Abstract
The mechanism for limb ischemic precondition (RLIPC)-induced suppression of reperfusion arrhythmia remains unknown. The purpose of this study was to examine the roles of the pro-survival reperfusion injury salvage kinase (RISK) and survivor activating factor enhancement (SAFE) pathways in this RLIPC-mediated antiarrhythmic activity. Male Sprague Dawley rats were assigned to sham-operated, control, or RLIPC groups. All rats except for the sham rats had 5 min of left main coronary artery occlusion with another 20 min of reperfusion. RLIPC was initiated by four cycles of limb ischemia (5 min) and reperfusion (5 min) on the bilateral femoral arteries. Hearts in every group were taken for protein phosphorylation analysis. RLIPC ameliorated reperfusion-induced arrhythmogenesis and reduced the incidence of sudden cardiac death during the entire 20-min reperfusion period (66.7% of control rats had SCD vs. only 16.7% of RLIPC-treated rats). RLIPC enhances ventricular ERK1/2 phosphorylation after reperfusion. RLIPC-induced antiarrhythmic action and ERK1/2 phosphorylation are abolished in the presence of the ERK1/2 inhibitor U0126. Limb ischemic preconditioning protects the heart against myocardial reperfusion injury-induced lethal arrhythmia. These beneficial effects may involve the activation of ERK1/2 in the RISK signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emergency revascularization, such as thrombolysis, percutaneous coronary intervention (PCI), or coronary artery bypass grafting (CABG), is an essential therapeutic strategy for patients with acute myocardial infarction. However, the restoration of blood flow can also lead to significant electrophysiological imbalance and cause lethal reperfusion arrhythmias. Therefore, developing new therapeutic strategies to prevent and treat reperfusion-induced arrhythmia is urgently needed.
Murry et al. proposed the concept of ischemic preconditioning (IPC) in 1986, which is a powerful form of endogenous cardioprotective strategy. IPC is an intervention whereby short episodes of vascular occlusion render the heart more resistant to the subsequent deleterious effects of sustained ischemia [27]. Later, in 1993, Przyklenk et al. found that preconditioning a certain region of the heart may protect a remote myocardium from subsequent ischemia [30]. Soon, this concept of remote preconditioning was extended by Kharbanda et al., who showed that the remote cardioprotective effect could be accomplished by short limb ischemia/reperfusion stimuli [20]. The noninvasive remote limb ischemic preconditioning (RLIPC) method is clinically feasible since the procedure can be easily performed in an arm with a blood cuff in patients. Thereafter, substantial evidence was sufficient to support the beneficial effects of limb conditioning in humans [5]. In addition to the limb, preclinical studies have also demonstrated that preconditioning the vascular bed, such as the intestine [39], liver [42], or kidney [19], can also protect the heart against myocardial I/R injury. Notably, several experimental studies have shown that RLIPC has cardioprotective properties in the setting of reperfusion-induced arrhythmia [28]. However, the exact mechanisms by which RLIPC confers antiarrhythmic effects per se have been less clear.
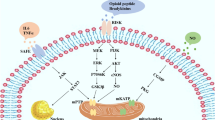
It has been demonstrated that the reperfusion-related RISK (pro-survival reperfusion injury salvage kinase) and SAFE (survivor activating factor enhancement) pathways are critically involved in ischemic preconditioning-induced cardioprotective interventions. The RISK pathway includes extracellular signal-regulated kinase (ERK) and protein kinase B (AKT), and their downstream glycogen synthase kinase-3β (GSK-3β). The SAFE pathway includes the signal transducer and activator of transcription 1 (STAT1), 3 (STAT3), and 5 (STAT5) molecules. Both pathways can be activated (or phosphorylated) upon preconditioning [11, 38]. We have previously used intermittent I/R stimuli to the liver to characterize the efficiency of remote liver preconditioning on myocardial I/R-induced sudden cardiac death. In that model, we found that liver preconditioning prohibited reperfusion-induced lethal arrhythmia via an ERK/GSK-3β-dependent mechanism [16].
There is no study showing the effect of remote limb preconditioning on the phosphorylation status of the RISK and SAFE pathways in the context of cardiac I/R injury-induced arrhythmia. We therefore examined the phosphorylation pattern of the vital signaling molecules within these two pathways in a rat model of RLIPC followed by myocardial I/R. Second, with the use of a specific antagonist, we aimed to further identify the potential pathway in antiarrhythmic activity afforded by remote limb ischemic preconditioning.
Materials and methods
Ethical approval
This study was reviewed and approved by the Institutional Animal Care and Use Committee of Sichuan University (Approval No. 2015035A). Sprague Dawley (SD) male rats 10–12 weeks old (weighing 220–250 g) were obtained from Chengdu Dashuo Experimental Animal Research Center (Chengdu, China). Rats were housed in a pathogen-free animal house at 20–25 °C with a 12-h light:12-h dark cycle. A standard rat chow diet was used before the experiment.
Experimental groups
Fifty-three rats were assigned to three groups. Rats in the sham group received only sham surgery (n = 8); rats in the control group (CON, n = 12) had myocardial I/R injury. Rats in the RLIPC group (RLIPC, n = 12) received remote limb ischemic preconditioning stimuli before cardiac ischemia. To further examine whether ERK1/2 was involved in RLIPC-mediated antiarrhythmic effects, the ERK1/2 inhibitor U0126 (0.5 mg/kg, Sigma, St. Louis, MO, USA) was applied via the femoral vein 30 min ahead of left main coronary artery ligation to control (CON + U0126, n = 10) and RLIPC (RLIPC + U0126, n = 11) rats. Body temperature was maintained with a heating blanket. At the end of the experiment, the surviving animals were euthanized by the injection of an overdose of sodium pentobarbital (200 mg/kg, i.p.). Death was ascertained by the cessation of heartbeat and respiration.
Surgical procedures
As previously described [15], sodium pentobarbital (50 mg/kg) was used for anesthesia (intraperitoneal injection). The depth of anesthesia was assessed by checking the pedal withdrawal reflex, spontaneous movements, and pinna reflex. Next, a tracheostomy was performed, and the rats were mechanically ventilated with a tidal volume of 8 ml/kg by a rodent ventilator (Chengdu Taimeng Technology Co., Ltd., Chengdu, China). Rats were given 10 min of stabilization before cardiac manipulation. Then, a thoracotomy was conducted, and the proximal main left coronary artery was ligated with a 6–0 silk suture (Ethicon, Somerville, NJ, USA). Later, the snare was loosened for reperfusion. Reperfusion was achieved by the absence of epicardial cyanosis. All rats except for the sham group had 5-min left main coronary artery occlusion followed by 20-min reperfusion in our study. For the induction of limb ischemic preconditioning, four cycles of limb ischemia (5 min) and reperfusion (5 min) were performed on bilateral femoral arteries with an atraumatic microvascular clip 30 min ahead of myocardial ischemia. Sodium pentobarbital (20 mg/kg, ip) was given every 30 min throughout the experiment for the maintenance of anesthesia. Analgesia was conducted with nalbuphine (2 mg/kg, S.C.).
Arrhythmia parameters
A limb lead II standard electrocardiography system was used throughout the entire experiment in our study (Powerlab/8sp system, AD Instruments, Colorado Springs, CO, USA). The incidence of sudden cardiac death (SCD) was recorded. Arrhythmia parameters included ventricular tachycardia (VT), polymorphic VT (PVT), sustained ventricular tachycardia over 1 min (SVT), ventricular fibrillation (VF), AV block (AVB), VT duration (the sum of all VT durations during the reperfusion period), longest VT duration (LVT, the longest episode of VT occurred during reperfusion), and latency to the first recorded VT/VF episode. All parameters were recorded and analyzed by LabChart 7.2.1 software (AD Instruments, Colorado Springs, CO, USA).
Serum analysis
Blood samples were taken from the heart after euthanasia. Serum was obtained by centrifugation (1000 g, 10 min, 4 °C). Levels of serum cardiac troponin I (cTnI) and catecholamine were measured with ELISA kits according to the manufacturers’ instructions (Quanzhou Ruixin Biological Technology Co., Ltd., China).
Heart tissue collection
The main left coronary artery was then reoccluded at the end of the experiment. The perfused and nonperfused heart regions were stained with 1% Evans blue (Sigma, St. Louis, MO, USA). The nonischemic heart tissues were stained blue, and the ischemic regions were not stained. The ischemic tissues (area at risk) were taken and kept in a –80 °C freezer.
Western blotting
Heart tissues were homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) before centrifugation at 10,000 × g for 10 min at 4 °C (n = 5 per group). A bicinchoninic acid (BCA) assay kit was used to determine the protein concentration (Pierce, Rockford, IL, USA). Proteins were separated on a 12% SDS-PAGE 1.5-mm thick gel before transferring onto nitrocellulose membranes (BioTrace™ NT, Pall Corporation, Port Washington, NY, USA) at 80v for 1 h. The membrane was then incubated for 1 h at room temperature with 5% nonfat milk dissolved in phosphate buffered saline and 0.1% Tween-20. Next, the membrane was incubated with primary antibodies against phosphorylated glycogen synthase kinase-3β (Ser9) (p-GSK-3β), total GSK-3β, phosphorylated Akt (ser473) (p-AKT), total Akt, extracellular signal-regulated kinase 1/2 (ERK1/2) (Thr202/Tyr204) (p-ERK), total ERK1/2, phosphorylated STAT-1 (Tyr701) (p-STAT1), total STAT-1, phosphorylated STAT-5 (Tyr694) (p-STAT5), total STAT-5, phosphorylated STAT-3 (Tyr705) (p-STAT3), and total STAT-3 (all 1:1000 dilution, from Cell Signaling, Danvers, MA, USA). After incubation at 4 °C overnight, the membranes were washed and incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG secondary antibody (all 1:5000 dilution, Bio-Rad, Hercules, CA, USA) for 2 h at room temperature. Bands were visualized using the chemiluminescence ECL detection kit (Millipore, Billerica, MA, USA) by the Amersham Imager 600 system (GE Healthcare, Little Chalfont, UK). ImageJ Data Acquisition Software (National Institutes of Health, Bethesda, MD, USA) was used for quantification of protein band densities.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). GraphPad Prism version 8.0 (GraphPad, La Jolla, CA, USA) was used for data analysis. The incidences of arrhythmias were compared using Fisher’s exact test. Data were tested for normal distribution using the Kolmogorov-Smirnoff test. The differences between two groups were determined by unpaired Student’s t test. For comparisons of groups over three, statistical analyses were performed using one-way analysis of variance (ANOVA) following the post hoc test of the Student–Newman–Keuls test (variance was equal) or Dunnett’s T3 test (variance was not equal). The homogeneity of variance was examined by Levene’s test. Differences with p values < 0.05 were considered statistically significant. All p values were two-sided.
Results
RLIPC decreases serum levels of cTnI post I/R
Cardiac troponin I (cTnI) is highly specific for perioperative myocardial tissue damage. We found that cardiac reperfusion injury increased plasma cTnI levels post I/R (0.4 ± 0.2 ng/ml in CON, vs. 0.2 ± 0.1 ng/ml in sham, p < 0.01), suggesting impaired cardiac function in control rats after cardiac reperfusion. However, RLIPC treatment (0.2 ± 0.1 ng/ml) significantly decreased serum levels of cTnI compared to control treatment (p < 0.01, Fig. 1A). Cardiac ischemia may cause catecholamine release and abnormal sympathetic nerve excitability [35]. Therefore, plasma catecholamine levels were also measured in our study. No difference was detected regarding serum catecholamine levels among groups in our study (192.7 ± 22.9 ng/ml in sham, 193.2 ± 34.5 ng/ml in CON, 187.2 ± 24.4 ng/ml in RLIPC, p > 0.05).
The effect of remote ischemic preconditioning on the release of cardiac troponin I (cTnI) and catecholamine. A Post I/R mean serum levels of cardiac troponin I in RLIPC-treated control rats (n = 8–10). **p < 0.01 vs. sham; ##p < 0.01 vs. CON. sham, sham-operated; CON, control; RLIPC, remote ischemic preconditioning. B Post I/R mean serum levels of catecholamine in RLIPC-treated control rats (n = 8–10). **p < 0.01 vs. sham; ##p < 0.01 vs. CON
RLIPC ameliorates reperfusion-induced arrhythmogenesis
To confirm whether RLIPC may affect cardiac susceptibility to arrhythmias post I/R, we characterized the surface ECGs using a standard limb lead II recording system. Cardiac reperfusion injury was conducted via ligation/reperfusion of the left coronary artery in each heart. Representative ECG tracings are presented in Fig. 2. The analysis of arrhythmia parameters is shown in Table 1. Rats in the control group developed various categories of arrhythmia post I/R, including ventricular tachycardia (VT), sustained VT over 1 min, polymorphic VT (PVT), atrioventricular block (AVB), ventricular fibrillation (VF), or sudden cardiac death (SCD). During the entire 20-min reperfusion period, the incidence of arrhythmia or VT was prevalent in the control (12 out of 12, 100%) but not in the RLIPC group (7 out of 12, 58.3%, p < 0.05). In the control group, death was caused by lethal arrhythmias, such as VF or severe AVB. A total of 66.7% of control rats (8 out of 12) developed SCD. However, reperfusion stimuli provoked only two cases of SCD in the RLIPC-treated rats (2 out of 12, 16.7%, p < 0.05). Furthermore, VF also occurred more frequently in control rats (8 out of 12, 66.7%) than in RLIPC rats (2 out of 12, 16.7%, p < 0.05). Additionally, significant differences were detected in the incidence of sustained VT (CON: 9/12; RLIPC: 2/12, p < 0.05), PVT (CON: 8/12; RLIPC: 2/12, p < 0.05), and AVB (CON: 10/12; RLIPC: 3/12, p < 0.05) between the CON and RLIPC groups. Importantly, we found that I/R could deteriorate cardiac conductance at a very early stage of cardiac reperfusion in control rats. Five minutes after the onset of reperfusion, five control rats (41.7%) developed SCD and VF vs. 0 (0%) RLIPC rats (p < 0.05). Furthermore, VT was commonly seen in all control rats (12/12, 100%). However, only half of the RLIPC developed VT at the early stage of reperfusion (7/12, 58.3%, p < 0.05). We found that RLIPC rats rarely developed SVT or PVT during the first 5 min of reperfusion (SVT: 8.3%; PVT: 0%), while the ratio of control rats exhibiting SVT (50%) or PVT (50%, p < 0.05) was significantly increased when compared to the RLIPC group. Meanwhile, a similar pattern for VF induction was found during the first 10 min of reperfusion between the control (8 out of 12, 66.7%) and RLIPC groups (0 out of 12, 0%, p < 0.01), as well as the SCD incidence (CON: 7/12, 58.3%, RLIPC: 0/12, 0%, p < 0.01).
Interestingly, we found that the VT duration of RLIPC-treated rats (48.1 ± 32.5 s) was significantly shorter than that of control rats (139.4 ± 70.1 s, p < 0.01, Fig. 3A) throughout reperfusion. Additionally, the longest episode of VT duration was > 2.95-fold longer in control rats (96.3 ± 56.2 s) than that in RLIPC-treated rats (32.6 ± 26.0 s, p < 0.05, Fig. 3B). In addition, rats in the control group developed VT (18.5 ± 9.8 s) or VF (256.4 ± 92.5) earlier than those in the RLIPC group (VT: 35.6 ± 21.7 s, p < 0.05, Fig. 3C; VF: 722.0 ± 99.0, p < 0.001, Fig. 3D) after commencing reperfusion. Taken together, these results suggested that rats treated with RLIPC were less susceptible to cardiac reperfusion-induced ventricular arrhythmias.
RLIPC reduces cardiac susceptibility to reperfusion-induced arrhythmogenesis. A Mean VT durations during the 20-min postischemia reperfusion in rats with (n = 12) or without (n = 12) RLIPC treatment. **p < 0.01 between groups. CON, control; RLIPC, remote ischemic preconditioning. B The longest VT (LVT) durations during the 20 min of postischemia reperfusion *p < 0.05 between groups. C The starting time of the first episode of VT after the onset of reperfusion. *p < 0.05 between groups. D The starting time of the first episode of VF after the onset of reperfusion. ***p < 0.001 between groups
RLIPC alters ventricular ERK1/2 phosphorylation after reperfusion
Evidence has shown that vital signaling molecules such as AKT, ERK1/2, and GSK-3β in the prosurvival reperfusion injury salvage kinase (RISK) pathway and STAT1, STAT3, or STAT5 in the survivor activating factor enhancement (SAFE) pathway can be phosphorylated/activated upon ischemic stimuli. We therefore examined the phosphorylation status of these proteins (via normalization to their corresponding total protein levels) in our study. We found that the total protein levels were similar among groups (all p > 0.05). As shown in Fig. 4A and B, GSK-3β and AKT were equally phosphorylated in the three experimental groups (all p > 0.05). Strikingly, although reperfusion injury did not change the ventricular phosphorylation status of ERK1/2 (p > 0.05 CON vs. sham), the activation of ERK1/2 (via phosphorylation) following the ischemic preconditioning stimulus was observed when compared with control hearts (p < 0.001 RLIPC vs. CON, Fig. 4C), indicating that ERK1/2 phosphorylation may participate in RLIPC-induced antiarrhythmic action. We did not find differences regarding the phosphorylation levels of STAT1 (Fig. 4D) and STAT5 (Fig. 4E) among the groups. Notably, control rats exhibited elevated STAT3 phosphorylation compared with sham rats post I/R (p < 0.001); however, RLIPC could not further enhance its phosphorylation level (p > 0.05 vs. CON, Fig. 4F). Taken together, these findings indicate that the SAFE pathway may not be involved in RLIPC-mediated cardioprotection in our study.
RLIPC alters ERK1/2 phosphorylation post I/R. A Left: Western blots of ventricular phosphorylated GSK-3β and total GSK-3β (A), phosphorylated AKT and total AKT (B), phosphorylated ERK1/2 and total ERK1/2 (C), phosphorylated STAT1 and total STAT1 (D), phosphorylated STAT5 and total STAT5 (E), and phosphorylated STAT3 and total STAT3 (F) from RLIPC-treated and control rats post I/R. Right: mean band densities of p-GSK-3β/GSK-3β (A), p-AKT/AKT (B), p-ERK1/2/ERK1/2 (C), p-STAT1/STAT1 (D), p-STAT5/STAT5 (E), and p-STAT3/STAT3 (F) (n = 5 per group). ***p < 0.001 vs. sham, ###p < 0.001 vs. CON. sham, sham-operated group; CON, control group
Inhibition of ERK1/2 phosphorylation abolishes RLIPC-induced antiarrhythmic action
We found that ERK1/2 was phosphorylated in the RLIPC group after reperfusion. To further clarify the role of ERK1/2, we administered U0126, an ERK1/2 inhibitor, to control and RLIPC rats before cardiac manipulation. Figure 5 shows the typical electrocardiogram tracings after myocardial I/R, and Table 2 illustrates the incidence of different kinds of ventricular arrhythmias. We found that all classes of arrhythmia were similarly induced upon reperfusion stimuli in the CON (10 out of 10, 100%) and RLIPC (11 out of 11, 100%) groups when U0126 was present. ERK1/2 inhibitor largely abolished the RLIPC-induced anti-arrhythmic effect. During the entire 20-min reperfusion period, 7 rats in the RLIPC + U0126 group developed VF or SCD vs. only 2 in the RLIPC group without inhibitor (p < 0.05). In the RLIPC + U0126 group, death was caused by lethal arrhythmias, such as VF or severe AVB. Specifically, VT occurred more frequently in RLIPC rats treated with U0126. Seven out of 12 (58.3%) RLIPC rats developed VT, while the ratio of U0126-treated RLIPC rats exhibiting VT was 100% (11 out of 11, p < 0.05 vs. RLIPC + U0126). Consistent with this, significant differences were detected in the incidence of SVT (RLIPC: 2/12; RLIPC + U0126: 7/11, p < 0.05), PVT (RLIPC: 2/12; RLIPC + U0126: 7/11, p < 0.05), or AVB (RLIPC: 3/12; RLIPC + U0126: 9/11, p < 0.05) between RLIPC groups with or without an inhibitor. Importantly, we also found that rats in the RLIPC + U0126 group experienced episodes of lethal ventricular tachycardia or fibrillation in the early phase of reperfusion. All U0126-treated RLIPC rats developed arrhythmia during the first 5 or 10 min of reperfusion (11 out of 11, 100%), while only 58.3% of the RLIPC rats developed arrhythmia (7 out of 12, 50%, p < 0.05). A total of 36.4% or 63.6% of RLIPC rats treated with U0126 (5 min: 4 out of 11; 10 min: 7 out of 11) developed VF or SCD. However, the reperfusion stimuli could not induce any VF or SCD in the RLIPC-treated rats without inhibitor (0 out of 12, 0%, p < 0.05 or p < 0.01). Furthermore, the severity of post I/R arrhythmia was significantly increased in the RLIPC + U0126 group during the early phase of reperfusion, as evidenced by the increased incidence of VT, SVT, and PVT (p < 0.05).
The effect of U0126 on exemplar surface ECGs post I/R. Arrhythmia: the presence of the first abnormal cardiac rhythm after myocardial reperfusion; VT, ventricular tachycardia; AVB, AV block, PVT, polymorphic VT; VF, ventricular fibrillation; sinus: sinus rhythm. CON, control, RLIPC: remote ischemic preconditioning, U0126: ERK1/2 inhibitor
We also examined the effect of U0126 on VT durations (Fig. 6A) and longest VT durations (Fig. 6B). We observed significant differences between the U0126-treated and nontreated RLIPC groups (all p < 0.05). In addition, U0126 treatment did modify the latency to first run of VT (Fig. 6C, p < 0.05) or VF episodes (Fig. 6D, p < 0.001) after the onset of cardiac reperfusion when compared with non-U0126-treated RLIPC ones. Taken together, our results suggest that pharmacological inhibition of ERK1/2 phosphorylation impairs the antiarrhythmic action of RLIPC.
The effect of U0126 on reperfusion-induced arrhythmogenesis. A Mean VT durations during the 20 min of postischemia reperfusion. #p < 0.05 vs. RLIPC rats. CON, control; RLIPC, remote ischemic preconditioning; U0126, ERK1/2 inhibitor. Values for control, RLIPC rats are repeated from Fig. 3A for comparison. B The longest VT (LVT) durations during the 20 min of postischemia reperfusion. #p < 0.05 vs. RLIPC rats. Values for control, RLIPC rats are repeated from Fig. 3B for comparison. C The starting time of the first episode of VT after the onset of reperfusion. #p < 0.05 vs. RLIPC rats. Values for control, RLIPC rats are repeated from Fig. 3C for comparison. D The starting time of the first episode of VF after the onset of reperfusion. ###p < 0.001 vs. RLIPC rats. Values for control, RLIPC rats are repeated from Fig. 3D for comparison
U0126 diminishes RLIPC-induced ERK1/2 phosphorylation post I/R
We found in our study that limb preconditioning activated ERK1/2 via phosphorylation after reperfusion injury. We further tested whether pharmacological inhibition of ERK1/2 activation could reverse ventricular ERK1/2 phosphorylation status. As seen in Fig. 7, U0126 halved the ERK1/2 phosphorylation level from 1.2 ± 0.2 (RLIPC) to 0.50 ± 0.2 arbitrary units (RLIPC + U0126, p < 0.001), to a level similar to that of hearts in the control group without inhibitor (0.50 ± 0.2), suggesting that RLIPC-induced anti-arrhythmic activity was ERK1/2 dependent.
U0126 impaired RLIPC-induced ERK1/2 phosphorylation. Left: Western blots of ventricular phosphorylated ERK1/2 and total ERK1/2. Right: mean band densities of p-ERK1/2/ERK1/2 (n = 5 per group). ***p < 0.001 vs. RLIPC, CON. sham, sham-operated group; CON, control group; RLIPC, remote limb preconditioning group
Discussion
We demonstrate in the current study that remote limb ischemic preconditioning effectively attenuates the severity of reperfusion-induced arrhythmias and reduces the incidence of sudden cardiac death post I/R via an ERK1/2-dependent signaling mechanism.
The phenomenon of ischemic preconditioning (IPC) was first described by the research group of Murry et al. They showed that brief, nonlethal episodes of ischemia–reperfusion ahead of prolonged I/R limit infarction in a canine heart ischemia model [27]. IPC is a cornerstone concept in the field of perioperative cardioprotection. Thereafter, the cardioprotective effects of IPC have been demonstrated repeatedly in animals and humans [10, 13, 34]. Based on the concept of IPC, remote ischemic preconditioning opens a new era of organ protection [30]. Myocardial protection can be induced by brief I/R stimuli of ex-cardiac tissue or organs, such as the intestine, liver, and kidney [8, 24]. Specifically, cardiac ischemic tolerance can be induced by remote limb ischemic preconditioning (RLIPC). This procedure is normally performed in an arm using a blood pressure cuff, typically using 1–4 cycles of 3–5 min of limb I/R protocol. Given its simplicity and cost efficiency, RLIPC has a wide range of clinical possibilities. It can readily be initiated outside the operating room, in an ambulance, or at home. Sufficient experimental investigations support the cardioprotective effects of remote limb preconditioning, such as infarct limitation, improved systolic and diastolic function, reduced myocardial apoptosis, and cardiac histological damage following I/R [12]. Consistent with these results, clinical trials have also demonstrated that RLIPC effectively lowers cardiac troponin T [1] and I [7] levels and reduces chest pain scores and ST-segment deviation (CRISP trial) [14] during percutaneous coronary intervention. In addition, scattered preclinical studies have pointed out the beneficial effect of RLIPC on myocardial reperfusion-induced arrhythmogenesis [4, 28]. In accordance with these previous publications, we found in our study that rats treated with RLIPC were less susceptible to cardiac reperfusion-induced ventricular arrhythmias. For example, 66.7% of control rats had SCD during the entire 20-min reperfusion period, while only two cases of SCD in the RLIPC-treated rats accounted for only 16.7% of the RLIPC rat population. Unfortunately, the mechanism of RLIPC-induced anti-arrhythmia is less characterized. Previously, the infarct-sparing effect of remote preconditioning of the myocardium has been studied extensively. Several substances, such as hypoxia inducible factor-1α [3], KATP channels [21], matrix metalloproteinases [23], and multiple signaling pathways [31], have been proposed to mediate RLIPC-induced anti-infarction activity. In comparison, the mechanism of RLIPC on reperfusion-induced arrhythmogenesis is less characterized. Due to different experimental protocols and animal models, the effectors and key mediators of RLIPC in the setting of infarction and arrhythmia may not be the same; therefore, identifying the mechanism of RLIPC-induced antiarrhythmogenesis will be important for optimizing better therapeutic strategies against reperfusion arrhythmia.
Cardiac troponin I (cTnI) is a specific cardiac damage marker whose elevation is associated with deteriorated cardiovascular events such as myocardial dysfunction, infarction, or arrhythmia [36]. An observational study showed that the level of cTnI was correlated with the incidence of arrhythmias [25]. Importantly, it has been reported that RLIPC could reduce cTnI release in patients undergoing cardiac surgery [29, 41]. Consistent with these reports, we found in the current study that the serum level of cTnI was elevated after reperfusion; however, RLIPC effectively ameliorated its elevation. Interestingly, earlier studies showed that catecholamines were not necessary mediators of the early phase of ventricular arrhythmias [6]. It has been shown that adverse stress, such as ischemia or hypoxia, may lead to the release of catecholamine [35]. Nevertheless, the role of catecholamines in ischemic preconditioning is controversial. Banerjee et al. found that preconditioning was initiated in an alpha 1-adrenergic-dependent manner [2]. In contrast, Weselcouch et al. showed that endogenous catecholamines were irrelevant to ischemic preconditioning in isolated rat hearts [40]. Similarly, we did not detect a difference among groups in our study regarding the serum levels of catecholamine post I/R.
The RISK (pro-survival reperfusion injury salvage kinase) [33] pathway is a vital signaling pathway associated with I/R injury. The RISK pathway consists of extracellular signal-regulated kinase (ERK) and protein kinase B (AKT), and their downstream molecule glycogen synthase kinase-3β (GSK-3β). It is clear that protective medications or stimuli such as insulin, empagliflozin [17], volatile anesthetics, or ischemic conditioning can activate or phosphorylate the key molecules in the RISK pathway [32]. In the present study, we showed that the activation of ERK1/2 (via phosphorylation) was observed in limb-preconditioned rats, while the effect of limb preconditioning against reperfusion arrhythmia was largely abolished by the ERK1/2 inhibitor U0126. This is in accordance with our previous study regarding the anti-arrhythmic effect of liver preconditioning, in which we found that liver preconditioning increased the phosphorylation of cardiac ERK1/2 [16]. In contrast to the data obtained in our liver preconditioning study, however, we failed to detect alterations in the phosphorylation status of GSK-3β in RLIPC-treated rats compared with control rats post I/R in the current study. This discrepancy indicates that liver and limb preconditioning against reperfusion arrhythmia may not share the same mechanism. Meanwhile, we did not find a significant difference in AKT phosphorylation levels among the experimental groups after reperfusion. Similar to our data, Dow et al. showed that limb preconditioning induced cardioprotection against ventricular arrhythmias without altering the p-AKT levels [9].
The SAFE (survivor activating factor enhancement) [11] pathway is another key prosurvival pathway related to I/R injury, independent of the RISK pathway. It consists of the signal transducer and activator of transcription (STAT 1–6). Similar to the RISK pathway, the activation of STATs by various cardioprotective strategies may offer promising protective therapeutic targets to ameliorate I/R injury [11, 22]. It is not clear whether the activation of both the RISK and SAFE pathways can maximize the protective effect. In fact, many researchers have shown that these two pathways can be activated simultaneously or individually during the protection process. For example, Tamareille et al. showed that both RISK and SAFE could be activated if remote limb ischemic preconditioning was exerted in combination with local ischemic postconditioning in a rat model of myocardial I/R injury [38]. We also found that remote ischemic postconditioning protected testes against testicular torsion/detorsion via the RISK and SAFE-mediated signaling pathways [18]. In contrast, in a rat model of reperfusion arrhythmia, RISK (not SAFE) was activated upon empagliflozin treatment [17]. Meanwhile, we have previously found that SAFE (but not RISK) activation was required for remote liver preconditioning-induced lung protection [26]. Moreover, Skyschally et al. further found that remote preconditioning could pass the protection across species via the activation of STAT3 [37]. Importantly, it is unknown whether limb preconditioning-induced antiarrhythmic effects occur by modulating the phosphorylation status of these two pathways. In the current study, with the use of an inhibitor, we found that the RLIPC-induced antiarrhythmic effect was accomplished by the activation of the RISK pathway (ERK1/2 phosphorylation) but not via the activation of STAT1, STAT3, or STAT5 in the SAFE pathway. Taken together, these findings suggest that SAFE is a unique pathway whose activation upon protective stimuli contributes to protection regardless of the activation of the RISK pathway.
We acknowledge the potential limitations of this study. First, we interpreted our results based on the western blot data. Other techniques, for example, transcriptome analysis such as RNA Sequencing, proteomic mass spectrometry, etc., must be used in the future for the identification of target molecules. The protein phosphorylation status may change at different time points during the reperfusion period; therefore, the activation of other proteins in the RISK or SAFE pathway cannot be absolutely excluded if the time point varies. However, we showed that U0126 could successfully abolish the cardioprotective effect in the presence of RLIPC; thus, it is logical to consider that ERK1/2 activation was a key factor in RLIPC-mediated protection against reperfusion arrhythmia. Second, we used only one RLIPC protocol in our study, and whether different limb ischemic preconditioning or postconditioning strategies confer similar protein activation needs further investigation. Third, limb preconditioning may alter the release of humoral factors or other endocrine mediators, which are capable of initiating cardioprotection. Whether ERK1/2 activation may interact with these extracardiac factors could be a future topic.
In conclusion, limb ischemic preconditioning markedly ameliorates reperfusion-induced arrhythmogenesis in rats. ERK1/2 in the RISK pathway may play a major role in the mechanism of this protection.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmed RM, el Mohamed HA, Ashraf M, Maithili S, Nabil F, Rami R, Mohamed TI (2013) Effect of remote ischemic preconditioning on serum troponin T level following elective percutaneous coronary intervention. Catheter and cardiovasc interv : Off J Soc Card Angiography Interv 82:E647-653. https://doi.org/10.1002/ccd.24825
Banerjee A, Locke-Winter C, Rogers KB, Mitchell MB, Brew EC, Cairns CB, Bensard DD, Harken AH (1993) Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circ Res 73:656–670. https://doi.org/10.1161/01.res.73.4.656
Cai Z, Luo W, Zhan H, Semenza GL (2013) Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci USA 110:17462–17467. https://doi.org/10.1073/pnas.1317158110
Chen Z, Luo H, Zhuang M, Cai L, Su C, Lei Y, Zou J (2011) Effects of ischemic preconditioning on ischemia/reperfusion-induced arrhythmias by upregulatation of connexin 43 expression. J Cardiothorac Surg 6:80. https://doi.org/10.1186/1749-8090-6-80
Cho YJ, Kim WH (2019) Perioperative cardioprotection by remote ischemic conditioning. Int J Mol Sci 20. https://doi.org/10.3390/ijms20194839
Daugherty A, Frayn KN, Redfern WS, Woodward B (1986) The role of catecholamines in the production of ischaemia-induced ventricular arrhythmias in the rat in vivo and in vitro. Br J Pharmacol 87:265–277. https://doi.org/10.1111/j.1476-5381.1986.tb10180.x
Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP (2013) Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv 6:246–251. https://doi.org/10.1161/CIRCINTERVENTIONS.112.000184
Donato M, Bin EP, V DA, Gelpi RJ, (2021) Myocardial remote ischemic preconditioning: from cell biology to clinical application. Mol Cell Biochem 476:3857–3867. https://doi.org/10.1007/s11010-021-04192-4
Dow J, Bhandari A, Simkhovich BZ, Hale SL, Kloner RA (2012) The effect of acute versus delayed remote ischemic preconditioning on reperfusion induced ventricular arrhythmias. J Cardiovasc Electrophysiol 23:1374–1383. https://doi.org/10.1111/j.1540-8167.2012.02397.x
Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, Tenenbaum A, Motro M, Adler Y (2004) Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis 172:201–210. https://doi.org/10.1016/S0021-9150(03)00238-7
Hadebe N, Cour M, Lecour S (2018) The SAFE pathway for cardioprotection: is this a promising target? Basic Res Cardiol 113:9. https://doi.org/10.1007/s00395-018-0670-5
Hausenloy DJ, Yellon DM (2008) Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79:377–386. https://doi.org/10.1093/cvr/cvn114
Hausenloy DJ, Yellon DM (2016) Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 13:193–209. https://doi.org/10.1038/nrcardio.2016.5
Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M, Dutka DP (2009) Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation 119:820–827. https://doi.org/10.1161/CIRCULATIONAHA.108.809723
Hu Z, Chen M, Zhang P, Liu J, Abbott GW (2017) Remote ischemic preconditioning differentially attenuates post-ischemic cardiac arrhythmia in streptozotocin-induced diabetic versus nondiabetic rats. Cardiovasc Diabetol 16:57. https://doi.org/10.1186/s12933-017-0537-3
Hu Z, Hu S, Yang S, Chen M, Zhang P, Liu J, Abbott GW (2016) Remote liver ischemic preconditioning protects against sudden cardiac death via an ERK/GSK-3beta-dependent mechanism. PLoS ONE 11:e0165123. https://doi.org/10.1371/journal.pone.0165123
Hu Z, Ju F, Du L, Abbott GW (2021) Empagliflozin protects the heart against ischemia/reperfusion-induced sudden cardiac death. Cardiovasc Diabetol 20:199. https://doi.org/10.1186/s12933-021-01392-6
Hu Z, Liu Q, Yan Z, Wang Q, Liu J (2022) Protective effect of remote ischemic postconditioning in rat testes after testicular torsion/detorsion. Andrology. https://doi.org/10.1111/andr.13184
Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J (2005) Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol 100:404–412. https://doi.org/10.1007/s00395-005-0539-2
Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883. https://doi.org/10.1161/01.cir.0000043806.51912.9b
Kristiansen SB, Henning O, Kharbanda RK, Nielsen-Kudsk JE, Schmidt MR, Redington AN, Nielsen TT, Botker HE (2005) Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol 288:H1252-1256. https://doi.org/10.1152/ajpheart.00207.2004
Lecour S (2009) Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol 47:32–40. https://doi.org/10.1016/j.yjmcc.2009.03.019
Li SJ, Wu YN, Kang Y, Yin YQ, Gao WZ, Liu YX, Lou JS (2010) Noninvasive limb ischemic preconditioning protects against myocardial I/R injury in rats. J Surg Res 164:162–168. https://doi.org/10.1016/j.jss.2009.03.017
Lim SY, Hausenloy DJ (2012) Remote ischemic conditioning: from bench to bedside. Front Physiol 3:27. https://doi.org/10.3389/fphys.2012.00027
Liu Z, Cui L, Wang Y, Guo Y (2006) Cardiac troponin I and ventricular arrhythmia in patients with chronic heart failure. Eur J Clin Invest 36:466–472. https://doi.org/10.1111/j.1365-2362.2006.01655.x
Luo N, Liu J, Chen Y, Li H, Hu Z, Abbott GW (2018) Remote ischemic preconditioning STAT3-dependently ameliorates pulmonary ischemia/reperfusion injury. PLoS ONE 13:e0196186. https://doi.org/10.1371/journal.pone.0196186
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136. https://doi.org/10.1161/01.cir.74.5.1124
Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B (1997) Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol 273:H1707-1712. https://doi.org/10.1152/ajpheart.1997.273.4.H1707
Payne RE, Aldwinckle J, Storrow J, Kong RS, Lewis ME (2015) RIPC remains a promising technique for protection of the myocardium during open cardiac surgery: a meta-analysis and systematic review. Heart Surg Forum 18:E074-080. https://doi.org/10.1532/hsf.1251
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899. https://doi.org/10.1161/01.cir.87.3.893
Randhawa PK, Bali A, Jaggi AS (2015) RIPC for multiorgan salvage in clinical settings: evolution of concept, evidences and mechanisms. Eur J Pharmacol 746:317–332. https://doi.org/10.1016/j.ejphar.2014.08.016
Rossello X, Yellon DM (2016) A critical review on the translational journey of cardioprotective therapies! Int J Cardiol 220:176–184. https://doi.org/10.1016/j.ijcard.2016.06.131
Rossello X, Yellon DM (2018) The RISK pathway and beyond. Basic Res Cardiol 113:2. https://doi.org/10.1007/s00395-017-0662-x
Schmidt MR, Sloth AD, Johnsen J, Botker HE (2012) Remote ischemic conditioning: the cardiologist’s perspective. J Cardiovasc Med 13:667–674. https://doi.org/10.2459/JCM.0b013e328357bff2
Schomig A, Haass M, Richardt G (1991) Catecholamine release and arrhythmias in acute myocardial ischaemia. Eur Heart J 12(Suppl F):38–47. https://doi.org/10.1093/eurheartj/12.suppl_f.38
Skeik N, Patel DC (2007) A review of troponins in ischemic heart disease and other conditions. Int J Angiol : Off Publ Int Coll Angiol Inc 16:53–58. https://doi.org/10.1055/s-0031-1278248
Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G (2015) Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117:279–288. https://doi.org/10.1161/CIRCRESAHA.117.306878
Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croue A, Henrion D, Furber A, Prunier F (2011) RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol 106:1329–1339. https://doi.org/10.1007/s00395-011-0210-z
Wang YP, Maeta H, Mizoguchi K, Suzuki T, Yamashita Y, Oe M (2002) Intestinal ischemia preconditions myocardium: role of protein kinase C and mitochondrial K(ATP) channel. Cardiovasc Res 55:576–582. https://doi.org/10.1016/s0008-6363(02)00245-6
Weselcouch EO, Baird AJ, Sleph PG, Dzwonczyk S, Murray HN, Grover GJ (1995) Endogenous catecholamines are not necessary for ischaemic preconditioning in the isolated perfused rat heart. Cardiovasc Res 29:126–132
Yang L, Wang G, Du Y, Ji B, Zheng Z (2014) Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth 28:682–689. https://doi.org/10.1053/j.jvca.2013.05.035
Yang S, Abbott GW, Gao WD, Liu J, Luo C, Hu Z (2017) Involvement of glycogen synthase kinase-3beta in liver ischemic conditioning induced cardioprotection against myocardial ischemia and reperfusion injury in rats. J Appl Physiol 122:1095–1105. https://doi.org/10.1152/japplphysiol.00862.2016
Funding
This work was supported by Sichuan University West China Hospital setup funding to ZH.
Author information
Authors and Affiliations
Contributions
ZH designed the study. XC, HL, ZY, and ZH performed the experiments. XC, HL, ZY, JL, and ZH conducted acquisition, analysis, or interpretation of data for the work. ZH drafted the manuscript. All the authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was reviewed and approved by the Institutional Animal Care and Use Committee of Sichuan University (Approval No. 2015035A).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, X., Li, H., Yan, Z. et al. Ischemic limb preconditioning-induced anti-arrhythmic effect in reperfusion-induced myocardial injury: is it mediated by the RISK or SAFE pathway?. Pflugers Arch - Eur J Physiol 474, 979–991 (2022). https://doi.org/10.1007/s00424-022-02716-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-022-02716-5