Abstract
Transitory starch is formed in chloroplasts during the day and broken down at night. We investigated carbon export from chloroplasts resulting from transitory-starch breakdown. Starch-filled chloroplasts from spinach (Spinacia oleracea L. cv. Nordic IV) were isolated 1 h after the beginning of the dark period and incubated for 2.5 h, followed by centrifugation through silicone oil. Exported products were measured in the incubation medium to avoid measuring compounds retained inside the chloroplasts. Maltose and glucose made up 85% of the total exported products and were exported at rates of 626 and 309 nmol C mg−1 chlorophyll h−1, respectively. Net export of phosphorylated products was less than 5% and higher maltodextrins were not detected. Maltose levels in leaves of bean (Phaseolus vulgaris L. cv. Linden), spinach, and Arabidopsis thaliana (L.) Heynh. were low in the light and high in the dark. Maltose levels remained low and unchanged during the light/dark cycle in two starch-deficient Arabidopsis mutants, stf1, deficient in plastid phosphoglucomutase, and pgi, deficient in plastid phosphoglucoisomerase. Through the use of nonaqueous fractionation, we determined that maltose was distributed equally between the chloroplast and cytosolic fractions during darkness. In the light there was approximately 24% more maltose in the cytosol than the chloroplast. Taken together these data indicate that maltose is the major form of carbon exported from the chloroplast at night as a result of starch breakdown. We hypothesize that the hydrolytic pathway for transitory-starch degradation is the primary pathway used when starch is being converted to sucrose and that the phosphorolytic pathway provides carbon for other purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In leaves, transitory starch is formed in the chloroplasts during the day and broken down at night. Transitory starch acts as an energy reserve providing the plant with carbohydrate during the night when sugars cannot be made by photosynthesis and acts as an overflow, allowing photosynthesis to go faster than sucrose synthesis during the day.
During the day, carbon is exported from chloroplasts on the triose phosphate translocator (TPT; Flügge 1999). In light of work done by many groups in the previous decade, it appears unlikely that this is the route by which carbon is exported from chloroplasts at night. In a Flaveria linearis mutant with no cytosolic fructose bisphosphatase (FBPase), an enzyme required for the synthesis of sucrose from triose phosphate, no carbon was exported from leaves during the day and large amounts of starch accumulated; at night the starch was broken down (Sharkey et al. 1992). This indicated that plants have an alternative pathway for carbon export from chloroplasts but that it only operates at night. In potato plants with antisense genes for cytosolic FBPase, carbon export was shifted from day to night, with plant growth and tuber yield remaining unchanged from the wild type (WT) (Zrenner et al. 1996). Leaves of many species have high nighttime concentrations of the FBPase inhibitor fructose 2,6-bisphosphate (Stitt et al. 1985; Usuda et al. 1987; Servaites et al. 1989; Stitt 1990; Scott and Kruger 1994) and so it is likely that most export of carbon from chloroplasts at night is not as triose phosphate.
Further evidence of the limited role of triose phosphate export at night came from a series of experiments using plants with antisense genes for the TPT in potato and tobacco and a knock-out of the TPT gene in Arabidopsis. In all plants, starch accumulation was greater than in the WT during the day followed by increased starch breakdown during the night (Riesmeier et al. 1993; Heineke et al. 1994; Häusler et al. 1998; Schneider et al. 2002). The TPT antisense tobacco began starch breakdown approximately 5 h earlier than did the WT, while the time of synthesis remained unchanged (Häusler et al. 1998). The potato antisense TPT plants showed only a slight reduction in height, and tuber yield was unchanged compared to WT (Riesmeier et al. 1993). However, CO2 assimilation rates in transgenic tobacco plants with antisense repression of TPT were reduced by about 40% under non-photorespiratory conditions in comparison to the WT (Häusler et al. 2000a, 2000b). This shows that maximal photosynthetic rates require a fully operational TPT.
When ADP-glucose pyrophosphorylase activity was reduced using antisense technology, less starch was formed and more sucrose was produced during the day (Leidreiter et al. 1995a). Plants with antisense genes or knock-out mutations to both ADP-glucose pyrophosphorylase and TPT had severe growth effects, as would be expected if both major carbon sinks for photosynthesis were disrupted (Hattenbach et al. 1997; Schneider et al. 2002).
From these experiments it is clear that there are two pathways by which carbohydrates can leave the chloroplast: (i) triose phosphate export during the day, and (ii) an alternative export pathway resulting from starch breakdown at night. These pathways can compensate for each other to provide the plant with sugars. However, the question of whether the products of starch breakdown leave the chloroplast by the alternate pathway in plants with normal levels of FBPase and TPT still remained. To answer this question, nuclear magnetic resonance (NMR) was used to examine the incorporation of deuterium from deuterium-enriched water into the glucose moiety of sucrose at night. The experiments demonstrated that in tomato and bean, at least 75%, if not all, of the carbon converted from starch to sucrose at night is never broken down to the level of a triose phosphate (Schleucher et al. 1998). This demonstrated that during darkness carbon would have to be exported from the chloroplast as a hexose, a hexose phosphate, or higher maltodextrin. There are hexose phosphate/phosphate transporters but in most plants they are only present in heterotrophic plastids for hexose phosphate import into the plastid, and measured rates of hexose phosphate uptake by isolated chloroplasts are low (Flügge and Heldt 1991; Kammerer et al. 1998). Higher maltodextrins can probably be ruled out as well given the reported low permeability of the chloroplast envelope to them (Rost et al. 1996).
A putative glucose transporter has been characterized in spinach (Schäfer et al. 1977). More recently this transporter has been cloned from spinach, potato, tobacco, Arabidopsis, and maize (Weber et al. 2000). It was thought that the sex1 mutant of Arabidopsis, incapable of starch breakdown, was the result of a mutation in the glucose transporter (Caspar et al. 1991; Trethewey and ap Rees 1994). However, Yu et al. (2001) have recently shown that sex1 is defective in the glucan, water dikinase, R1, which phosphorylates starch, thereby controlling its degradation by an as yet unidentified mechanism (Ritte et al. 2002). Thus, at present the effect of blocking glucose export from chloroplasts is unknown.
It has been shown that maltose is transported into spinach chloroplasts and that glucose does not compete with this transport, indicating that there are separate maltose and glucose transporters (Herold et al. 1981; Rost et al. 1996). There have been numerous reports of 3-phosphoglycerate (PGA), triose phosphate, glucose, and maltose export as a result of starch breakdown (Heldt et al. 1977; Peavey et al. 1977; Stitt and ap Rees 1980; Stitt and Heldt 1981; Kruger and ap Rees 1983; Neuhaus and Schulte 1996). There has even been one report of isomaltose export (Servaites and Geiger 2002). Many of these studies concluded that the breakdown of starch is primarily phosphorolytic and triose phosphate export predominates.
In light of the recent genetic evidence against triose phosphate export at night we reinvestigated the form of carbon exported from the chloroplast at night. Upon finding that maltose is the major from of carbon exported from the chloroplast at night we measured the diel changes in the maltose levels in leaves of spinach, bean, and Arabidopsis. We also determined the subcellular concentrations of maltose in the light and dark. We conclude that hydrolytic starch degradation is the major pathway for conversion of starch to sucrose.
Materials and methods
Plant material and growing conditions
Bean (Phaseolus vulgaris L. cv Linden) and spinach (Spinacia oleracea L. cv Nordic IV) plants were grown under a 16- or 12-h photoperiod, 400 μmol photons m−2 s−1. A Conviron (Pembina, ND, USA) growth chamber with four 1,000-W metal-halide lamps supplemented with six 60-W incandescent lamps for 1 h at the beginning and end of the photoperiod was used. The temperature was 24 °C during the light period and 18 °C during the dark period. Humidity was maintained at a minimum of 60%. Plants used in experiments were between 3 and 5 weeks old. Wild-type (WT) Arabidopsis thaliana (L.) Heynh. (accessions Ler and Col-0) and the two starchless mutants stf1 (deficient in the plastidic isozyme of phosphoglucomutase, Kofler et al. 2000; genetic background Ler), and pgi1-1 (deficient in the plastidic isozyme of phosphoglucoisomerase, Yu et al. 2000; genetic background Col-0) were used. Arabidopsis plants were grown under the same conditions except that two of the metal-halide lamps were turned off providing 200 μmol photons m−2 s−1.
Metabolite assays
Metabolite determinations were made using NAD(P)(H) linked assays (Lowry and Passonneau 1972) in a Sigma ZFP 22 dual-wavelength filterphotometer (Sigma Instrumente, Berlin, Germany). Initially, α-glucosidase was used to assay for maltose, but we found this enzyme to be unreliable because of the lack of specificity, especially in the presence of a large amount of sucrose as is commonly found in plant material. Recently, maltose epimerase and maltose phosphorylase have become commercially available from the Kikkoman Corporation (Tokyo, Japan). These enzymes allow accurate and precise measurements of maltose, even in the presence of millimolar levels of sucrose, using reactions shown in Fig. 1 (Shirokane et al. 2000). All other enzymes used were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Isomaltose and higher maltodextrins were measured using an HPLC system consisting of a Waters (Milford, MA, USA) 626 pump, a Waters 717plus autosampler, a CarboPac PA1 4×50 mm guard column, a CarboPac PA1 250×4 mm column (Dionex, Sunnyvale, CA, USA), and a Waters 464 pulsed electrochemical detector with a gold electrode. For separation of isomaltose, 13 mM NaOH + 1 mM sodium acetate was used isocratically. For separation of the dextrins, 13 mM NaOH + 3.5 mM sodium acetate was used isocratically. The flow rate was 1 ml min−1. The settings of the pulse amperometric detector were the following: E 1=50 mV, T 1=0.4 s, E 2=750 mV, T 2=0.2 s, E 3=−150 mV, T 3=0.4 s. The range was set to 0.1 μA. Samples were first filtered through a 0.2-μm nylon syringe filter; injection volume was typically 10–50 μl.
Chloroplast isolation experiments
Chloroplasts were isolated from fully expanded leaves 1 h after the beginning of an 8-h dark period. Chloroplasts obtained from protoplasts following enzymatic digestion of the cell walls (Stitt et al. 1981) had contaminating maltase activity from the cellulase and/or pectinase preparations that could not be washed away, making this method unsuitable. For mechanical isolation of chloroplasts, approximately 20 spinach or bean leaves were cut into 1-cm2 pieces. These pieces were macerated in a Waring (Model 1043; Waring Products Company, Winsted, CN, USA) blender using four bursts of 1 s each. The buffer used in the blender maceration process consisted of 200 ml of ice-cold 30 mM Hepes buffer (pH 7.6) with 350 mM mannitol, 3 mM EDTA, and 0.5 g of acetone-washed fraction-V BSA. All BSA used in the chloroplast isolation procedure was fraction-V BSA because glucose contamination was found in other BSA preparations, and the BSA was washed with acetone to remove any lipids, which might disrupt the chloroplast membrane. Sorbitol was found to be an unsuitable osmoticum because of high levels of glucose contamination. After maceration, the leaf slurry was filtered through four layers of cheesecloth. The filtrate was then centrifuged at 5 °C in a Beckman JS-13.1 swinging-bucket rotor (Beckman Coulter, Fullerton CA, USA) for 10 min at 630 g. After centrifugation the tubes were placed on ice and the supernatant was poured off. The pellet was gently resuspended using a soft #3 paintbrush in a minimal amount of 30 mM Hepes buffer (pH 7.6) with 350 mM mannitol, 2 mM EDTA and 5 mg per ml BSA. One ml of this resuspended solution was then placed on top of a Percoll gradient. The Percoll gradient was made up of four steps using 2 ml of 80%, 60%, 40%, and 20% Percoll solutions with 30 mM Hepes (pH 7.6), 350 mM mannitol, 2 mM EDTA, and 5 mg per ml BSA. The Percoll gradient was centrifuged at 5 °C in a Beckman JS-13.1 swinging-bucket rotor for 20 min at 1,200 g to separate chloroplasts. After centrifugation, four to five distinct bands were observed in the gradient. We used the chloroplasts in the bottom band between the 60% and 80% Percoll solutions. These chloroplasts were removed using a long Pasteur pipette. Chloroplast intactness was assayed by measuring NADP-dependent glyceraldehyde-3-phosphate (GAP) dehydrogenase activity before and after rupturing the chloroplasts by osmotic shock. Chloroplasts isolated this way had about one-third of the amount of starch per mg chlorophyll as did intact leaves. At the end of the incubation the chloroplast fraction always had at least one-half of the amount of starch present in the chloroplasts before the incubation.
This chloroplast solution was then incubated in a 30 mM Hepes buffer (pH 7.6) with 350 mM mannitol, 2.5 mM MgCl2, 5 mM KH2PO4 and 5 mg per ml BSA on top of Fluka AR 200 silicone oil (Sigma–Aldrich) for 2.5 h in the dark (Fig. 2). We did not add ATP to our incubation media even though it has been shown to increase export rate (Neuhaus and Schulte 1996) because it made quantification of exported glucose more difficult due to conversion to glucose 6-phosphate (G6P) by the hexokinase on the outside of the chloroplast (Wiese et al. 1999). At the end of the incubation period, the microfuge tubes were centrifuged for 40 s to force the chloroplasts through the silicone oil, leaving the incubation buffer on top of the silicone oil. The use of silicone oil prevented the possibility of contamination of the incubation buffer by contents of chloroplasts that ruptured during centrifugation. The incubation buffer was analyzed for glucose, maltose, and other metabolites.
Leaf-punch experiments
Leaf punches (5 cm2) were taken from bean leaves at various times through the course of the light and dark period. The punches were plunged into a boiling solution of 0.75 ml of 80% ethanol for 20 min to stop all metabolism and extract the soluble carbohydrates. The ethanol was then carefully pipetted off and saved. The leaf punch was extracted with a second aliquot of ethanol solution. The combined sample was concentrated by evaporating the ethanol in a vacuum centrifuge overnight with no heat added. The sample was then resuspended in 300 μl H2O and assayed for various carbohydrates. This same procedure was used for spinach leaves except only two punches, area 3.73 cm2, were taken in the middle of light period and in the middle of the dark period.
Arabidopsis leaves of about the same age were harvested, in the middle of their light period and the middle of their dark period, and rapidly frozen in liquid nitrogen in prepared microfuge tubes filled with 80% ethanol. Frozen leaf material was weighed and quickly brought to boiling. Further treatment was as described for bean leaf punches.
Nonaqueous fractionation
Nonaqueous fractionation was carried out as described in Sharkey and Vanderveer (1989). A three-compartment analysis (chloroplast, cytosol, and vacuole) was done initially which predicted the distribution of maltose through the gradient assuming every possible distribution of maltose among the three compartments in steps of 1%. The predicted distribution that was most similar to the observed gradient distribution was taken to indicate the distribution of maltose among the three compartments. The results of the three-compartment distribution indicated that the maltose concentration in the vacuole was not significantly different from zero. Therefore, the more robust two-compartment analysis, assuming no maltose in the vacuole, was used (Gerhardt and Heldt 1984).
Results
Chloroplast isolation experiments
Chloroplasts isolated from spinach 1 h after the beginning of their dark period were 91% intact. These chloroplasts were incubated in the dark and found to primarily export maltose plus glucose (Fig. 3). Twice as much carbon was exported as maltose relative to glucose (Fig. 3). Some fructose export was observed, as well as a small amount of anions made up of G6P, dihydroxyacetone phosphate (DHAP), and PGA (Fig. 3). Similar results were also observed in chloroplasts isolated from bean (data not shown). The chloroplast incubation buffer was also tested by HPLC with pulsed amperometric detection for the presence of isomaltose and higher maltodextrins. Levels of isomaltose were below our detection limit estimated to be 33 nmol C mg−1 Chl h−1. Maltodextrins were not found (data not shown).
Maltose (Mlt), glucose (Glc), fructose (Fru), and anion: G6P, DHAP, GAP (Ani) accumulation in the incubation buffer. Accumulation in the buffer is taken as a measure of export rates from spinach (Spinacia oleracea) chloroplasts isolated 1 h after the end of the light period. On a carbon basis, the export rate of maltose is more than twice that of glucose. Values are mean ± SE (n=4)
Leaf-punch experiments
Metabolites were extracted from leaf punches taken at selected time points during the light and dark cycle of the plants. In bean plants, maltose levels remained low during the light period then rose by 3.5 μmol g−1 FW as soon as the lights went off (Fig. 4). Maltose levels remained high throughout the dark period. Corresponding with the onset of the light period, maltose levels dropped back down to the previous level (Fig. 4). The glucose level increased by more than 600 μmol m−2 before the light period then slowly decreased (Fig. 4). Maltose levels in spinach leaves were 2.5 times greater in the dark than in the light (Fig. 5).
The same trend was observed in Arabidopsis, with maltose levels three times greater in the dark than in the light (Fig. 6). Maltose levels were also assayed in two starchless mutants of Arabidopsis. The starchless mutants had approximately the same basal daytime level of maltose as the WT but did not show any increase in maltose levels during the dark period (Fig. 6).
Diel changes in maltose levels in Arabidopsis thaliana WT and two starchless mutants deficient in phosphoglucoisomerase (PGI) and phosphoglucomutase (PGM). Because the data did not vary much within each light regime, all samples taken when lights were on were grouped together (L) and all samples taken when lights were off were grouped together (D). The WT clearly shows an increase in maltose in the dark, but the starchless mutants have the same low level of maltose in both the light and dark. Values are mean ± SE (n=8)
Nonaqueous fractionation experiments
In the dark the distribution of maltose between the chloroplast and cytosol of bean leaves was roughly equal (Table 1). However, in the light, a higher proportion of the maltose was in the cytosol relative to the chloroplast (Table 1). Overall there was more total maltose in the dark than in the light, as reported in Fig. 4.
Discussion
We found that maltose is the major form of carbon exported from the chloroplast at night (Fig. 3). In addition to the present study there have been four studies quantitatively demonstrating maltose and glucose export from isolated chloroplasts (Heldt et al. 1977; Stitt and Heldt 1981; Neuhaus and Schulte 1996; Servaites and Geiger 2002). At least four other studies demonstrate maltose export qualitatively (Levi and Gibbs 1976; Peavey et al. 1977; Stitt and ap Rees 1980; Kruger and ap Rees 1983), and Ritte and Raschke (2003) have shown substantial maltose export from guard cell chloroplasts incubated in the light under conditions where starch is normally broken down.
Servaites and Geiger (2002) claim that half of the maltose export they observed was actually isomaltose based on the biphasic time-course for maltose hydrolysis by α-glucosidase. We looked for isomaltose using HPLC anion-exchange chromatography with pulsed amperometric detection and found no evidence for isomaltose export by isolated chloroplasts.
In our study and three of the previous studies in which the rate of metabolite export is quantifiable, the rate of metabolite export is much lower than the rate of starch breakdown measured in the intact leaf (Table 2). Heldt et al. (1977) and Stitt and Heldt (1981) had relatively high rates of carbon export from chloroplasts but they included metabolites retained in the chloroplasts as well as those exported from the chloroplasts, and their incubation period started immediately after imposing darkness.
Export of triose phosphates and PGA from isolated chloroplasts has been reported in the past (Levi and Gibbs 1976; Heldt et al. 1977; Peavey et al. 1977; Stitt and ap Rees 1980; Stitt and Heldt 1981). However, these experiments were carried out by feeding 14HCO3 to isolated chloroplasts and then measuring the liberation of labeled products for the first few minutes to 1 h after imposing darkness. The metabolism of chloroplasts may require some time to switch from the daytime route of carbon export to the nighttime route of carbon export from the chloroplast. In three of the previous studies (Heldt et al. 1977; Peavey et al. 1977; Stitt and ap Rees 1980), triose phosphate export was not linear with time and export stopped after 30 min in the dark. On the other hand, PGA export continued in these studies. It is not clear how PGA can be converted to sucrose in the cytosol because there would be no reducing power available. It is also unclear how PGA is made in chloroplasts at night. Phosphorolytic starch degradation could produce triose phosphates if a source of ATP were available for the phosphofructokinase reaction. But the plastidic NADP-GAP dehydrogenase is a light-activated enzyme that should have low or no activity at night.
In our experiments chloroplasts were isolated well after the normal dark period had begun and so may be more representative of the normal nighttime metabolism. We did observe low amounts of PGA export from chloroplasts (<5% of the carbon exported), which may indicate low levels of triose phosphate metabolism to PGA inside chloroplasts at night to provide reducing power for other metabolic pathways inside the chloroplast (e.g. fatty acid synthesis). The exported PGA could be used in glycolysis in the cytosol but could not be converted to sucrose without a supply of reducing power. Thus, we conclude that the initial burst of triose phosphate export immediately following imposition of darkness and the longer-term export of PGA are not the main routes for carbon export in support of sucrose synthesis at night.
Neuhaus and Schulte (1996) isolated chloroplasts from C3 and crassulacean acid metabolism (CAM)-induced Mesembryanthemum crystallinum L. and, similar to our study, they isolated the chloroplasts after the beginning of the dark period. When chloroplasts isolated from C3 plants were incubated in the presence of 5 mM Pi, maltose export was abolished and was replaced by triose phosphate and PGA export. Adding ATP to the external medium restored maltose export, eliminated PGA export, but stimulated triose phosphate export. We hypothesize that triose phosphate conversion to PGA provides the ATP needed for the phosphofructokinase reaction when only Pi is present in the medium. Maltose was the primary export product of Mesembryanthemum chloroplasts in the C3 state but substantially more triose phosphate and PGA was exported than we found for spinach or bean chloroplasts. This may reflect a species difference and we note that Mesembryanthemum chloroplasts in the CAM mode exported triose phosphate, PGA, and G6P even without the addition of Pi to the incubation medium.
Isolated chloroplasts have given us valuable insight into the products of starch degradation in the dark. However, isolating starch-laden chloroplasts is a difficult endeavor and this may explain the discrepancy between export rates from isolated chloroplasts and starch breakdown rates measured in intact leaves. Thus, the isolated-chloroplast studies, while highly suggestive, must be backed up with additional data in order to establish that maltose metabolism is critical to transitory-starch degradation.
The finding that maltose is higher at night than during the day in bean, spinach, and Arabidopsis (Figs. 4, 5, 6) and that starchless mutants do not show this diel change in maltose level further supports the critical role of maltose in starch conversion to sucrose at night.
There is a substantial amount of maltose in both the cytosol and chloroplast (Table 1). The volume of the chloroplast and cytosol in bean is unknown but in barley, spinach, and potato the chloroplast volume is greater than the cytosol volume. They are reported as follows: barley, 4.6 pl cell−1 chloroplast and 2.06 pl cell−1 cytosol; spinach, 66 μl mg−1 Chl and 24 μl mg−1 Chl; potato, 35 μl mg−1 Chl chloroplast and 22 μl mg−1 Chl cytosol (Winter et al. 1993, 1994; Leidreiter et al. 1995b). If true for Phaseolus, this would indicate a higher concentration of maltose in the cytosol than in the chloroplast. In the light there is clearly more maltose in the cytosol fraction. This could be explained by active transport of maltose out of chloroplasts as is known to occur in Escherichia coli (Boos and Shuman 1998) or perhaps binding of maltose on maltose-binding proteins, that would lower the effective concentration but would still be measured in our assay. Our assay also measured both α- and β-maltose, and one or the other could be metabolically inactive in the cytosol. If one-third to one-half of the amount of maltose in the cytosol in the light were metabolically inactive both day and night, then there would be a higher concentration of maltose in the chloroplast than in the cytosol at night, allowing for its export by diffusion. Otherwise, maltose export from the chloroplast could be by active transport.
Given the results of this work, it appears that a major pathway for transitory-starch breakdown for carbon export at night is hydrolytic rather than phosphorolytic. A deficiency in a chloroplastic endoamylase was found to impair starch breakdown at night and caused a starch-excess phenotype (sex4; Zeeman et al. 1998; Zeeman and ap Rees 1999). Kruger and ap Rees (1983) reported maltose phosphorylase activity in pea chloroplasts. However, we have not been able to find a gene encoding a maltose phosphorylase in the Arabidopsis genome, and this putative maltose phosphorylase activity has been suggested to be a minor side reaction of the plastidic D-enzyme (Kakefuda and Duke 1989).
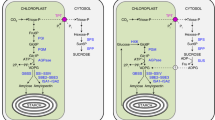
Most β-amylase activity in leaves is located outside of the chloroplast and in a number of studies no evidence for chloroplastic β-amylase activity was found (Levi and Preiss 1978; Stitt et al. 1978; Kakefuda et al. 1986; Caspar et al. 1989). An Arabidopsis mutant, ram1, with almost a complete loss of β-amylase activity was found to accumulate WT levels of starch and soluble sugars and have no impact on growth (Laby et al. 2001). Thus, it was not clear that maltose should be the product of transitory-starch breakdown. However, the β-amylase protein affected by the ram1 mutation is localized to phloem sieve elements (Laby et al. 2001) and so is not relevant to transitory-starch metabolism. Lao et al. (1999) demonstrated that there is a gene in Arabidopsis encoding a chloroplast-targeted β-amylase. Scheidig et al. (2002) demonstrated that, in potato, antisense plants for this chloroplast-targeted β-amylase led to a starch-excess phenotype. Our own analysis of the Arabidopsis genome indicates there could be genes for as many as four plastid-targeted β-amylases. Given this information, we propose a model for transitory-starch degradation (Fig. 7). In the model, starch is first broken down to maltodextrins, perhaps by α-amylase or α-glucosidase (Sun et al. 1995). Maltose is produced by β-amylase action on these maltodextrins. When the maltodextrins are shortened to maltotriose, chloroplastic D-enzyme can interconvert maltotriose units, resulting in longer maltodextrins and glucose (Kakefuda and Duke 1989). A lack of chloroplastic D-enzyme activity led to a massive accumulation of maltotriose in the dark, suggesting a critical role of the chloroplastic D-enzyme in starch breakdown (Critchley et al. 2001).
If maltose is exported from the chloroplast, then there must be enzymes in the cytosol to metabolize it. Lu and Sharkey (2003) showed that there is an enzyme analogous to malQ in E. coli and that, at least in Arabidopsis, maltose may be metabolized using the maltose/maltodextrin system described in E. coli (Boos and Shuman 1998). Two lines of Arabidopsis with the amylomaltase (malQ analog) knocked out accumulate 20–90 times more maltose and grow more slowly than WT plants.
The phosphorolytic pathway for starch breakdown could be used to supplement the Calvin cycle when CO2 is unavailable. Evidence for this idea comes form the observation that when plants are held in the light in CO2-free air CO2 is released a result of photorespiration. We have observed photorespiratory production of CO2 to proceed for at least 5 h (data not shown). This pathway could also be used to provide carbon to the Calvin cycle in the morning. This pathway would also provide carbon for other reactions in the oxidative pentose phosphate pathway, which may consume an unknown fraction of the carbon liberated from starch at night (Stitt and ap Rees 1979). Thus the hydrolytic and phosphorolytic pathways for transitory-starch breakdown serve different purposes and the hydrolytic pathway is the primary pathway for starch conversion to sucrose at night.
The cost of converting starch to sucrose can be a substantial fraction of respiration at night (Bouma et al. 1995). Depending upon the pathway, this cost can vary from two to three ATP molecules (counting the UTP used by UDP-glucose pyrophosphorylase as an ATP). If maltose is made via the hydrolytic pathway and exported from the chloroplast and the energy in the glucose–glucose bond can be preserved, then the cost of maltose export would be two ATPs per sucrose. If maltose is broken down in the cytosol to glucose molecules, then the cost of maltose export would be three ATPs per sucrose, the same as glucose export. Similarly, if maltose must be exported by active transport, presumably the energy savings of the maltose/maltodextrin system would be offset by the cost of active transport.
In conclusion, maltose is a major form of carbon export as a result of transitory-starch breakdown in the chloroplast at night. Maltose and glucose are the major products exported between 1 and 3 h after the lights are turned off. It is likely that the two different pathways for transitory-starch degradation are specialized, with the hydrolytic pathway the predominant pathway by which starch is broken down for subsequent sucrose synthesis at night.
Abbreviations
- CAM:
-
crassulacean acid metabolism
- Chl:
-
chlorophyll
- DHAP:
-
dihydroxyacetone phosphate
- FBPase:
-
fructose bisphosphatase
- GAP:
-
glyceraldehyde-3-phosphate
- G6P:
-
glucose 6-phosphate
- PGA:
-
3-phosphoglycerate
- TPT:
-
triose phosphate translocator
- WT:
-
wild type
References
Boos W, Shuman H (1998) Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev 62:204–229
Bouma TJ, De Visser R, Van Leeuwen PJ, DeKock MJ, Lambers H (1995) The respiratory energy requirements involved in nocturnal carbohydrate export from starch-storing mature source leaves and their contribution to dark respiration. J Exp Bot 46:1185–1194
Caspar T, Lin T-P, Monroe J, Bernhard W, Spilatro S, Preiss J, Somerville C (1989) Altered regulation of β-amylase activity in mutants of Arabidopsis with lesion in starch metabolism. Proc Natl Acad Sci USA 86:5830–5833
Caspar T, Lin T-P, Kakefuda G, Benbow L, Preiss J, Somerville C (1991) Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol 95:1181–1188
Critchley J, Zeeman S, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knockout mutation in Arabidopsis thaliana. Plant J 26:89–100
Flügge U-I (1999) Phosphate translocators in plastids. Annu Rev Plant Physiol Plant Mol Biol 50:27–45
Flügge U-I, Heldt HW (1991) Metabolite translocators of the chloroplast envelope. Annu Rev Plant Physiol Plant Mol Biol 42:129–144
Gerhardt R, Heldt HW (1984) Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol 75:542–547
Hattenbach A, Müller-Röber B, Nast G, Heineke D (1997) Antisense repression of both ADP-glucose pyrophosphorylase and triose phosphate translocator modifies carbohydrate partitioning in leaves. Plant Physiol 115:471–475
Häusler RE, Schlieben NH, Schulz B, Flügge U-I (1998) Compensation of decreased triose phosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204:366–376
Häusler RE, Schlieben NH, Nicolay P, Fischer K, Fischer KL, Flügge U-I (2000a) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum L.) I. Comparative physiological analysis of tobacco plants with antisense repression and overexpression of the triose phosphate/phosphate translocator. Planta 210:371–382
Häusler RE, Schlieben NH, Flügge U-I (2000b) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum L.) II. Assessment of control coefficients of the triose phosphate/phosphate translocator. Planta 210:383–390
Heineke D, Kruse A, Flügge U-I, Frommer WB, Riesmeier JW, Willmitzer L, Heldt HW (1994) Effect of antisense repression of the chloroplast translocator on photosynthesis metabolism in transgenic potato plants. Planta 193:174–180
Heldt HW, Chon CJ, Maronde D, Herold A, Stankovic ZS, Walker DA, Kraminer A, Kirk MR, Heber U (1977) Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol 59:1146–1155
Herold A, Leegood RC, McNeil PH, Robinson SP (1981) Accumulation of maltose during photosynthesis in protoplasts isolated from spinach leaves treated with mannose. Plant Physiol 67:85–88
Kakefuda G, Duke SH (1989) Characterization of pea chloroplast D-enzyme (4-α-d-glucanotransferase. Plant Physiol 91:136–143
Kakefuda G, Duke SH, Hostak MS (1986) Chloroplast and extrachloroplastic starch-degrading enzymes in Pisum sativum L.. Planta 168:17–182
Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge U-I (1998) Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate phosphate antiporter. Plant Cell 10:105–117
Kofler H, Häusler RE, Schulz B, Gröner F, Flügge UI, Weber A (2000) Molecular characterisation of a new mutant allele of the plastidic phosphoglucomutase and complementation of the mutant with the wild-type cDNA. Mol Genet Genomics 263:978–986
Kruger NJ, ap Rees T (1983) Maltose metabolism by pea chloroplasts. Planta 158:179–184
Laby RJ, Kim D, Gibson SI (2001) The ram1 mutant of Arabidopsis exhibits severely decreased β-amylase activity. Plant Physiol 127:1798–1807
Lao NT, Schoneveld O, Mould RM, Hibberd JM, Gray JC, Kavanagh TA (1999) An Arabidopsis gene encoding a chloroplast-targeted β-amylase. Plant J 20:519–527
Leidreiter K, Heineke D, Heldt HW, Müller-Röber B (1995a) Leaf-specific antisense inhibition of starch biosynthesis in transgenic potato plants leads to an increase in photoassimilate export from source leaves during the light period. Plant Cell Physiol 36:615–624
Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt HW (1995b) Subcellular volumes and metabolite concentrations in potato (Solanum tuberosum cv désirée) leaves. Bot Acta 108:439–444
Levi C, Gibbs M (1976) Starch degradation in isolated chloroplasts. Plant Physiol 57:933–935
Levi C, Preiss J (1978) Amylopectin degradation in pea chloroplast extracts. Plant Physiol 61:218–220
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, Orlando, pp 1–291
Lu Y, Sharkey TD (2003) The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta DOI 10.1007/s00425-003-1127-z
Neuhaus HE, Schulte N (1996) Starch degradation in chloroplasts isolated from C3 or CAM (crassulacean acid metabolism)-induced Mesembryanthemum crystallinum L.. Biochem J 318:945–953
Peavey DG, Steup M, Gibbs M (1977) Characterization of starch breakdown in the intact spinach chloroplast. Plant Physiol 60:305–308
Riesmeier JW, Flügge U, Schulz B, Heineke D, Heldt HW, Willmitzer L, Frommer WB (1993) Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci USA 90:6160–6164
Ritte G, Raschke K (2003) Metabolite export of isolated guard cell chloroplasts of Vicia faba. New Phytol 159:195–202
Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M (2002) The starch-related R1 protein is an alpha-glucan, water dikinase. Proc Natl Acad Sci USA 99:7166–7171
Rost S, Frank C, Beck E (1996) The chloroplast envelope is permeable for maltose but not for maltodextrins. Biochim Biophys Acta 1291:221–227
Schäfer G, Heber U, Heldt HW (1977) Glucose transport into spinach chloroplasts. Plant Physiol 60:286–289
Scheidig A, Fröhlich A, Schulze S, Lloyd JR, Kossmann J (2002) Downregulation of a chloroplast-targeted β-amylase leads to a starch-excess phenotype in leaves. Plant J 30:581–591
Schleucher J, Vanderveer PJ, Sharkey TD (1998) Export of carbon from chloroplasts at night. Plant Physiol 118:1439–1445
Schneider A, Häusler RE, Kolukisaoglu Ü, Kunze R, van der Graaff E, Schwacke R, Catoni E, Desimone M, Flügge U-I (2002) An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J 32:685–699
Scott P, Kruger NJ (1994) Fructose-2,6-bisphospahte levels in mature leaves of tobacco (Nicotiana tabacum) and potato (Solanum tuberosum). Planta 193:16–20
Servaites JC, Geiger D (2002) Kinetic characteristics of chloroplast glucose transport. J Exp Bot 53:1–11
Servaites JC, Geiger DR, Tucci MA, Fondy B (1989) Leaf carbon metabolism and metabolite levels during a period of sinusoidal light. Plant Physiol 89:403–408
Sharkey TD, Vanerveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91:679–684
Sharkey TD, Savitch LV, Vanderveer PJ, Micallef BJ (1992) Carbon partitioning in a Flaveria linearis mutant with reduced cytosolic fructose bisphosphatase. Plant Physiol 100:210–215
Shirokane Y, Ichikawa K, Suzuki M (2000) A novel enzymic determination of maltose. Carbohydr Res 329:699–702
Stitt M (1990) Fructose-2,6-bisphosphate in plants. Annu Rev Plant Physiol 41:153–185
Stitt M, ap Rees T (1979) Capacities of pea chloroplasts to catalyse the oxidative pentose phosphate pathway and glycolysis Phytochemistry 18:1905–1911
Stitt M, ap Rees T (1980) Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim Biophys Acta 627:131–143
Stitt M, Heldt HW (1981) Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol 68: 755–761
Stitt M, Bulpin PV, ap Rees T (1978) Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta 544:200–214
Stitt M, Wirtz W, Heldt HW (1981) Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta 593:85–102
Stitt M, Wirtz W, Gerhardt R, Heldt HW, Spencer C, Walker D, Foyer C (1985) A comparative study of metabolite levels in plant leaf material in the dark. Planta 166:354–364
Sun ZT, Duke SH, Henson CA (1995) The role of pea chloroplast alpha-glucosidase in transitory starch degradation. Plant Physiol 108:211–217
Trethewey RN, ap Rees T (1994) The role of the hexose transporter in the chloroplasts of Arabidopsis thaliana L. Planta 195:168–174
Usuda H, Kalt-Torres W, Kerr PS, Huber SC (1987) Diurnal changes in maize leaf photosynthesis. II. Levels of metabolic intermediates of sucrose synthesis and the regulatory metabolite fructose 2,6-bisphosphate. Plant Physiol 83:289–293
Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge U-I (2000) Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell 12:787–801
Wiese A, Gröner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flügge U-I, Weber A (1999) Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett 461:13–18
Winter H, Robinson DG, Heldt HW (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191:180–190
Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193:530–535
Yu T-S, Lue W-L, Wang S-M, Chen J (2000) Mutation of Arabidopsis plastid phosphoglucose isomerase affects leaf starch synthesis and floral initiation. Plant Physiol 123:319–325
Yu T-S, Kofler H, Häusler RE, Hille D, Flügge U-I, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, Steup M, Lue W-L, Chen J, Weber A (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation In plants, and not in the chloroplast hexose transporter. Plant Cell 13:1907–1918
Zeeman SC, ap Rees T (1999) Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant Cell Environ 22:1445–1453
Zeeman SC, Northrop F, Smith AM, ap Rees T (1998) A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme Plant J 5:357–365
Zrenner R, Krause KP, Apel P, Sonnewald U (1996) Reduction of the cytosolic fructose-1,6-bisphosphatase in transgenic potato plants limits photosynthetic sucrose biosynthesis with no impact on plant growth and tuber yield. Plant J 9:671–681
Acknowledgements
This research was supported by the US Department of Energy under grant DE-FG02-99ER 20345. S.E.W. was supported in part by the Wisconsin Center for Space Automation and Robotics of UW-Madison. We thank Peter Vanderveer for help in starting this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weise, S.E., Weber, A.P.M. & Sharkey, T.D. Maltose is the major form of carbon exported from the chloroplast at night. Planta 218, 474–482 (2004). https://doi.org/10.1007/s00425-003-1128-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-003-1128-y