Abstract
Earlier, we reported that mutation in the Male Sterile33 (MS33) locus in Arabidopsis thaliana causes inhibition of stamen filament growth and a defect in the maturation of pollen grains [Fei and Sawhney (1999) Physiol Plant 105:165–170; Fei and Sawhney (2001) Can J Bot 79:118–129]. Here we report that the ms33 mutant has other pleiotropic effects, including aberrant growth of all floral organs and a delay in seed germination and in flowering time. These defects could be partially or completely restored by low temperature or by exogenous gibberellin A4 (GA4), which in all cases was more effective than GA3 Analysis of endogenous GAs showed that in wild type (WT) mature flowers GA4 was the major GA, and that relative to WT the ms33 flowers had low levels of the growth active GAs, GA1 and GA4, and very reduced levels of GA9, GA24 and GA15, precursors of GA4. This suggests that mutation in the MS33 gene may suppress the GA biosynthetic pathway that leads to GA4 via GA9 and the early 13-H C20 GAs. WT flowers also possessed a much higher level of indole-3-acetic acid (IAA), and a lower level of abscisic acid (ABA), relative to ms33 flowers. Low temperature induced partial restoration of male fertility in the ms33 flowers and this was associated with partial increase in GA4. In contrast, in WT flowers GA1 and GA4 were very much reduced by low temperature. Low temperature also had little effect on IAA or ABA levels of ms33 flowers, but did reduce (>2-fold) IAA levels in WT flowers. The double mutants, ms33 aba1-1 (an ABA-deficient mutant), and ms33 spy-3 (a GA signal transduction mutant) had flower phenotypes similar to ms33. Together, the data suggest that the developmental defects in the ms33 mutant are unrelated to ABA levels, but may be causally associated with reduced levels of IAA, GA1 and GA4, compared to WT flowers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Analyses of various nuclear-coded genic (GMS) and cytoplasmic (CMS) male-sterile mutants in plants have shown that pollen development in angiosperms is controlled by a large number of nuclear and mitochondrial genes (reviewed in Kaul 1988; Shivanna and Sawhney 1997). In Arabidopsis, nearly 900 male-sterile mutants, induced by T-DNA insertion or ethyl methane sulfonate (EMS) mutagenesis, had defects in anther and pollen development, including aberrations in anther dehiscence (Sanders et al. 1999). Although many genes expressed during anther development are housekeeping genes, some are designated as pollen-specific and are expressed at early and late stages of microsporogenesis (Mascarenhas 1990). Additionally, in a few male-sterile mutants, other aspects of plant development are also affected, suggesting that some of the genes controlling pollen development may not be specific, i.e., they have pleiotropic effects. For example, the single gene 7B-1 mutant in tomato is male-sterile, but it also exhibits reduced de-etiolation of hypocotyl growth and increased resistance to abiotic stresses i.e., high osmoticum, various salts, and low temperature (Fellner and Sawhney 2000; Fellner et al. 2001). Conversely, some of the dwarf mutants in maize (Phinney and Spray 1982; Dellaporta and Calderon-Urrea 1994), tomato (Koornneef et al. 1990) and Arabidopsis (Koornneef and van der Veen 1980) are also male-sterile and have pleiotropic effects, e.g., delayed seed germination and flowering. This suggests that certain genes controlling pollen and stamen development may also be expressed at other stages of plant development, and that their effects may be mediated via common factors required for several developmental processes.
Plant hormones are implicated in various developmental processes, including seed germination, vegetative growth, flowering induction and reproductive organ development (Davies 1995). The various roles of plant hormones have been determined by research involving application, both in situ and in vitro, and analyses of endogenous hormones (see, e.g., Pharis and King 1985; Sawhney and Shukla 1994). In addition, mutants exhibiting defects in hormone biosynthesis or hormone signaling have been used to support the role of hormones in developmental processes, e.g., dwarfing, delayed seed germination and male sterility (Koornneef and van der Veen 1980; Koornneef et al. 1990; Jacobsen and Olszewski 1991, 1993; Wilson and Somerville 1995). For example, the gibberellin (GA) deficient, ga1-1 and ga1-3 mutants, and the GA-insensitive, gai mutant of Arabidopsis exhibit multiple defects, including delayed seed germination and flowering, loss of apical dominance, effects on plant stature and defects in male and female reproductive organ development (Koornneef et al. 1982; Wilson et al. 1992; Sun and Kamiya 1994; Goto and Pharis 1999). Similarly, male-sterile mutants in tomato, Brassica spp. and rice possess altered levels and/or altered metabolism of hormones e.g., GAs, cytokinins, and abscisic acid (ABA), suggesting that stamen and pollen development requires a critical balance of several key hormones (reviewed in Sawhney and Shukla 1994). A recent study with transgenics containing constructs of CKX1, a cytokinin oxidase gene, and GAI, a gene that regulates sensitivity to GA, has provided molecular evidence in support of the role of hormones in stamen and pollen development (Huang et al. 2003).
Pollen and stamen development is also influenced by environmental factors. In a number of plant species temperature, photoperiod and water stress can affect the expression of male sterility (e.g., Sawhney 1983; Murai and Tsunewaki 1993; Saini 1997) and it has been suggested that these effects may be mediated through changes in endogenous hormones (Singh et al. 1992; Singh and Sawhney 1998). That said, conflicting results have been reported with respect to the role played by environmental factors in different male sterile systems. For example, low temperature restores partial to complete fertility in the sl-2 mutant of tomato (Sawhney 1983) and in the msp mutant of soybean (Carlson and Williams 1985). In contrast, in the nap and pol male-sterile mutants of Brassica, it is high temperature that stimulates normal pollen development (Fan and Stefansson 1986). Thus, the interrelationships of environmental factors and hormones with pollen development are not entirely clear.
A single gene recessive male sterile33 (ms33) mutant in Arabidopsis, isolated by EMS mutagenesis, has defects in both stamen filament elongation and pollen development (Dawson et al. 1993). We showed earlier that the inhibition of filament growth in ms33 was related to inhibition of rapid cell elongation immediately before anthesis, and that exogenous GA or low temperature was able to restore normal filament growth (Fei and Sawhney 1999). Pollen development in ms33 is impaired near the maturation stage and this was, in part, related to increased vacuolation of pollen grains, likely as a result of inadequate pollen desiccation (Fei and Sawhney 2001).
The objectives of the present study were to determine (1) whether the ms33 mutant in Arabidopsis has pleiotropic effects, i.e., other than those on pollen and stamen development, (2) whether hormones and temperature influence the expression of various phenotypic traits in the mutant, and if so, (3) whether the pleiotropic effects in the ms33 mutant are related to changes in endogenous hormone content and/or hormone signaling.
Materials and methods
Plant material and growth conditions
Seeds of wild type (WT) Arabidopsis thaliana Landsberg erecta and the ms33 mutant, produced by EMS mutagenesis (Dawson et al. 1993), were provided by B. Mulligan of the University of Nottingham, United Kingdom. Seeds of the spy-3 mutant (Columbia ecotype) were obtained from the Arabidopsis Biological Research Center, Ohio State University, Columbus, Ohio, and seeds of the aba1-1 mutant (Landsberg erecta=ler, ecotype) were obtained from the Nottingham Arabidopsis Stock Centre. Seeds were sown in 15 cm diameter plastic pots containing Tera-lite Redi-earth mix (Grace, Ajax, Ontario, Canada). Pots were then exposed to 4°C in the dark for 3 days prior to transfer to a growth chamber under 22°C/18°C (day/night) temperatures and a 16h/8 h light/dark photoperiod. Fluorescent tubes provided the light source (Osram Sylvania, Versailles, Ky.) at 120–150 μmol m−2 s−1.
Seed germination
Fifty seeds of WT and pure line of the ms33 mutant (obtained from low temperature treatment as described below) were germinated in light or in the dark at a constant 24°C for 7 days in 6 cm diameter Petri dishes lined with two layers of filter paper. Each dish contained 2 ml distilled water (control) or 2 ml of one of the following solutions (all solutions had 0.02% (v/v) Tween-20); 10−4 M GA3, 10−3 M GA3, 10−4 M GA4, 10−3 M GA4, 10−4 M paclobutrazol (PP333) and a mixture of 10−4 M GA4 and 10−4 M PP333 solution. For low temperature treatments, Petri dishes were exposed to either 4°C or 15°C in the dark for 3 days, and then transferred to 24°C in the dark for germination. Germination for dark experiments was examined under a dim green light. Each experiment was repeated three times.
Analyses of endogenous plant hormones
Sample collection
The mature flowers of ms33 mutant and WT plants grown at normal temperature (22°C/18°C) and low temperature (15°C/11°C) were collected separately prior to anthesis. The samples were ground in liquid N2 and freeze-dried and the lyophilized flowers were stored at −80°C until subsequent analysis.
GA analysis
Extraction
Dry sample (1 g) was extracted with 20 ml 80% (v/v) aqueous methanol, with the following deuterated internal standards of GAs: [2H2]-GA1 10 ng, [2H2]-GA4 20 ng, [2H2]-GA8 20 ng, [2H2]-GA9 20 ng, [2H2]-GA15 20 ng, [2H2]-GA20 20 ng, [2H2] GA24 20 ng, [2H2]-GA44 20 ng and [2H2]-GA53 20 ng, added to the methanolic extract for subsequent quantification of endogenous GAs by the isotope dilution method using gas chromatography-mass spectrometry-selected ion monitoring (GC-MS-SIM) for appropriate diagnostic m/z ions as in Fujioka et al. 1988 (modified by D. Pearce, cited in Zhang 1990). After an initial filtration, the tissue residue was re-extracted twice with 20 ml 80% aqueous methanol. All filtrates were then combined and taken to dryness in vacuo on a rotary flash evaporator at 35°C. The dried residue was subsequently dissolved in 5 ml 80% aqueous methanol for further purification.
Purification
The 5 ml sample solution was loaded onto a column filled with 3 g Prep C18 (125 Å, 55–105 μm). The column was eluted with 20 ml 80% aqueous methanol. The eluate was collected and dried as above. The residue was then dissolved in methanol and transferred into a 50 ml beaker containing 1 g Celite. After drying with a warm air blower, the Celite+sample was loaded onto a column made of 5 g ICN-deactivated (20% water by weight) Silica (32–100 mesh). This column was eluted with 80 ml ethyl acetate:hexane (95:5, v/v) saturated with 0.5 M formic acid and the eluate was dried (Koshioka et al. 1983). The dried residue was then dissolved in 0.4 ml methanol and 0.6 ml 1% (v/v) aqueous acetic acid, then the [3H]-GAs ([3H]-GA1, [3H]-GA4 and [3H]-GA9, about 3 million Bq each) were added as radiotracers. The sample solution was then subjected to reversed phase high performance liquid chromatography (HPLC) with a Radial-Pak μBondapak C18 cartridge (RCM, 8×100 mm, 5 μm, Waters, Milford, Mass.). After the sample was injected, the column was eluted isocratically with a solvent mixture of 10% methanol and 100% methanol (40:60, v/v) for 40 min at a flow rate of 1 ml/min. The eluate was collected in 1 ml fractions and a 10 μl aliquot of each fraction was mixed with 5 ml scintillation cocktail to detect radioactivity. The fractions were then grouped based on peaks of radioactivity. All fraction groupings were dried separately and each residue was subsequently dissolved in 1 ml methanol:acetic acid (99.9:0.1 v/v). The samples were further purified using a Nucleosil N(CH3)2 HPLC column (4.6×150 mm, 5 μm; Alltech, Deerfield Ill.), eluted with methanol:acetic acid (99.9:0.1 v/v) for 40 min at a flow rate of 1 ml/min. The eluate was collected as 1 ml fractions and the radioactivity in each fraction was measured and combined as above, then dried.
Methylation and silylation of GAs
GA9 and GA15 were methylated with diazomethane. GA1, GA3, GA4 and GA20 were first methylated with diazomethane, and then derivatized with bis-trimethyl-silyltrifluoroacetamide (BSTFA). The methylation procedure was accomplished by first dissolving the sample in 10 ml methanol, then adding 90 ml ethereal CH2N2. The mixture was left at room temperature for 15 min and the solvent removed under a flow of N2. For silylation, the samples were dissolved in 50 µl GC-MS grade pyridine followed by 50 µl BSTFA with 1% trimethylchlorosilane (TMCS). The reaction vial was flushed with N2 then left at 80°C for 30 min. The samples were dried under a flow of N2.
Quantitative analysis
Each GA was quantified by GC-MS-SIM by dissolving each sample in two drops of hexane and a 2 μl aliquot was then introduced into a GC-MS [Hewlett Packard (HP) 5890 II; MS, HP 5970 A] by on-column injection into a retention gap of a 0.5 m ×0.32 mm deactivated fused silica capillary DB1-15 N column (15 m ×0.25 mm, 0.25 μm methyl silicone film). The oven was heated from 60°C to 200°C at 20°C/min and from 200°C to 280°C at 5°C/min. Data acquisition was controlled by a HP 300 Series computer.
Analyses of indole-3-acetic acid and abscisic acid
Extraction and purification
The procedures for extraction and purification by a reversed-phase C18 open column for indole-3-acetic acid (IAA) and abscisic acid (ABA) were the same as those used for GAs, except that 200 ng [13C6]-IAA and 100 ng [2H6]-ABA were added as quantitative stable isotope labeled internal standards.
The dry residue from the last step was dissolved in 10 ml 1% acetic acid. The solution was partitioned with 10 ml ethyl acetate (saturated with 2% acetic acid) three times. The ethyl acetate solutions were combined and dried. The residue was dissolved in 1 ml methanol and 1 ml 1% acetic acid. About 3 million Bq each of [3H]-IAA and [3H]-ABA of high specific radioactivity was added as a radiotracer for use in locating fractions after HPLC. The combined fraction expected to contain ABA and IAA was loaded onto the Sep-Pak C18 Cartridges with a syringe. The column was eluted with 15 ml 50% aqueous methanol, and the eluate collected and dried. Subsequently it was dissolved in 0.1 ml methanol, and then mixed with 0.9 ml 1% acetic acid. The sample solution was injected into HPLC with a C18 RCM (see above) column (8×110 mm, 5 μ). The solvent system for elution was 10% methanol in Pump A and 100% methanol in pump B at a flow rate of 2 ml/min. The following elution program was set: 0–10 min, 100% of pump A; 10–40 min, 30% of pump A and 70% of pump B with a linear gradient; 40–50 min, 0% pump A and 100% pump B. The first four fractions were 10 ml, followed by three fractions of 5 ml, five fractions of 2 ml and seven fractions of 5 ml. The radioactivity in each fraction was measured as above and radioactive fractions combined and dried as detailed above.
Methylation and quantification
IAA and ABA were methylated with diazomethane and quantified by GC-MS-SIM using the isotope dilution method as detailed above for GAs. All equipment used was the same as that for GAs. The temperature in GC was programmed from 15°C to 195°C at 15°C/min, and from 195°C to 275°C at 5°C/min.
Construction of double mutants
Homozygous recessive ms33 mutant plants were crossed with homozygous recessive aba1-1 and spy-3 mutants, separately, to generate ms33/ms33 aba1-1/aba1-1 and ms33/ms33 spy-3/spy-3 double mutants. The F2 seeds were collected and sown in pots. Novel phenotypes with characteristics of both the parent mutants were identified from the F2 population. Other phenotypes in the F2 population were also scored. Chi-square analysis was used to determine the significance of the dihybrid ratio (9:3:3:1).
Application of GA4
A 10−4 M GA4 solution containing 0.02% Tween-20 (v/v) was sprayed to drip off, twice a week for 2 weeks, onto the ms33 plants, beginning ca. 1 week prior to anthesis.
Results
Vegetative growth and flowering in ms33 and WT
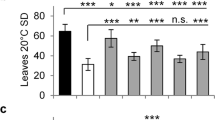
WT seeds sown under normal growth conditions (22°C/18°C and 16h/8 h photoperiod, day/night) began to germinate after 2 days (see below). After 3 weeks of vegetative growth, when the ninth leaf had emerged, plants began to bolt and differentiate flowers. Each plant produced a primary inflorescence and 4–5 secondary inflorescences. Subsequently a number of siliques were developed. The major contributor to plant height was the peduncle.
For the ms33 mutant seeds (obtained from plants grown under the low-temperature treatment) there was a delay in germination, which led to a delay in vegetative growth and flowering by approximately 5 days. However, if ms33 seeds were stratified at 4°C for 3 days, the vegetative growth and flowering time of ms33 plants were similar to WT plants. There were no gross phenotypic differences between the vegetative organs of ms33 and WT plants that developed from seeds exposed, or not exposed, to 4°C prior to germination.
Morphology of ms33 and WT flowers
In ms33 flowers all the floral organs were present, and in the same order, as in WT flowers. Before anthesis, the petal and stamen lengths of ms33 floral buds were similar to WT, but their sepals and gynoecium were longer than in WT and the gynoecium protruded through the buds (Table 1, Fig. 1a, b). Immediately before anthesis, there was rapid growth of petals in WT flowers and the long stamens extended beyond the level of the stigma at anthesis (Fig. 1c). However, in ms33 floral buds petal elongation was delayed by approximately 7 days and growth of stamens was inhibited such that at maturity the stamens barely reached the mid-position of the gynoecium (Fig. 1d). A comparison of floral organs at anthesis showed no difference in final petal lengths between ms33 and WT flowers. However, the mutant stamens were significantly shorter, and sepals and gynoecium longer, than the corresponding WT organs (Table 1, see also Fei and Sawhney 1999).
The low temperature treatment, i.e., 15°C/11°C, had different effects on the growth of floral organs in ms33 and WT. Although the growth of sepals was unaffected by low temperature for both types of flowers, the petal length increased in both ms33 and WT by ca. 15% and 18%, respectively (Fig. 2). For stamen length there was a much greater increase in the mutant (74%), compared to only a 10% increase for the WT, and the mutant stamen length at low temperature was similar to that of WT at normal temperatures (see also Fei and Sawhney 1999). In contrast, carpel length was reduced in ms33 flowers by about 8% and increased in WT flowers by 30% (Fig. 2).
Seed germination
In light, WT seeds showed approximately 10% germination after 2 days at 24°C, with maximum (100%) germination at 4 days. However, the germination of ms33 seeds was delayed for 3 days and maximum germination (98% average) was reached at day 7 (Fig. 3a). In the dark, germination was delayed for both WT and ms33 seed, e.g., after 7 days maximum germination in WT was, on average, 66% and in the mutant 28% (Fig. 3a).
Germination of WT and ms33 seeds with different treatments at 24°C for 7 days. a WT and ms33 seeds germinated in white light and in the dark. b WT and ms33 seeds were stratified either at 15°C or 4°C for 3 days, and then germinated in the dark. c WT seeds germinated in the presence of different concentrations of gibberellin A3 (GA3) or GA4 in the dark. d ms33 seeds germinated in the presence of different concentrations of GA3 or GA4 in the dark. Fifty seeds were sown for each treatment. Each value is a mean of three replicates. Bars SE
Pretreatment of WT and ms33 seeds at either 15°C or 4°C under moist conditions for 3 days followed by 24°C in the dark resulted in enhanced germination in both genotypes. WT seeds exposed to 15°C showed approximately 75% germination at 2 days in the dark in comparison to no germination in the controls, i.e., 24°C (Fig. 3b). Similarly, ms33 seeds exposed to 15°C showed approximately 55% germination after 2 days versus no germination under 24°C. However, after 7 days the germination percentage for ms33 seeds pretreated at 15°C increased to 63% compared to 28% in the control (24°C) seeds. Seeds exposed to 4°C showed reduced germination relative to the 15°C treatment for both WT and ms33 seeds, but germination was still higher than that at normal (24°C) temperatures (Fig. 3b).
As expected, applied GAs stimulated seed germination in the dark for both WT and ms33 seeds. WT seeds treated with 10−4 M GA3 showed a small increase in germination compared to the control after 1 week (Fig. 3c), but at 10−3 M GA3 germination was enhanced to 100% at day 3. However, GA4 was much more effective in stimulating germination than GA3 at the same concentration, e.g., with 10−3 M or 10−4 M GA4, 100% germination was obtained in WT seed after 2 and 3 days, respectively (Fig. 3c).
GAs also stimulated the germination of ms33 seeds in the dark. After 1 week, 10−4 M GA3 increased the germination to approximately 40%, and 10−3 M GA3 to 90%, compared to 28% for the dark control (Fig. 3d). In contrast, with 10−3 M or 10−4 M GA4, ms33 seeds showed 95% germination at 3 and 5 days, respectively (Fig. 3d). Thus, GA4, even at a low concentration, both enhanced the rate and increased the final percent germination compared to GA3, for both WT and ms33 seeds.
The germination of both the WT and ms33 seeds was totally inhibited by treatment with 10−4 M PP333, an inhibitor of GA biosynthesis. This inhibition could be entirely overcome by the addition of 10−4 M GA4, but only partially by 10−4 M GA3 (Fig. 4).
Endogenous GAs in ms33 and WT flowers
A profile of endogenous GAs in WT and ms33 flowers for plants grown at normal temperatures included gibberellins A1,4,8,9,15,20,24 (GA44 and GA53 were undetectable in both WT and ms33 flowers). In WT flowers, GA4 was the major GA followed by GA24, GA15, GA8, GA9 and GA1 (Table 2). In ms 33 flowers most of the GAs were reduced in amount, relative to WT, except for GA8, an inactive metabolite of GA1. In particular, GA4 levels were 12-fold lower in ms33 than in WT and GA9 and GA15, two GA4 precursors, were 6- and 8-fold lower in ms33 than in WT (Table 2).
In low-temperature-grown WT flowers, GA levels, especially GA1, GA4 and GA20, declined relative to GAs in WT flowers grown at normal temperature (Table 2). In contrast, in ms33 flowers grown at low temperature, GA4 levels increased relative to those in ms33 flowers from normal temperatures. The levels of other GAs in ms33 flowers at low temperature tended to be reduced, or were no different, than in flowers grown at normal temperature. Thus, low temperature severely reduced GA levels in WT flowers, but had mixed effects on GA levels in ms33 flowers.
Endogenous IAA and ABA in ms33 and WT flowers
WT flowers from plants grown at normal temperatures contained strikingly higher IAA levels, approximately 6-fold higher, than ms33 flowers from plants grown at normal temperatures (Fig. 5a). However, at low temperatures the IAA content of WT flowers was reduced to less than one-half of that found in WT flowers grown under normal temperatures (Fig. 5a). Even so, IAA content in WT flowers of low temperature plants was still two times higher than that in ms33 flowers grown at normal or low temperatures (Fig. 5a). In ms33 flowers, IAA levels were not affected by low temperature (Fig. 5a).
The relative content of ABA was lower in WT than in ms33 flowers at normal temperatures (Fig. 5b). At low temperature the levels of ABA in WT flowers increased by approximately 55%, but there was little effect of low temperature on ABA levels in ms33 flowers, thus resulting in near equal levels for the mutant and WT flowers grown at low temperature (Fig. 5b).
Double mutants
The differences in endogenous hormones levels in WT and ms33 mutant flowers grown at normal and low temperatures suggested that the ms33 mutation affects either the biosynthetic (or catabolic) or signal transduction pathways of hormones, especially for GAs. A genetic approach was used to further investigate the role of hormones by constructing double mutants of ms33 with the following Arabidopsis mutants, spindly-3 (spy-3), a GA-signal transduction mutant (Jacobsen and Olszewski 1993), and aba1-1, an ABA-deficient mutant (Koornneef et al. 1982).
ms33 aba1-1
The aba1-1 (Landsberg ecotype background) mutant has low ABA content and is characterized by reduced peduncle length and plant height, an increase in transpiration rate and wilting of plants even under normal temperatures (Koornneef et al. 1982). Under our growth conditions the mean height of aba1-1 plants was 7.6±0.5 cm (n=30 plants), compared to 23.0±0.2 cm (n=30 plants) for WT (see also Fig. 6a). However, the floral phenotype of the aba1-1 mutant was similar to WT, and the flowers were both male- and female-fertile (data not shown). As described earlier, the height (primarily peduncle length) of the ms33 mutant is similar to WT, but the flowers have short stamens, they produce non-viable pollen, and have a higher level of ABA at normal temperatures than the WT.
Representative plants of WT, ms33, aba1-1 and ms33 aba1-1 identified from the F2 generation after 6 weeks of growth. b A double mutant ms33 aba1-1 flower with shortened stamens. c Representative plants of WT (Landsberg), WT (Columbia), ms33, spy-3 and ms33 spy-3 identified in the F2 generation after 30 days of growth at normal conditions. d Double mutant ms33 spy-3 flower showing shortened stamens Bars a 1 cm; b, d 1 mm; c 5 cm
The double mutant ms33 aba1-1 was identified in the F2 progeny. In a total of 923 F2 plants, four phenotypes were identified: 543 tall plants which had long stamens in their flowers and normal silique development with seeds (WT), 172 tall plants with short stamens and no silique development (ms33), 154 short plants which had long stamens in their flowers, normal silique development, but were wilty (aba1-1), and 54 plants of a novel additive phenotype that were short, wilty, had flowers with short stamens (Fig. 6b) and showed no silique development (ms33 aba1-1) (Fig. 6a). The ratio of these phenotypes was 9.8:3.1:2.8:1 (X 2=3.43, P>0.25).
ms33 spy-3
The spy-3 (Columbia ecotype) is a GA signal transduction mutant and the phenotype of a homozygous recessive spy-3 plant is similar to WT plants treated with GAs, i.e., plants exhibit long hypocotyls, elongated peduncles, light green leaves and early flowering (Jacobsen and Olszewski 1993). Under our normal temperature conditions, the mean height of spy-3 plants was 52.2±0.4 cm (n=30 plants), compared to 32.9±0.65 (n=30 plants) in the WT (Columbia) (see also Fig. 6c). The height of ms33 was 24.6±0.3 cm (n=30 plants) at maturity.
In 1,472 plants of the F2 progeny from a cross of ms33 and spy-3, four different phenotypes were identified: 844 plants were of normal height and produced siliques with seeds (WT), 257 plants were of normal height, but their flowers had shortened stamens and were male sterile (ms33), 286 plants were tall and produced siliques and seeds (spy-3), and 85 plants were tall and male sterile with shortened stamens in their flowers (Fig. 6d). The last category was novel and showed the additive phenotype of ms33 and spy-3 mutations (Fig. 6c, d). The ratio of phenotypes of these plants was 9.8:3.0:3.3:1.0 (X 2=2.36, P>0.25).
Restoration of male fertility in ms33
The ms33 plants were grown at the following temperatures [(day/night (°C)]: 12/10, 15/11, 18/15, 30/24 and 22/18 (control) with a photoperiod of 16/8 h (day/night). Only under 15°C/11°C was there a partial reversion to male fertility in ms33 flowers, as evidenced by the development of some siliques with seeds. However, the number of siliques developed on ms33 plants varied and approximately 43% of the mutant plants (in a population of 180 plants) produced seeds. WT plants were unaffected, in terms of silique and seed development, by these low (15°C/11°C) temperatures. A partial restoration of male fertility was also obtained when GA4 (10−4 M) was applied to ms33 plants under normal (22°C/18°C) temperature conditions. The seeds produced from both these treatments were sown under normal temperature conditions, and all plants that developed showed the ms33 phenotype. Thus, both applied GA4 and low temperatures could give a partial restoration of male fertility in the ms33 mutant.
Discussion
The phenotype of the ms33 mutant in Arabidopsis was described earlier as exhibiting inhibition of stamen filament growth and abnormalities in pollen development (Dawson et al. 1993; Fei and Sawhney 1999, 2001). Here we show that the ms33 mutant has other pleiotropic effects i.e., aberrant growth of all floral organs, and delayed seed germination and flowering time. However, there were no apparent differences in the morphology of vegetative parts, i.e., leaves and stem, of the WT and ms33. Although the growth of all floral organs was affected in ms33, the effects were different, i.e., delayed petal expansion, inhibition of stamen filament growth, but enhanced sepal and gynoecium growth. Thus, the MS33 gene function is required for normal growth of all floral organs, not just for stamens. As reported elsewhere, the inhibition of stamen filament growth in ms33 was caused by inhibition of cell elongation prior to anthesis, and this effect could be rescued by low temperature or exogenous GA, suggesting that the mutant may have a defect in GA biosynthesis or signaling (Fei and Sawhney 1999). Similarly, in the ga1-1 mutant in Arabidopsis, the stunted growth of the stamen filament could be restored by exogenous GA (Goto and Pharis 1999). The delay in corolla expansion in ms33 may also be due to a delay in cell elongation caused by a defect in endogenous GAs since in other systems corolla expansion is known to involve rapid cell elongation immediately before anthesis and this process is regulated by GAs (reviewed in Greyson 1994; Goto and Pharis 1999). At low temperature, the growth of all mutant floral organs was comparable to the WT; thus the effects of low temperature may also be mediated through changes in endogenous GAs (discussed below).
The ms33 mutation also delayed seed germination and flowering time for plants grown at normal temperatures. Kinetic studies showed that both in light and dark seed germination was delayed in ms33, relative to WT. In the dark, both the rate of germination and percentage germination were reduced for both WT and ms33, compared to in the light. Exposing seeds to low temperature before germination enhanced the rate of germination as well as the final percentage germination in both WT and ms33 seeds in the dark, with the 15°C treatment yielding a better response than 4°C. In Arabidopsis, it is common to stratify seeds with 4°C for increased germination (Weigel and Glazebrook 2002) but, as our results show, 15°C treatment is better than 4°C. Low temperature is known to increase endogenous levels of GAs (Hazebroek et al. 1993; Ma et al. 1996) and it also enhances the responsiveness of Arabidopsis seeds to applied GA (Derkx and Karssen 1993). Thus, in the ms33 mutant the increase in germination at low temperature may be due to an increase in endogenous GAs and/or enhanced GA signaling.
Exogenous GAs can often replace the need for environmental stimuli, e.g., temperature pretreatment or the requirement of light for germination. This suggests that endogenous GAs are important intermediates in the environment-induced stimulation of germination. The absolute dependence on applied GA for germination of both the ga1-1 mutant of Arabidopsis, and the gib-1 mutant of tomato also strongly favors a key role of GA in the control of germination (Karssen et al. 1989). In WT and ms33 seed, we found that GA4 was more effective than GA3 in promoting seed germination (Fig. 3c, d). Treatment of seed with PP333 (Fig. 4), an inhibitor of GA biosynthesis, further supports the suggestion that GA4 is a more effective promoter of seed germination than GA3 in both WT and ms33 seed.
Analysis of endogenous GAs showed that the major GA in WT flowers was GA4, which is also a major GA in Arabidopsis leaves (Talon et al. 1990), seeds (Derkx et al. 1994) and germinating seeds (Ogawa et al. 2003). WT flowers contained 12 times more GA4 and 4 times more GA1 than ms33 flowers. Relatively high levels of GAs have been associated with normal flower development, in particular stamen and petal development (e.g., Murakami 1975; Dathe and Sembdner 1980). Conversely, some male-sterile mutants are known to contain relatively low levels of GAs compared to their WT counterparts (Sawhney 1974; Nakajima et al. 1991), and in male-sterile anthers of rice, GA4 was specifically low (Nakajima et al. 1991). Further, applied GAs are known to induce fertility in some male-sterile mutants of tomato, barley, and Cosmos, and in GA-deficient, male-sterile mutants in tomato (reviewed in Sawhney and Shukla 1994). Thus, there is a good correlation of low endogenous GAs with male sterility. Since exogenous GA4 can partially restore fertility in the ms33 mutant and since low temperature also partially restores fertility and increases GA4 levels in ms33, these findings lend support to the view that low levels of GA4 are at least the partial cause of male sterility in ms33.
The ms33 flowers also contained low levels of IAA and high level of ABA compared to WT. Auxins have been shown to have a role in flower development and in petal growth (Moe 1971; Kopecewicz et al. 1979). Applied ABA is known to induce male sterility in some species (reviewed in Sawhney and Shukla 1994) and a male-sterile mutant in tomato contains high ABA content (Singh and Sawhney 1998). Thus, a low level of IAA and high ABA could be possible factors in the delay of petal expansion, pollen abortion and inhibition of stamen filament growth in ms33 flowers. However, relative to WT, the mutant had only a modest increase (25%) in ABA levels. Hence, it does not seem likely that elevated ABA levels is causal for the phenotypic lesions in mutant flowers. Further, the double mutant ms33 aba1-1 showed the male-sterile phenotype suggesting that reduction in ABA content alone is not sufficient for the restoration of fertility in ms33.
The low level of GA4 and its precursors in ms33 flowers suggests that the ms33 mutation may alter GA biosynthesis. In Arabidopsis, there are at least two major GA biosynthetic pathways from GA12, the first GA in GA biosynthesis in plants (Talon et al. 1990; Finkelstein and Zeevaart 1994; Sponsel 1995): (1) the early 13-hydroxylation of GA12 that leads to GA53, GA44, GA19, GA20, and GA1, and (2) the early non-hydroxylation of GA12 leading to GA15, GA24, GA9 and GA4 (GA20 can also originate from GA9, and GA1 from either GA20 or GA4). It thus appears that in both WT and mutant flowers only the early non-13-hydroxylation pathway is operative. Further, since in the ms33 mutant GA4 level is severely reduced (Table 2), we conclude that a product of the MS33 gene allows GA biosynthesis within this pathway to be maintained at a high level, thereby maintaining high levels of GA1 and especially GA4.
While low temperature reduced levels of GA4 by 2.5-fold in WT flowers, these lowered levels are still relatively high (almost 10 ng/g DW), and WT plants grown under low temperature produce normal flowers. Hence, levels of 10–23 ng/g DW of GA4 in WT must be sufficient to support normal floral organ growth. These results are consistent with the conclusion that GA4 is an important native GA in a wide range of developmental processes in Arabidopsis, especially floral organ growth (Goto and Pharis 1999).
The question of whether GA signaling rather than GA biosynthesis is affected in the ms33 mutant was addressed by constructing a double mutant of ms33 with a GA signal transduction mutant spy-3. The spy-3 mutant has the same phenotype as WT plants treated with GA, i.e., tall plants and early flowering (Jacobsen and Olszewski 1993). In the double mutant ms33 spy-3, the phenotype of plants was similar to spy-3, i.e., long peduncles and early flowering. Additionally, the phenotype of the double mutant flowers was similar to that of ms33, i.e., flowers were male-sterile and stamen length was inhibited. These results show that the stimulation of the GA signaling pathway by spy-3 does not overcome the inhibition of stamen filament growth and the abortion of pollen development. Thus, the expression of male sterility in ms33 seems likely not to be related to a blockage in the GA signal transduction pathway.
Abbreviations
- ABA :
-
Abscisic acid
- GA :
-
Gibberellin
- GC-MS-SIM :
-
Gas chromatography-mass spectrometry-selected ion monitoring
- IAA :
-
Indole-3-acetic acid
- ms33 :
-
Male sterile33 mutant
- PP333 :
-
Paclobutrazol
- WT :
-
Wild type
References
Carlson DR, Williams CB (1985) Effect of temperature on the expression of male sterility in partially male-sterile soybean. Crop Sci 25:646–648
Dathe W, Sembdner G (1980) Endogenous plant hormones of the broad bean, Vicia faba L. II. Gibberellins and plant growth inhibitors in floral organs during their development. Biochem Physiol Pflanz 175:599–610
Davies PJ (1995) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant hormones and their role in plant growth and development, 2nd edn. Kluwer, London, pp 1–12
Dawson J, Wilson ZA, Aarts MGM, Braithwaite AF, Briaty LG, Mulligan BJ (1993) Microspore and pollen development in six male-sterile mutants of Arabidopsis thaliana. Can J Bot 71:629–638
Dellaporta SL, Calderon-Urrea A (1994) The sex determination process in maize. Science 266:1501–1505
Derkx MPM, Karssen CM (1993) Effects of light and temperature on seed dormancy and gibberellin-stimulated germination in Arabidopsis thaliana: studies with gibberellin-deficient and -insensitive mutants. Physiol Plant 89:360–368
Derkx MPM, Vermeer E, Karssen CM (1994) Gibberellins in seeds of Arabidopsis thaliana: biological activities, identification and effects of light and chilling on endogenous levels. Plant Growth Regul 15:223–234
Fan Z, Stefansson BR (1986) Influence of temperature on sterility of two cytoplasmic male-sterility systems in rape (Brassica napus L.). Can J Plant Sci 66:221–227
Fei H, Sawhney VK (1999) Role of plant growth substances in MS33-controlled stamen filament growth in Arabidopsis. Physiol Plant 105:165–170
Fei H, Sawhney VK (2001) Ultrastructural characterization of male sterile33 (ms33) mutant in Arabidopsis affected in pollen desiccation and maturation. Can J Bot 79:118–129
Fellner M, Sawhney VK (2000) Seed germination in a tomato male-sterile mutant is resistant to osmotic, salt and low temperature stresses. Theor Appl Genet 102:215–221
Fellner M, Zhang R, Pharis RP, Sawhney VK (2001) Reduced de-etiolation of hypocotyl growth in a tomato mutant is associated with hypersensitivity to, and high endogenous levels of, abscisic acid. J Exp Bot 52:725–738
Finkelstein RR, Zeevaart JAD (1994) Gibberellin and abscisic acid biosynthesis and response. In: Meyerowitz EM, Somerville CR (eds) Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 523–553
Fujioka S, Yamane H, Spray CR, Gaskin P, MacMillan J, Phinney BO, Takahashi N (1988) Qualitative and quantitative analysis of gibberellins in vegetative shoots of normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol 88:1367–1372
Goto N, Pharis RP (1999) Role of gibberellins in the development of floral organs of the gibberellin-deficient mutant, ga1-1, of Arabidopsis thaliana. Can J Bot 77:944–954
Greyson RI (1994) The development of flowers. Oxford University Press, New York
Hazebroek JP, Metzger JD, Mansager ER (1993) Thermoinductive regulation of gibberellin metabolism in Thlaspi arvense L. II. Cold induction of enzymes in gibberellin biosynthesis. Plant Physiol 102:547–552
Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, Molloy KP, Ness LA (2003) Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol 131:1270–1282
Jacobsen SE, Olszewski NE (1991) Characterization of the arrest in anther development associated with gibberellin deficiency of the gib-1 mutant of tomato. Plant Physiol 97:409–414
Jacobsen SE, Olszewski NE (1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5:887–896
Karssen CM, Zagórski SZ, Kepczynski J, Groot SPC (1989) Key role for endogenous gibberellins in the control of seed germination. Ann Bot 63:71–80
Kaul MLH (1988) Male sterility in higher plants. Springer, Berlin Heidelberg New York
Koornneef M, van der Veen JH (1980) Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 58:257–263
Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 61:385–393
Koornneef M, Bosma TDG, van der Veen JH, Zeevaart JAD (1990) The isolation and characterization of gibberellin-deficient mutants in tomato. Theor Appl Genet 80:852–857
Kopcewicz J, Centkowska G, Kriesel K, Zatorska Z (1979) Phytohormones level in the leaves of Hyoscyamus niger L. during variable photoperiods at the time of flower initiation and differentiation. Acta Soc Bot Pol 48:245–258
Koshioka M, Takeno K, Beall FD, Pharis RP (1983) Purification and separation of plant gibberellins from their precursors and glucosyl conjugates. Plant Physiol 73:398–406
Ma HP, Blake PS, Browning G (1996) Changes in content of endogenous jasmonate and gibberellins A3, A4 and A7 measured by gas chromatograph-mass spectrometry during stratification of apple (Malus pumila L.) Seeds. Acta Phytophysiol Sin 22:81–86
Mascarenhas JP (1990) Gene expression in the male gametophyte. In: Blackmore S, Knox RB (eds) Microspores. Evolution and ontogeny. Academic Press, New York, pp 265–280
Moe R (1971) The relationship between flower abortion and endogenous auxin content of rose shoots. Physiol Plant 24:374
Murai K, Tsunewaki K (1993) Photoperiod-sensitive cytoplasmic male sterility in wheat with Aegilops crassa cytoplasm. Euphytica 67:41–48
Murakami Y (1975) The role of gibberellins in the growth of floral organs of Mirabilis jalapa. Plant Cell Physiol 16:337–345
Nakajima M, Yamaguchi I, Kizawa S, Murofushi N, Takahashi N (1991) Semi-quantification of GA1 and GA4 in male sterile anthers of rice by radioimmunoassay. Plant Cell Physiol 32:511–513
Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15:1591–1604
Pharis RP, King RW (1985) Gibberellins and reproductive development in higher plants. Annu Rev Plant Physiol 36:517–568
Phinney B, Spray C (1982) Chemical agents and gibberellin pathway in Zea mays. In: Wareing PF (ed) Plant growth substances. Academic Press, London, pp 101–110
Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10:67–73
Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Sawhney VK (1974) Morphogenesis of the stamenless-2 mutant in tomato III. Relative levels of gibberellins in the normal and mutant plants. J Exp Bot 25:1004–1009
Sawhney VK (1983) Temperature control of male sterility in a tomato mutant. J Hered 74:51–54
Sawhney VK, Shukla A (1994) Male sterility in flowering plants: Are plant growth substances involved? Am J Bot 81:1640–1647
Shivanna KR, Sawhney VK (1997) Pollen biotechnology for crop production and improvement. Cambridge University Press, Cambridge, UK
Singh S, Sawhney VK (1998) Abscisic acid in a male sterile tomato mutant and its regulation by low temperature. J Exp Bot 49:199–203
Singh S, Sawhney VK, Pearce DW (1992) Temperature effects on endogenous indole-3-acetic acid levels in leaves and stamens of the normal and male sterile stamenless-2 mutant of tomato (Lycopersicon esculentum Mill.). Plant Cell Environ 15:373–377
Sponsel VM (1995) The biosynthesis and metabolism of gibberellins in higher plants. In: Davies PJ (ed) Plant hormones: physiology, biochemistry and molecular biology. Kluwer, London, pp 66–97
Sun TP, Kamiya Y (1994) The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthase A of gibberellin biosynthesis. Plant Cell 6:1509–1518
Talon M, Koornneef M, Zeevaart JAD (1990) Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci USA 87:7983–7987
Weigel D, Glazebrook J (2002) Arabidopsis: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Wilson RN, Somerville CR (1995) Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol 108:495–502
Wilson RN, Heckman JW, Somerville CR (1992) Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100:403–408
Zhang R (1990) Hormonal basis of rapid growth in Pinus. MSc Thesis, University of Calgary, Canada
Acknowledgements
The Authors thank Dr. B. Mulligan of University of Nottingham (UK) for kindly providing the ms33 seed, and Dr. L.N. Mander of the Australian National University for deuterated GAs. H. Fei acknowledges the support of a University of Saskatchewan Graduate scholarship during the course of this work. This research was supported by NSERC of Canada Discovery grants to V.K.S. and R.P.P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fei, H., Zhang, R., Pharis, R.P. et al. Pleiotropic effects of the male sterile33 (ms33) mutation in Arabidopsis are associated with modifications in endogenous gibberellins, indole-3-acetic acid and abscisic acid. Planta 219, 649–660 (2004). https://doi.org/10.1007/s00425-004-1270-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1270-1