Abstract
In wild-type Nicotiana plumbaginifolia Viv. and other higher plants, nitrate reductase (NR) is regulated at the post-translational level and is rapidly inactivated in response to, for example, a light-to-dark transition. This inactivation is caused by phosphorylation of a conserved regulatory serine residue, Ser 521 in tobacco, and interaction with divalent cations or polyamines, and 14-3-3 proteins. The physiological importance of the post-translational NR modulation is presently under investigation using a transgenic N. plumbaginifolia line. This line expresses a mutated tobacco NR where Ser 521 has been changed into aspartic acid (Asp) by site-directed mutagenesis, resulting in a permanently active NR enzyme [C. Lillo et al. (2003) Plant J 35:566–573]. When cut leaves or roots of this line (S521) were placed in darkness in a buffer containing 50 mM KNO3, nitrite was excreted from the tissue at rates of 0.08–0.2 μmol (g FW)−1 h−1 for at least 5 h. For the control transgenic plant (C1), which had the regulatory serine of NR intact, nitrite excretion was low and halted completely after 1–3 h. Without nitrate in the buffer in which the tissue was immersed, nitrite excretion was also low for S521, although 20–40 μmol (g FW)−1 nitrate was present inside the tissue. Apparently, stored nitrate was not readily available for reduction in darkness. Leaf tissue and root segments of S521 also emitted much more nitric oxide (NO) than the control. Importantly, NO emission from leaf tissue of S521 was higher in the dark than in the light, opposite to what was usually observed when post-translational NR modulation was operating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrate reductase (NR, EC 1.6.6.1) catalyses a key step in nitrogen assimilation:
NR is known to be regulated post-translationally by phosphorylation of a conserved serine residue, Ser 543 in spinach (Douglas et al. 1995; Bachmann et al. 1996) and Ser 534 in Arabidopsis (Su et al. 1996), corresponding to Ser 521 in Nicotiana tabacum. In the presence of Mg2+ or spermidine the phosphorylated form of NR interacts with 14-3-3 proteins, and NR activity is then inhibited (Provan et al. 2000; Athwal and Huber 2002). This regulatory mechanism assures rapid inactivation of NR under certain conditions, like a sudden decrease in light intensity. The nitrite formed in the reaction step catalysed by NR is further converted into ammonium by nitrite reductase (NiR) and then incorporated into organic compounds by glutamine synthetase and glutamate synthase (GOGAT). NiR and GOGAT are closely coupled to photosynthesis because, in leaves, reduced ferredoxin generated in photosynthesis is used as the electron donor for these enzymes. In darkened leaves, or root tissue, reducing power for NiR and GOGAT must be generated by other means than photosynthesis, but still involves ferredoxin or a ferredoxin-like protein (Meyer and Stitt 2001). Rapid down-regulation of NR due to, for instance, a sudden decrease in light intensity has been claimed to be necessary to avoid accumulation of nitrite, which is toxic to the plant (Riens and Heldt 1992; Huber et al. 2002; Lillo 2004), and the work presented here indeed demonstrates that the Ser 521 can be important for cessation of nitrite production.
In roots from many plant species, anaerobic conditions result in nitrite excretion (Botrel et al. 1996; Allègre 2003). This efflux of nitrite has been ascribed to an activation of NR by dephosphorylation, probably triggered in part by a decrease in cytosolic pH (Botrel and Kaiser 1997; Allègre 2003). In barley roots, nitrite assimilation was also impaired in anaerobic conditions (Botrel et al. 1996). It has been known for a long time that supplementary nitrate increases a plant’s resistance to hypoxia and anoxia, and recently it has been shown that an impairment of NR activity reduced the resistance of tomato plants to anaerobiosis (Allègre 2003). Also, under anoxia, tobacco mutants deficient in root NR showed much higher ethanol and lactate fermentation than wild-type (WT) roots (Stoimenova et al. 2003a, 2003b). These observations are in agreement with nitrate reductase taking part in regeneration of NAD+ for the glycolytic pathway since respiration cannot recycle the NAD+ under anoxic conditions.
NR also catalyses the conversion of nitrite into nitric oxide (NO), a gaseous free radical exerting numerous physiological effects in plants (Kaiser et al. 2004). NO can further react with superoxide, formed by NR or other enzymes in the plant, to form peroxynitrite, a highly toxic and reactive compound (Ruoff and Lillo 1990; Yamasaki and Sakihama 2000; Rockel et al. 2002; Kaiser et al. 2004).
We have previously produced several N. plumbaginifolia transgenic lines (S521) expressing a tobacco NR with the regulatory Ser 521 changed to aspartic acid (Asp 521). Under optimal growth conditions nitrite did not accumulate in response to a light–dark transition in S521 leaves although NR was always in its active form (Lillo et al. 2003). The reason for this could be that NR is restrained in situ also by other mechanisms than phosphorylation and association with 14-3-3 proteins. Restriction in substrate availability can indeed be important. Although the K m value for NADH is very low for spinach and tobacco NR, i.e.1–2 μM (Sanchez and Heldt 1990; Lillo et al. 1997), it has been suggested that NADH may decrease in darkness and be limiting for nitrate reduction (Kaiser et al. 2000). The present work shows that although NR in S521 is in its active form in darkness, formation of nitrite is still restricted, but will increase to higher levels when external nitrate is provided. The compartmentation of nitrate into a metabolic and a storage pool appears to be important for regulation of nitrogen assimilation, and may prevent conversion of nitrate to nitrite under conditions that cannot support adequate further metabolism of nitrite. This complex regulation of nitrate compartmentation and reduction is apparently aimed at avoiding accumulation of toxic compounds.
Materials and methods
Plant material
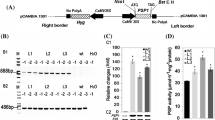
After germination in sand or agar, plants were grown in perlite overlaid by a thin layer of soil for about 6 weeks. The plants were in the rosette stage and had approximately seven leaves when leaf samples were harvested. Seeds were provided by Unité de Nutrition Azotée des Plantes INRA, Versailles, France. Plants tested were: WT Nicotiana plumbaginifolia Viv., transgenic N. plumbaginifolia (var. viviani) with the full length tobacco NR (C1) (Vincentz and Caboche 1991), and S521-7 where tobacco NR Ser 521 was mutated into Asp; otherwise, the S521-7 line resembles the C1 line (Lillo et al. 2003). In C1 and S521, NR was constitutively expressed using the cauliflower mosaic virus 35S promoter. All the NR activity detected in these transgenic plants is derived from the transgene expression, as the endogenous NR gene is inactivated by a retrotransposon insertion (Leprince et al. 2001). Plants were grown at 20°C with a 12-h photoperiod at 80 μmol photons m−2 s−1, and were irrigated with Hoagland solution containing 15 mM KNO3 three times a week, and always the day before harvesting.
Nitrite excretion
Leaves were cut into pieces of approximately 0.50 cm2. Roots and leaves were washed in deionised water and cut into 0.7-cm-long pieces. The tissue, 5×1 g, was placed in a 100-ml Erlenmeyer flask with 20 ml of 50 mM Mes buffer pH 6.5 (Hepes pH 7.0 was also used, and gave the same results) and various concentrations of KNO3. The Erlenmeyer flasks were kept in darkness under slow agitation. Samples of the buffer were taken every hour for measurement of nitrite content, and the tissue was used for assay of NR activity.
Nitrate measurements
Crude extracts were diluted 100 times, and all the nitrate was converted into nitrite enzymatically by adding NR purified from N. plumbaginifolia. Nitrite was then determined as described below. Alternatively, aliquots of 20–25 mg of freeze-dried powder were extracted successively with 1 ml of 80% (v/v in water) ethanol, 1 ml of 60% ethanol and finally with 1 ml of water (1 h of extraction for each step) at 4°C. The three successive extractions were pooled and homogenised. An aliquot (2×1 ml) of the supernatant was then evaporated in a Speed-Vac and resuspended in 1 ml of water for nitrate determination. Nitrate concentrations were determined by HPLC (Dionex analyser, DX-120; HPLC AS14 column; 3.5 mM Na2CO3 and 1 mM NaHCO3 as eluant). Peaks were identified and quantified in an integrator (Peak-net station) by comparison with standard nitrate concentrations (10 μM to 1 mM).
Extraction and assay of NR
Leaves, 1 g, were homogenised with 4 ml of 0.1 M Hepes–KOH (pH 7.5), 3% (w/v) polyvinylpolypyrrolidone, 1 mM EDTA and 7 mM cysteine. The assay mixture contained 50 mM Hepes–KOH (pH 7.5), 100 μM NADH, 5 mM KNO3 with 2 mM EDTA or 6 mM MgCl2. The assay volume was 0.70 ml. Activity was measured in crude extracts by determining NO2 − formation by the addition of 1% sulphanilamide and 0.02% N-(1-naphthyl)-ethylene-diamine dihydrochloride in 1.5 M HCl (Lillo and Henriksen 1984). The activity state is defined as NR assayed in the presence of Mg2+ (and 14-3-3 proteins) as a percentage of NR activity measured in the presence of EDTA, and reflects how much of the enzyme is in the non-phosphorylated active form. Assays were run at 25°C.
Gas-phase NO measurements
N. plumbaginifolia plants were cultivated on vermiculite under a 16 h light/8 h dark regime at 25°C day/22°C night temperature. Three leaves were harvested for NO emission and weighed. The leaves with freshly cut petioles were placed in a beaker with Hoagland nutrient solution (15 mM nitrate) and mounted in a chamber with a transparent lid and 4 l air volume. A constant flow of measuring gas (1.5 l min−1) was pulled through the chamber and subsequently through the chemiluminescence detector (CLD 770 AL ppt; Eco-Physics, Dürnten, Switzerland; detection limit 20 ppt; 1 min time resolution) by a vacuum pump connected to an ozone destroyer. The ozone generator of the chemiluminescence detector was supplied with dry oxygen (99%). The measuring gas (air or nitrogen) was made NO-free by conducting it through a custom-made charcoal column (1 m long, 3 cm internal diameter, particle size 2 mm). Calibration was routinely carried out with NO-free air (0 ppt NO) and with various concentrations of NO (1–35 ppb) adjusted by mixing the calibration gas (500 ppb NO in nitrogen; Messer Griesheim, Darmstadt, Germany) with NO-free air. Flow controllers (FC-260; Tylan General, Eching, Germany) were used to adjust all gas flows. Light was provided by a 400-W HQi-lamp (Schreder, Winterbach, Germany) above the cuvette. Quantum flux density was 200 μmol m−2 s−1 PAR). Air temperature in the cuvette was continuously monitored, and was usually about 20°C in the dark and 23–25°C in the light.
NO emission from roots
Roots were harvested from hydroponically grown N. plumbaginifolia plants, washed briefly and cut into segments of approx. 0.5 cm. 2 g of root segments were immersed in a beaker with 20 ml Hoagland nutrient solution and this beaker was placed in the measuring cuvette. NO was measured under constant shaking (150 rpm), as described.
Results
Nitrite excretion from leaf tissue
Leaves of N. plumbaginifolia were harvested in the morning after 3 h of light, cut into narrow pieces, and placed in Erlenmeyer flasks in a buffer with various concentrations of nitrate. The flasks were kept in darkness under slow agitation. Figure 1a shows nitrite excretion from leaf tissue with 50 mM nitrate in the buffer. For S521 tissue, nitrite was excreted almost linearly with time during the 5 h of the experiment, whereas nitrite excretion from the control plant expressing a WT NR with the Ser 521 intact (C1) was very low, and stopped completely after 3 h. Total NR activity (assayed in the presence of EDTA) showed a 45% decrease during the first 4 h for C1 and 22% for S521 (Fig. 1b). NR activity tested in the presence of Mg2+ (actual NR activity) is believed to reflect the in situ NR activity more adequately than NR assayed in the presence of EDTA (Kaiser et al. 2000). The actual NR activity shown in Fig. 1c was similar at time zero for C1 and S521, but NR activity in C1 then decreased sharply, whereas actual activity in S521 was almost constant. This is correlated with the data showing nitrite excretion (Fig. 1a). Mean values for nitrite excretion during the first hour were 0.04 and 0.08 μmol (g FW)−1 h−1 for C1 and S521, respectively. During the next 2 h nitrite excretion from S521 was 10 times higher than for C1. Thereafter nitrite excretion remained high for S521, but stopped completely for C1. As expected, the NR activity state was always high for S521. For C1, the activity state was relatively low at the start of the experiments and decreased further during the first 2 h (Fig. 1 d). Post-translational inactivation of NR therefore appears to be important for cessation of nitrite formation under these conditions.
Nitrite excretion and NR activity in cut leaf tissue during 5 h darkness in the presence of 50 mM exogenous nitrate for S521 (▲) and C1 (●) Nicotiana plumbaginifolia plants. a Nitrite excretion. b Total NR activity, measured in the presence of EDTA. c Actual NR activity, measured in the presence of Mg2+. d Activity state of NR. Means ± SE, n=3
Figure 2 shows the effect of different exogenous concentrations of nitrate on nitrite excretion from S521 and C1 leaf tissue. When no nitrate was added to the buffer wherein the tissue was immersed, nitrite excretion was low also in S521 plants. After the first 2 h, during the 2- to 5-h time span, nitrite excretion from S521 tissue with no exogenous nitrate was only 12% of that from tissue exposed to 50 mM exogenous nitrate. For C1 tissue, nitrite excretion was close to zero regardless of the exogenous concentrations of nitrate. Different batches of plants varied with respect to nitrite excretion as well as NR activity. As shown in Fig. 1, some nitrite excretion could sometimes be observed from C1 plants during the first 3 h of incubation, although excretion from C1 plants was very low compared with the S521 mutant. To examine how different concentrations of nitrate affected nitrite excretion, samples were harvested from the same batch of plants and tested at various nitrate concentrations. Figure 2 represents one out of several experiments showing a strong effect of nitrate concentration on nitrite excretion for the S521 plants, and zero or very low nitrite excretion from C1 regardless of nitrate concentration.
Nitrite excretion from N. plumbaginifolia cut leaf tissue immersed in various concentrations of nitrate during 5 h of darkness. The data presented represent one out of three similar repeats. Filled symbols S521, open symbols control plant C1. Nitrate concentrations tested were: 0 (●, ○), 1 (■, □), 10 (◆, ◇), and 50 mM (▲, △)
Nitrite content was also measured after boiling the tissue in the incubation buffer, which releases nitrite from within the cells. These measurements did not show a different nitrite formation rate compared with the data presented in Figs. 1a or 2; hence, not much nitrite accumulated within the tissue of S521 or C1 plants (data not shown). This was also confirmed in the samples prepared for NR activity measurements because, although slightly increasing, these samples did not show high background values for nitrite at the start of the NR assay.
Nitrate content of leaves was determined, and showed that S521 leaves contained 20–40 μmol nitrate (g FW)−1, i.e. 25–100 times more than the amount of nitrate that was actually reduced to nitrite in the experiments presented in Figs. 1 and 2. This indicates that the endogenous nitrate is not easily available for NR since high exogenous nitrate concentrations were necessary for sustained nitrite formation.
Nitrite excretion from root tissue
Nitrite excretion from root pieces was much higher in S521 plants compared with control (C1) plants (Fig. 3a). Actual NR activity was about 4 times higher in S521 than in control plants (Fig. 3c). The nitrate content of roots always exceeded 30 μmol (g FW)−1. When comparing roots submerged in buffers with different concentrations of nitrate it was found that nitrite excretion depended on high exogenous nitrate concentrations also for roots. Without exogenous nitrate, nitrite excretion stopped after 1 h (Fig. 4). Three different repeats were made of this experiment, showing basically the same results, but maximal excretion rates for S521 tissue (in 50 mM nitrate) varied from 0.65 to 1.0 μmol (g FW)−1 during the 5-h time span. Nitrite excretion always stopped after 1 h when no exogenous nitrate was present. Nitrite excretion from C1 roots was also tested and showed close to zero excretion rates for all nitrate concentrations used (Figs. 3a, 4).
Nitrite excretion and NR activity in cut roots of N. plumbaginifolia during 3 h darkness in the presence of 50 mM exogenous nitrate for S521 (▲) and C1 (●) plants. a Nitrite excretion. b Total NR activity, measured in the presence of EDTA. c Actual NR activity, measured in the presence of Mg2+. d Activity state of NR. Means ± SE, n=3
Nitrite excretion from N. plumbaginifolia cut root tissue immersed in various concentrations of nitrate during 5 h of darkness. The data presented represent one out of three similar experiments. Filled symbols S521, open symbols the control plant C1. Nitrate concentrations tested were: 0 (●, ○), 1 (■, □), 10 (◆, ◇), and 50 mM (▲, △)
NO formation
As a side reaction, NR can use nitrite as a substrate and form NO by a one-electron transfer reaction (Yamasaki and Sakihama 2000; Rockel et al. 2002; Kaiser et al. 2004). We therefore tested the formation of NO under conditions that could lead to (transient) accumulation of nitrite due to lack of post-translational regulation of NR in S521. NO emission from C1 was low in the dark, increased during the light phase and remained high in the second dark phase (Fig. 5). Quite in contrast, in S521 leaves, NO emission was generally much higher in the dark than in the light. Significant differences in NO2 − concentrations in situ were not found between S521 and C1 under these conditions (data not shown).
Higher NO emission from S521 than for C1 was also found for root pieces floating in 15 mM nitrate solution (Fig. 6). Generally, NO emission from roots exposed to normal air was not detectable, but under anaerobic conditions 8 and 1 nmol (g FW)−1 h−1 were emitted from S521 and C1 roots, respectively. Nitrite concentrations in the roots increased during 2 h of anaerobiosis from about 1 to 40 nmol (g FW)−1 in S521 and from 0.02 to 27 nmol (g FW)−1 in C1 plants. It seems that in the roots of the S521 line a significant proportion of the stored nitrite is reduced to NO (compare the level of nitrite to the maximal emission rate in Fig. 6). After returning to aerobic conditions, nitrite concentrations decreased only slowly (not shown), whereas NO emission ceased almost immediately (Fig. 6). Thus, the difference in anoxic NO emission between S521 and C1 roots was much higher than would be expected from the differences in the nitrite concentration.
Discussion
Previous work had shown that transforming the E23 N. plumbaginifolia NR-deficient mutant with a reporter gene or structural NR gene linked to the NR promoter often led to no or very low expression (Meyer and Stitt 2001), and to assure proper expression the mutated NR gene was placed under control of the 35S promoter. Mutated NR is therefore expressed constitutively in all kinds of cells (ectopic expression), even in cells that might have little or no NR activity. The results for the S521 plants were always compared with the C1 plants in which the non-mutated NR gene was also linked to the 35S promoter. Generally, we found WT and C1 plants to give the same results (data not shown), with the exception of NO emission under anoxia (Fig. 6). Under anoxia, 12% of the NO emission could have been caused by ectopic expression of NR.
Excretion of nitrite by anaerobic roots or by roots treated with inhibitors of respiration has been often reported (Lee 1979; Mann et al. 1979; Dry et al. 1981; Botrel et al. 1996). Similarly, tissue incubated in darkness and anoxia has been used for the so-called “in vivo NR activity test” where nitrite accumulation in the medium is also detected (Mann et al. 1979; Lillo and Henriksen 1984). This increase in nitrite accumulation in both roots and shoots sometimes has been linked to a decrease in ATP and/or glucose 6-phosphate concentration which was then supposed to restrict nitrite reduction in plastids, and to an acidification of the cytosol which is known to activate NR. Indeed, only a partial reduction in nitrite assimilation was observed in anoxic barley roots (Botrel et al. 1996). We show here that nitrite excretion from detached leaves and roots of N. plumbaginifolia is also linked to the loss of post-translational regulation of NR resulting from mutation of the regulatory Ser residue. Nitrite excretion, although low, was also detected at the beginning of the experiment when using leaf tissue of the control transgenic N. plumbaginifolia line, but this nitrite excretion stopped after 3 h of incubation in the dark. A concomitant decrease in NR activation state was also observed. It thus seems that the major factor controlling nitrite production is the modulation of NR activity by phosphorylation and 14-3-3 binding. Interestingly, even in the deregulated transgenic plants (S521), nitrite was not found to accumulate in leaves under optimal growth conditions (Lillo et al. 2003). In mature plant cells the total cell volume of the cytosol will make up only 5–10% of the cell, and the concentrations of nitrate or nitrite in different compartments can be very different from the values obtained by averaging the total content of the whole tissue. Local accumulation of nitrite may therefore occur, and be, for instance, 20 times higher than the averaged values obtained. Because of this dilution effect by the whole cell, increase of cytosolic nitrite can be hard to determine, and accumulation of nitrite is only seen under more extreme conditions.
The present work shows that when fresh nitrate is provided externally to cut leaf or root tissue nitrite formation is stimulated in the S521 plants (Figs. 2, 4). Even though high nitrate concentrations were present in both root and leaf tissues a source of external nitrate was necessary for sustained nitrite excretion. This indicates that compartmentation of nitrate into a metabolic pool (cytosolic) and a storage pool (vacuole) is important for regulation of nitrate reduction. The concept of two nitrate pools, a large storage pool in the vacuole and a small metabolically available pool in the cytosol, was launched a long time ago (Ferrari et al. 1973; Martinoia et al. 1981). Although a substantial mobilisation of vacuolar stored nitrate has been shown to occur in N-starved barley roots (van der Leij et al. 1998), it seems that in our conditions this remobilisation did not occur, or at least not at a rate sufficient for sustaining maximal nitrite production. Alternatively, the reduction of exogenous nitrate into nitrite by NR could be restricted to cells of the epidermal layer, which implies that the available vacuolar nitrate pool is much lower than when calculated from the total organ.
In leaves of the S521 line, NO emission was generally higher than in the control C1 transgenic line, both in the light and in the dark. Importantly, the dark/light pattern of NO emission was also changed by the mutation of Ser 521. In leaves of WT plants, NO emission was usually higher in the light than in the dark (Fig. 5, also compare Rockel et al. 2002), whereas in S521, the pattern was opposite (higher in dark than in light). No accumulation of nitrite was seen, but the higher rate of NO emission in the S521 line could be due to a slight increase in nitrite formation and to a higher rate of NO formation from nitrite by the constitutively active NR in S521 plants. It has previously been shown for other species that production of NO by NR occurs in tissue where nitrite concentration is high (Morot-Gaudry-Talamain et al. 2002; Rockel et al. 2002). However, in agreement with previous results (Lillo et al. 2003), accumulation of nitrite was not observed in leaves during 30 min of darkness in the WT, or in transgenic N. plumbaginifolia unless extra nitrate was added. Apparently, the assay for nitrite is not sufficiently sensitive to detect any increase during the time span (30 min) generally used to see optimal inactivation of NR, although nitrite may increase locally. Measurements of NO were, however, very sensitive and showed increasing emission of NO during 30 min of darkness.
A much higher NO emission than in C1 was also detected in anaerobic S521 roots, together with a small nitrite accumulation. Interestingly, an increased NO production has been detected also in anoxic alfalfa root cultures (Dordas et al. 2003) and it has been suggested that NO may be involved in the regulation of anoxic metabolism (Stoimenova et al. 2003a, 2003b). The present work shows a clear correlation between in vivo formation of NO and regulation of NR. However, direct evidence that NO emitted is the product of nitrate reduction remains to be obtained, and to fully evaluate the effects of abolishing phosphorylation of the regulatory Ser 521, 15N labelling in the S521 line will be done. This will allow us to quantify the rate of nitrate reduction (to nitrite and also NO) in both the C1 and S521 lines, and to follow the metabolic perturbations resulting from the NR deregulation.
Plants have evolved various complementary mechanisms to avoid the potential problem of nitrite accumulation under certain conditions when further assimilation of nitrite is slow. As shown in the experiments presented here, mutation of the regulatory serine in NR and thereby constitutively high NR activity can lead to formation and excretion of nitrite. It is also apparent that the post-translational regulation of NR not only regulates NR with respect to nitrite production but also functions to regulate NO emission.
Abbreviations
- NR :
-
Nitrate reductase
- NO :
-
Nitric oxide
- Ser :
-
Serine
- WT :
-
Wild type
References
Allègre A (2003) Approche physiologique et biomoléculaire du rôle de la nitrate réductase dans la résistance à l’asphyxie racinaire de la tomate. Thesis, Institut National Polytechnique de Toulouse, France
Athwal GS, Huber SC (2002) Divalent cations and polyamines bind to loop 8 of 14-3-3 proteins, modulating their interaction with phosphorylated nitrate reductase. Plant J 29:119–129
Bachmann M, Shiraishi N, Campbell WH, Yoo B-C, Harmon AC, Huber SC (1996) Identification of Ser-543 as the major regulatory phosphorylation site in spinach leaf nitrate reductase. Plant Cell 8:505–517
Botrel A, Kaiser WM (1997) Nitrate reductase activation state in barley roots in relation to the energy and carbohydrate status. Planta 201:496–501
Botrel A, Magné C, Kaiser WM (1996) Nitrate reduction, nitrite reduction and ammonia assimilation in barley roots in response to anoxia. Plant Physiol Biochem 34:645–652
Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 35:763–770
Douglas P, Morrice N, MacKintosh C (1995) Identification of a regulatory phosphorylation site in the hinge 1 region of nitrate reductase from spinach (Spinacea oleracea) leaves. FEBS Lett 377:113–117
Dry I, Wallace W, Nicholas DJD (1981) Role of ATP in nitrite reduction in roots of wheat and pea. Planta 152:234–238
Ferrari TE, Yoder OC, Filner P (1973) Anaerobic nitrite production by plant cells and tissues: evidence for two nitrate pools. Plant Physiol 51:423–431
Huber SC, MacKintosh C, Kaiser WM (2002) Metabolic enzymes as targets for 14-3-3 proteins. Plant Mol Biol 50:1053–1063
Kaiser WM, Kandlbinder A, Stoimenova M, Glaab J (2000) Discrepancy between nitrate reduction rates in intact leaves and nitrate reductase in leaf extracts: What limits nitrate reduction in situ? Planta 210:801–807
Kaiser WM, Planchet E, Stoimenova M, Sonoda M (2004) Modulation of nitrate reductase activity and (eco)physiological implications. In: Stulen I, Amancio Z (eds) N metabolism and plant adaption to the environment, chapter 7. Kluwer, Dordrecht
Lee RB (1979) The release of nitrite from barley roots in response to metabolic inhibitors, uncoupling agents and anoxia. J Exp Bot 30:119–133
Leprince A-S, Grandbastien M-A, Meyer C (2001) Retrotransposons of the Tnt1B family are mobile in Nicotiana plumbaginifolia and can induce alternative splicing of the host gene upon insertion. Plant Mol Biol 47:533–541
Lillo C (2004) Light regulation of nitrate uptake, assimilation and metabolism. In: Stulen I, Amancio Z (eds) N metabolism and plant adaption to the environment, chapter 6. Kluwer, Dordrecht
Lillo C, Henriksen A (1984) Comparative studies of diurnal variations of nitrate reductase activity in wheat, oat and barley. Physiol Plant 62:89–94
Lillo C, Kazaic S, Ruoff P, Meyer C (1997) Characterization of nitrate reductase from light- and dark-exposed leaves. Plant Physiol 114:1377–1383
Lillo C, Lea US, Leydecker M-T, Meyer C (2003) Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in constitutive activation of the enzyme in vivo and nitrite accumulation. Plant J 35:566–573
Mann AF, Hucklesby DP, Hewitt EJ (1979) Effect of aerobic and anaerobic conditions on the in vivo nitrate reductase assay in spinach leaves. Planta 146:83–89
Martinoia E, Heck U, Wiemken A (1981) Vacuoles as storage compartment for nitrate in barley leaves. Nature 289:292–294
Meyer C, Stitt M (2001) Nitrate reduction and signalling. In: Lea PJ, Morot-Gaudry JF (eds) Plant nitrogen. Springer, Berlin Heidelberg New York, pp 37–59
Morot-Gaudry-Talamain Y, Rockel P, Moureaux T, Quilleré I, Leydecker M-T, Kaiser WM, Morot-Gaudry JF (2002) Nitrite accumulation and NO emission in relation to cellular signaling in NiR antisense tobacco. Planta 215:708–715
Provan F, Aksland L-M, Meyer C, Lillo C (2000) Deletion of the nitrate reductase N-terminal domain still allows binding of 14-3-3 proteins but affects their inhibitory properties. Plant Physiol 123:757–764
Riens B, Heldt HW (1992) Decrease of nitrate reductase activity in spinach leaves during a light–dark transition. Plant Physiol 98:573–577
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Ruoff P, Lillo C (1990) Molecular oxygen as electron acceptor in the NADH-nitrate reductase system. Biochem Biophys Res Commun 172:1000–1005
Sanchez J, Heldt HW (1990) On the regulation of spinach nitrate reductase. Plant Physiol 92:684–689
Stoimenova M, Hänsch R, Mendel R, Gimmler H, Kaiser WM (2003a) The role of nitrate reduction in the anoxic metabolism of roots. I. Characterization of root morphology and normoxic metabolism of wild type tobacco and a transformant lacking root nitrate reductase. Plant Soil 253:145–153
Stoimenova M, Liboureligl, Ratcliffe RG, KaiserWM (2003b) The role of nitrate reduction in the anoxic metabolism of roots. II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant Soil 253:155–167
Su W, Huber SC, Crawford NM (1996) Identification in vitro of a post-translational regulatory site in the hinge 1 region of Arabidopsis nitrate reductase. Plant Cell 8:519–527
van der Leij M, Smith SJ, Miller AJ (1998) Remobilisation of vacuolar stored nitrate in barley root cells. Planta 205:64–72
Vincentz M, Caboche M (1991) Constitutive expression of nitrate reductase allows normal growth and development of Nicotiana plumbaginifolia plants EMBO J 10:1027–1035
Yamasaki H, Sakihama Y (2000) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett 468:89–92
Acknowledgements
This work was supported by the Marie Curie Host Fellowship QLK-3-CT-2001-60058 at the UNAP (U.S.L.), the Socrates/Erasmus program (F.tH.) and the Norwegian Research Council (F.P.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lea, U.S., ten Hoopen, F., Provan, F. et al. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in high nitrite excretion and NO emission from leaf and root tissue. Planta 219, 59–65 (2004). https://doi.org/10.1007/s00425-004-1209-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1209-6