypeptide (psbR), cysteine proteinase 1 (Hvcp1), aleurain, an aspartic proteinase (HvAP), carboxypeptidase III (cp-mIII), Clp protease ATP binding subunit (clpC) and proteasome α subunit (proteasome α) were determined. a Excised leaves were incubated under strong light (SL), weak light (WL) or in darkness (D). b Leaves were either untreated (C), girdled (G), or shift-girdled (SG). Ten microgram of total RNA was loaded in each lane. Comparable ethidium bromide staining of rRNA fractionated in an agarose gel indicates similar loading from lane to lane and lack of RNA degradation. Numbers represent transcript levels (measured as densities) in percent of the densest band in each row
In contrast to the two photosynthetic genes analyzed, mRNA levels of several genes coding for proteases or protease subunits were enhanced under carbon feast conditions in either excised leaves incubated under strong light, in attached girdled leaves, or in both of these treatments. Interestingly, while expression of photosynthetic genes was also repressed in dark-incubated excised tissue, and net proteolysis was accelerated by this treatment (Fig. 3a), the pattern of protease gene expression in dark-incubated tissue was completely different from that found under carbon feast conditions. Expression of the carboxypeptidase gene cp-mIII was positively correlated with carbohydrate levels in both excised (after 4 days) and attached leaves (after 4–12 days). The thiol protease gene HvCP1 was also strongly increased in both systems. However, its expression was also correlated with leaf age in attached leaves, and HvCP1 mRNA declined earlier in girdled than in control leaves (after 8–12 days). HvAP expression, while upregulated after 2 days in excised leaves and after 4 days in attached leaves under carbon feast conditions, also reacted to leaf excision (well visible at 6 days) and to leaf age (generally increased mRNA levels after 8–12 days in attached leaves). Somewhat surprisingly, overall HvAP expression was lower in the attached—than in the excised–leaf experiment (corresponding X-ray films were exposed longer), making data interpretation more difficult.
The three other protease genes investigated did not show consistent induction patterns in the two experimental systems. While genes coding for the thiol protease aleurain and for the α subunit of the proteasome complex were upregulated in girdled leaves after 4–8 days (in comparison to shift-girdled and untreated controls), their expression was not correlated with carbon feast conditions in the excised leaf system. In contrast, a gene coding for the ATP- binding subunit of the plastidial Clp protease was only weakly expressed throughout the experiment in attached plant material, but was induced in strong light-incubated excised leaves.
Discussion
Carbon feast conditions and senescence induction in excised and attached leaves
It has been convincingly demonstrated that carbohydrate accumulation is one of the signals inducing leaf senescence in intact plants (Masclaux et al. 2000; Yoshida 2003; Jongebloed et al. 2004; Pourtau et al. 2004). Based on this knowledge, the development of an experimental approach allowing the manipulation of leaf carbohydrate levels is a logical first step in the identification and characterization of proteases involved in the degradation of major leaf proteins at the onset of the senescence process. While both model systems presented in Figs. 1–6 clearly demonstrate accelerated leaf senescence under carbon feast conditions, the selective interruption of phloem export from attached leaves by girdling appears more relevant. First, xylem connections with the rest of the plant remain intact, allowing the continued import of xylem-borne metabolites and signals such as phytohormones. However, it should be noted that complete blockage of phloem export from girdled leaves also leads to some differences with natural leaf senescence, especially after the initial carbohydrate buildup. Accumulation of metabolites such as amino acids (starting at 4 days; Fig. 1f), which are exported from naturally senescing leaves, may influence processes in girdled leaves during later phases (after 8–12 days). Second, some data (not shown) indicate leakage of metabolites from the cut ends of excised leaves; while this fact may explain decreases in soluble carbohydrate and amino acid levels between 6 and 8 days (Fig. 1), it also complicates data interpretation. Third, using the shift-girdling control, both control and girdled leaves can be incubated under exactly the same light conditions, excluding the influence of light-borne signals from the system. Finally, Figs. 1–6 clearly indicate that all parameters assayed are very close in shift-girdled and untreated (control) leaves.
With respect to data interpretation, time courses of metabolite levels, total protein levels, protease activities and protease transcript levels show important differences. In girdled leaves, a considerable buildup of carbohydrates and proteases (both activities and transcripts) precedes increases in amino acids and decreases in chlorophylls and total proteins. Considering that the carbohydrate increase between 0 and 4 days is quantitatively more important than increases in protease activities (Figs. 1 and 4), these time courses are in agreement with causal relationships between carbon feast conditions and protease induction, and (subsequently) between protease induction and accelerated net proteolysis. It should also be noted that carbohydrate levels start to decrease in later phases of senescence (between 8 and 12 days), possibly reflecting a switch to respiratory consumption of sugars after dismantling of the photosynthetic apparatus. The described temporal correlations are less clear-cut for excised leaves, most likely due to the fact that excision induces senescence in all described treatments.
In summary, while the comparison of two experimental systems represents a clear advantage for the interpretation of our results, the girdling treatment appears more representative of the processes occurring at the onset of natural leaf senescence.
Does dark incubation of excised leaves lead to carbon famine?
Due to the fact that carbon feast and not famine conditions are an important trigger of natural leaf senescence (Yoshida 2003), this manuscript emphasizes proteolytic processes induced under carbon feast. However, as dark incubation has been utilized as a means to induce leaf senescence in a number of past studies (e.g. Feller and Fischer 1994; Kleber-Janke and Krupinska 1997; Weaver and Amasino 2001), this treatment was included for comparison. It might be expected that this approach, similarly to the situation encountered in isolated non-photosynthetic tissues such as root tips (Brouquisse et al. 1992), leads to carbon famine. However, our data (Fig. 1a, c) do not support this hypothesis, at least not for excised leaves. A <20% decrease in soluble and insoluble carbohydrates was observed during 8 days in this plant material. Interestingly, Gut and Matile (1988a, b) showed an increase in lipid degradation and in key enzymes of the glyoxylate cycle in dark-incubated barley leaf segments, suggesting the conversion of thylakoid lipids into carbohydrates by gluconeogenesis under these conditions. Therefore, while our data indicate that chlorophylls are degraded (although not as fast as under strong light, Fig. 1g), that net proteolysis is even higher in dark-incubated leaves than in those exposed to strong light (Fig. 3a), and that proteolytic activity was induced to some degree in this plant material (Figs. 4e, g, 5a), these processes are most likely not causally related to sugar (i.e., carbon famine) signaling. If correct, this interpretation indicates that different signals are involved in senescence induction in excised (dark-incubated) source and sink tissues.
Leaf senescence and protease induction under carbon feast conditions
The most conspicuous result from this study is the fact that both biochemical (Figs. 4, 5) and molecular (Fig. 6) approaches indicate that several proteases, potentially located in different cellular compartments, are induced under carbon feast conditions. Biochemically, one or several aminopeptidases, carboxypeptidases and endopeptidases are strongly upregulated under carbon feast conditions in excised and/or attached leaves; assayed endopeptidase(s) may also react to leaf excision. Serial Northern blots (Fig. 6) suggest that carbon feast conditions are a factor in the induction of two thiol protease genes (HvCP1, aleurain), an aspartic protease, a serine carboxypeptidase, and possibly genes coding for subunits of the plastidial Clp and cytosolic/nuclear proteasome complexes. Interpretation of the molecular data is complicated by the fact that some of the investigated genes behave differently in the two utilized model systems (aleurain, clpC and proteasome α subunit), and that some genes (HvCP1, aleurain, HvAP) also react to other stimuli besides carbon feast (such as leaf age or leaf excision). Since it has been suggested that multiple internal and external signals influence the onset and velocity of leaf senescence (Fischer et al. 1994; Noodén et al. 2004; Woo et al. 2004), this is not surprising.
In functional terms, carboxypeptidase activity (Fig. 4d) and gene expression data (Fig. 6b) represent the most interesting result from this study. First, cp-mIII expression is specific for girdled leaves (with almost no expression in controls throughout the experiment) and correlates well with the enzyme assay data, making it a candidate gene for the measured activity. Second, this gene is also highly upregulated after 4 days in strong light-incubated excised leaves (Fig. 6a), and only reacts to leaf excision after 6 days. Third, recent data from our laboratory, using a quantitative trait locus (QTL) mapping approach, have implicated one or several carboxypeptidases (assayed with the same substrate as in this study) in N recycling from senescing barley leaves (Yang et al. 2004). Together, data from both studies indicate that one or more carboxypeptidases play an important role in N remobilization at the onset of leaf senescence; therefore, it appears likely that a detailed functional analysis of cp-mIII (and, possibly, closely related genes) will prove rewarding. Intriguingly, while this has not yet been directly demonstrated for the product of cp-mIII, carboxypeptidases have previously been located in lytic vacuolar compartments (Brouquisse et al. 2001), adding another interesting facet to the ongoing discussion about the importance of such organelles for senescence-associated N remobilization.
Similarly to carboxypeptidases, thiol (such as HvCP1 and aleurain) and aspartic proteases have previously been located in vacuolar compartments (Brouquisse et al. 2001). Biochemically, aleurain is an aminopeptidase (Holwerda and Rogers 1992); while the preferred substrates of HvCP1 and HvAP are unknown, thiol and aspartic proteases are often endoproteolytic enzymes (Brouquisse et al. 2001; Beers et al. 2004). Carbon feast conditions clearly influence regulation of these genes, but they also react to other stimuli such as leaf excision or leaf age, and none of them appear directly correlated with the activities shown in Fig. 4. An induction of aspartic and thiol protease gene expression has previously been shown in senescing tissues (Buchanan-Wollaston 1997); specifically, Scharrenberg et al. (2003) have recently demonstrated that HvCP1 is upregulated during natural leaf senescence. Additionally, a corn cysteine proteinase (ccp1; GenBank accession number Q10716) with a high degree of sequence homology with HvCP1 (82% sequence identity) is upregulated in corn root tips under carbon famine conditions (Chevalier et al. 1995), suggesting that these thiol proteases may be involved in N salvaging under different physiological conditions.
In contrast to the enzymes discussed above, other proteases studied in this manuscript have been located to plastidial compartments (Clp protease, some aminopeptidases), cytosol (some aminopeptidases, proteasome) and nucleus (ubiquitin-proteasome pathway; Brouquisse et al. 2001). With regard to their potential plastidial location, the strong induction of aminopeptidase(s) under carbon feast conditions is intriguing. However, using the same aminopeptidase substrate as in this study, the QTL mapping approach pointing to a functional role for carboxypeptidase(s) in whole-plant N recycling (Yang et al. 2004) did not indicate such a role for barley leaf aminopeptidases. Results obtained for the Clp protease and a regulatory subunit of the proteasome are not consistent between the two experimental approaches utilized (Fig. 6). Based on the discussion (above) indicating that data shown in Fig. 6b are more relevant for processes occurring during natural leaf senescence, a detailed analysis of proteasome function under carbon feast conditions may furnish additional information on the metabolic role of this important enzyme complex. In this context, regulatory functions for the ubiquitin/proteasome pathway during leaf senescence have recently been proposed (Smalle and Vierstra 2004).
In conclusion, our data clearly demonstrate an upregulation of several proteases, located in different cellular compartments, under carbon feast conditions. Considering that Jongebloed et al. (2004) have demonstrated sieve tube occlusion and increasing carbohydrate levels at the onset of natural leaf senescence, our experimental approach, by enhancing and coordinating this signal in large numbers of leaves, greatly facilitates the identification of protease genes induced during this phase. As the photosynthetic apparatus is dismantled early during the senescence process (Hörtensteiner and Feller 2002), at least some of these genes are likely to be involved in the bulk degradation of plastidial proteins, which is one of the hallmarks of leaf senescence. Others may be involved in the specific degradation of regulatory proteins, or in (quantitatively) less important proteolytic processes in the cytosol and other cellular compartments. Additionally, while previously published results indicate the importance of plastidial proteases for photosynthetic protein degradation (Feller and Fischer 1994; Hörtensteiner and Feller 2002), our data, together with those from a previous study from our laboratory (Yang et al. 2004) suggest that a functional involvement of lytic vacuolar compartments in this process should be further investigated.
Notes
The ATP binding subunit of the Clp protease has been designated ClpA in E. coli, and ClpC in plants. See Adam et al. (2001) for a recent review
References
Adam Z, Adamska I, Nakabayashi K, Ostersetzer O, Haussuhl K, Manuell A, Zheng B, Vallon O, Rodermel SR, Shinozaki K, Clarke AK (2001) Chloroplast and mitochondrial proteases in Arabidopsis. A proposed nomenclature. Plant Physiol 125:1912–1918
Beers EP, Jones AM, Dickerman AW (2004) The S8 serine, C1A cysteine and A1 aspartic protease families in Arabidopsis. Phytochemistry 65:43–58
Brouquisse R, James F, Pradet A, Raymond P (1992) Asparagine metabolism and nitrogen distribution during protein degradation in sugar-starved maize root tips. Planta 188: 384–395
Brouquisse R, Masclaux C, Feller U, Raymond P (2001) Protein hydrolysis and nitrogen remobilization in the plant life and senescence. In: Lea PJ, Morot-Gaudry J-F (eds) Plant nitrogen. Springer, Berlin Heidelberg New York, pp. 275–293
Buchanan-Wollaston V (1997) The molecular biology of leaf senescence. J Exp Bot 48:181–199
Chevalier C, Bourgeois E, Pradet A, Raymond P (1995) Molecular cloning and characterization of six cDNAs expressed during glucose starvation in excised maize (Zea mays L.) root tips. Plant Mol Biol 28:473–485
Crafts-Brandner SJ, Below FE, Harper JE, Hageman RH (1984a) Differential senescence of maize hybrids following ear removal. I. Whole plant. Plant Physiol 74:360–367
Crafts-Brandner SJ, Below FE, Wittenbach VA, Harper JE, Hageman RH (1984b) Differential senescence of maize hybrids following ear removal. II. Selected leaf. Plant Physiol 74:368–373
Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D (1999) Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11:1253–1266
Feller U, Fischer A (1994) Nitrogen metabolism in senescing leaves. Crit Rev Plant Sci 13:241–273
Feller UK, Soong TS-T, Hageman RH (1977). Leaf proteolytic activities and senescence during grain development of field-grown corn (Zea mays L.). Plant Physiol 59: 290–294
Fischer A, Brouquisse R, Raymond P (1998) Influence of senescence and carbohydrate levels on the pattern of leaf proteases in purple nutsedge (Cyperus rotundus). Physiol Plant 102:385–395
Fröhlich V, Feller U (1992) Effect of phloem interruption on endopeptidase and aminopeptidase activities in flag leaves of field-grown wheat. Biochem Physiol Pflanzen 188:13–21
Gut H, Matile P (1988a) Breakdown of galactolipids in senescent barley leaves. Bot Acta 102:31–36
Gut H, Matile P (1988b) Apparent induction of key enzymes of the glyoxylic acid cycle in senescent barley leaves. Planta 176:548–550
Holwerda BC, Rogers JC (1992) Purification and characterization of aleurain. A plant thiol protease functionally homologous to mammalian cathepsin H. Plant Physiol 99:848–855
Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53:927–937
Jongebloed U, Szederkényi J, Hartig K, Schobert C, Komor E (2004) Sequence of morphological and physiological events during natural ageing and senescence of castor bean leaf: sieve tube occlusion and carbohydrate back-up precede chlorophyll degradation. Physiol Plant 120:338–346
Kleber-Janke T, Krupinska K (1997) Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions. Planta 203:332–340
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47:509–540
Koroleva O, Farrar JF, Tomos AD, Pollock CJ (1998) Carbohydrates in individual cells of epidermis, mesophyll, and bundle sheath in barley leaves with changed export or photosynthetic rate. Plant Physiol 118:1525–1532
Krapp A, Quick WP, Stitt M (1991) Ribulose-1,5-bisphosphate carboxylase-oxygenase, other Calvin-cycle enzymes, and chlorophyll decrease when glucose is supplied to mature spinach leaves via the transpiration stream. Planta 186:58–69
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Masclaux C, Valadier M-H, Brugière N, Morot-Gaudry J-F, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211:510–518
McCarty DR (1986) A simple method for extraction of RNA from maize tissue. Maize Genet Coop Newsl 60:61
Mitsuhashi W, Crafts-Brandner S J, Feller U (1992) Ribulose-1, 5-bis-phosphate carboxylase/oxygenase degradation in isolated pea chloroplasts incubated in the light or in the dark. J Plant Physiol 139:653–658
Moore B, Zhou L, Rolland F, Hall Q, Cheng W-H, Liu Y-X, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300:332–336
Noodén LD, Guiamét JJ, John I (2004) Whole plant senescence. In: Noodén LD (eds) Plant cell death processes. Academic, San Diego, pp. 227–244
Peoples MB, Dalling MJ (1988) The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In: Noodén LD, Leopold AC (eds) Senescence and aging in plants. Academic, San Diego, pp. 181–217
Pourtau N, Marès M, Purdy S, Quentin N, Ruël A, Wingler A (2004) Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 219:765–772
Prober JM, Trainor GL, Dam RJ, Hobbs FW, Robertson CW, Zagursky RJ, Cocuzza AJ, Jensen MA, Baumeister K (1987) A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science 238:336–341
Scharrenberg C, Falk J, Quast S, Hussühl K, Humbeck K, Krupinska K (2003) Isolation of senescence-related cDNAs from flag leaves of field grown barley plants. Physiol Plant 118:278–288
Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55:555–590
Smidansky ED, Martin JM, Hannah LC, Fischer AM, Giroux MJ (2003) Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 216:656–664
Weaver LM, Amasino RM (2001) Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiol 127:876–886
Woo HR, Lim PO, Nam HG, Noodén LD (2004) Genes that alter senescence. In: Noodén LD (eds) Plant cell death processes. Academic, San Diego, pp. 73–90
Yang L, Mickelson S, See D, Blake TK, Fischer AM (2004) Genetic analysis of the function of major leaf proteases in barley (Hordeum vulgare L.) nitrogen remobilization. J Exp Bot 55:2607–2616
Yoshida S (2003) Molecular regulation of leaf senescence. Curr Opin Plant Biol 6:79–84
Acknowledgements
Work presented in this study has been supported by award number 0101019 from the USDA-NRI Competitive Grants Program, and by grant agreement #04–10 from the Montana Board of Research and Commercialization Technology to A.M.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parrott, D., Yang, L., Shama, L. et al. Senescence is accelerated, and several proteases are induced by carbon “feast” conditions in barley (Hordeum vulgare L.) leaves. Planta 222, 989–1000 (2005). https://doi.org/10.1007/s00425-005-0042-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0042-x