Abstract
Chemical stimulation of kidney causes sympathetic activation and pressor responses in rats. The excitatory renal reflex (ERR) is mediated by angiotensin type 1 receptor (AT1R) and superoxide anions in hypothalamic paraventricular nucleus (PVN). The aim of this study is to determine whether interleukin-1β (IL-1β) in the PVN mediates the ERR, and whether the IL-1β production in the PVN is dependent on the AT1R-superoxide anion signaling. Experiments were performed in adult rats under anesthesia. The ERR was induced by renal infusion of capsaicin, and evaluated by the responses of the contralateral renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP). Inhibition of IL-1β production with MCC950 in the PVN dose-dependently inhibited the capsaicin-induced ERR and sympathetic activation. The PVN microinjection of IL-1 receptor antagonist IL-1Ra or specific IL-1β antibody abolished the capsaicin-induced ERR, while IL-1β enhanced the ERR. Renal infusion of capsaicin promoted p65-NFκB phosphorylation and IL-1β production in the PVN, which were prevented by PVN microinjection of NADPH oxidase inhibitor apocynin or the superoxide anion scavenger tempol. The PVN microinjection of NFκB inhibitor BMS-345541 abolished the capsaicin induced-ERR and IL-1β production, but not the NADPH oxidase activation and superoxide anion production. Furthermore, capsaicin-induced p65-NFκB phosphorylation and IL-1β production in the PVN were prevented by AT1R antagonist losartan, or angiotensin converting enzyme inhibitor captopril. These results indicate that capsaicin-induced ERR and sympathetic activation are mediated by IL-1β in the PVN. The IL-1β production in the PVN is dependent on the AT1R-mediated superoxide anion generation and NFκB activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sympathetic nervous system hyperactivity is closely associated with various diseases, such as hypertension, chronic kidney disease, myocardial ischemia, and chronic heart failure [6, 14]. The kidneys are richly innervated by efferent nerve and afferent sensory nerve, and the afferent activity from chemoreceptors and baroreceptors in the kidney to the brain plays major roles in modulating sympathetic outflow and blood pressure [2, 18]. Afferents in the ischemic kidney contribute to hypertension and renal dysfunction in rat model of renovascular hypertension [20]. Our recent studies have shown that chemical stimulation of the kidney in rats with capsaicin activates renal afferents and causes an excitatory renal reflex (ERR), which results in pressor response and sympathetic activation [40].
Paraventricular nucleus (PVN) of hypothalamus is an important integration center for regulating blood pressure and sympathetic activity [33, 41, 48]. The PVN receives various afferent activities and sends descending fibers to the rostral ventrolateral medulla (RVLM) and the intermediolateral column (IML) of spinal cord for control of sympathetic activity and blood pressure. The integration of inhibitory inputs and excitatory inputs in the PVN is responsible for the sympathetic overactivity in hypertension [5, 36, 47]. It has been found that renal afferent activity induces the excitation of some PVN neurons [37] and increases sympathetic activity [47]. We have found that bilateral PVN microinjection of kainic acid to destroy the neurons in the PVN abolished the capsaicin-induced ERR [40]. These findings indicate that the PVN is crucial in regulating the ERR and sympathetic activity.
Angiotensin II (Ang II), reactive oxygen species (ROS), and interleukin-1β (IL-1β) in the PVN are involved in the control of sympathetic outflow and blood pressure [22, 30, 42]. Scavenge excessive ROS attenuates inflammation via inhibiting NOD-like receptor protein 3 (NLRP3) inflammasome activation in several peripheral tissue and organs [32]. Recently, we have shown that ERR is mediated by angiotensin type 1 receptor (AT1R) and superoxide anions in the PVN [28]. However, it is unknown whether IL-1β in the PVN is involved in mediating the capsaicin-induced ERR, and whether the IL-1β production in the PVN depends on the AT1R-mediated superoxide anion generation. This study is to determine the roles and mechanisms of IL-1β in the PVN in mediating the capsaicin-induced ERR and sympathetic activation.
Methods
Animals
Male SD rats weighing between 300 and 340 g were used in this study. Procedures were authorized by Experimental Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Laboratory Animals (NIH). The rats were caged in a temperature-controlled room with 12:12-h light-dark cycle. Laboratory chow and tap water were available ad libitum.
General procedures
Rats were anesthetized with a mixture of α-chloralose (40 mg/kg) and urethane (800 mg/kg) via intraperitoneal injection. The depth of anesthesia was evaluated by the corneal reflex and the paw withdrawal reflex [44]. The rats were kept in supine position, and a vertical incision in the middle of the neck was made to expose the trachea and carotid artery. Endotracheal intubation was performed for positive pressure ventilation with room air using a ventilator (51600, Stoelting, Chicago, IL, USA). A PE50 catheter was inserted into the right common carotid artery for continuous blood pressure recording using an 8SP PowerLab system with data acquisition software (ADInstruments Inc., Bella Vista, NSW, Australia). The MAP was simultaneously recorded with RSNA. The rats were allowed to stabilize more than 30 min before experimental intervention. At the end, the rat was euthanized with rapid intravenous injection of pentobarbital sodium (100 mg/kg).
RSNA recording
RSNA was continuously recorded as we reported previously [40]. A left flank incision was made in the rat with prone position to expose renal nerve. The nerve was isolated and cut at the distal end to eliminate its afferent activity. The renal nerve was put on a pair of platinum electrodes and immersed in paraffin oil at 37 °C. The signals of the renal nerve were amplified using a Warner Instrument 4-Channel Differential Amplifier (Hamden, CT, USA) filtered with a band-pass between 100 and 3000 Hz. The RSNA was integrated using LabChart 8 software (ADInstruments Inc.) at a 100-ms time constant. The RSNA data were obtained by deducting the background noise, which were calculated as the difference of the values before and after cutting the central end of the nerve.
Evaluation of the ERR
Renal infusion of capsaicin was used to induce ERR in rats as we previously described [40]. In brief, the right kidney was exposed via a flank incision. A stainless steel tube with 0.31-mm outer diameter was inserted into the kidney horizontally from the lateral margin to the renal hilum. The tube tip was kept at the border of the cortex and medulla of the kidney, where the tube met a slight resistance. A PE50 catheter was used to connect the tube with a programmable pressure injector (PM2000B, MicroData Instrument, NJ, USA). ERR was induced by renal infusion of capsaicin at 1.0 μL/min (1 nmol/μL) for 20 min. The same amount of vehicle was used for control. The RSNA and MAP responses to capsaicin were used to evaluate the ERR.
PVN microinjection
Rat in a prone position was fixed with a stereotaxic frame (Stoelting Co., Chicago, IL, USA). According to the Paxinos and Watson rat atlas, the microinjection site is 0.4 mm lateral to the midline, 1.8 mm caudal to the bregma, and 7.9 mm below the skull surface. Bilateral PVN microinjections (50 nL for each side of the PVN) were carried out within 1 min with glass micropipettes (tip size 50 μM). Equal volume of Evans Blue was microinjected into the PVN for histological identification of the microinjection sites at the end of the experiment. The data were excluded for these rats whose microinjection sites were outside the PVN.
Measurement of NADPH oxidase activity and superoxide anion content
Coronal sections of the brain at the PVN level were made at a 450-μM thickness using a cryostat microtome (CM1900, Leica Instruments, Wetzlar, Germany). The bilateral PVN in the sections was punched out with a 15-gauge needle. The punched PVN tissues were homogenized and then centrifuged in RIPA lysis buffer. The total protein concentration in the supernatant was measured using a BCA protein assay kit (Pierce, Rockford, IL, USA). Lucigenin-derived chemiluminescence was used to determine NADPH oxidase activity [7, 29, 38] and superoxide anion content [16, 19, 27]. Briefly, the photon emission was started by adding both dark-adapted lucigenin (5 μM) and NADPH (100 μM) for measurement of NADPH oxidase activity, and by adding dark-adapted lucigenin (5 μM) for measurement of superoxide anion content. Light emission was measured using a model TD-20/20 luminometer (Turner Biosystems, Sunnyvale, CA, USA) ten times within 10 min. The chemiluminescence value of the buffer containing 5 μM of lucigenin was measured as background value. Both NADPH oxidase activity and superoxide anion content were expressed as relative mean light unit (MLU) per minute and milligram of protein.
Western blot analysis
The protein expressions of IL-1β, TNF-α, p65, and phosphorylated p65 (P-p65) were examined with Western blot analysis. Protein was separated with SDS-PAGE and then transferred onto a polyvinylidene difluoride (PVDF) membrane. IL-1β antibody was obtained from Abcam (Cambridge, MA, USA). P-p65 antibody was purchased from Affinity Biosciences (Cincinnati, OH, USA). Antibody against TNF-α, p65, β-actin, and peroxidase-conjugated goat anti-rabbit secondary antibody was bought from ProteinTech Group (Rosemont, IL, USA). The immunoblots were visualized with an enhanced chemiluminescence reagent (Millipore Corporation, Billerica, MA, USA). IL-1β and TNF-α levels were expressed as the ratio to β-actin levels. The P-p65 protein level was normalized to the p65 protein.
Chemicals
Capsaicin and MCC950 were purchased from MedChemExpress (Monmouth Junction, NJ, USA). IL-1β was acquired from PeproTech Chemical (Rocky Hill, NJ, USA). BMS-345541 was bought from Selleck Chemicals (Houston, TX, USA). Losartan, captopril, tempol, apocynin, and IL-1Ra were obtained from Sigma (St Louis, MO, USA). Capsaicin, captopril, or apocynin were dissolved in the PBS containing 1% DMSO, and other chemicals were dissolved in the PBS.
Statistics
RSNA and MAP changes were determined by a 1-min average of the maximal responses to chemicals. All the data were expressed as mean ± SE. One-way and two-way ANOVA were used for multiple comparisons followed by Bonferroni’s post hoc analysis. The diffidence in the baseline RSNA between the values before the PVN microinjection and the values after the microinjection was statistically analyzed with a paired t test. A P value less than 0.05 was considered to be statistically significant.
Results
Dose-effects of MCC950 on ERR
MCC950, a NLRP3 inflammasome inhibitor, was used to determine whether the production of inflammatory cytokines might be involved in the ERR. The renal infusion of vehicle or capsaicin was carried out 10 min after the pretreatment with bilateral PVN microinjection of PBS or different doses of MCC950 (1, 10, or 100 pmol). Low dose of MCC950 increased baseline renal sympathetic nerve activity (RSNA) and mean arterial pressure (MAP), but moderate and high dose of MCC950 had no significant effects on the baseline RSNA and MAP (Fig. 1a, Fig. S1). However, the MCC950 dose-dependently inhibits the ERR evidenced by the reduced RSNA and MAP responses to capsaicin (Fig. 1b). Representative recordings showed that the ERR that was elicited by capsaicin lasts about 30 min (Fig. 2a), which was prevented by the pretreatment with high dose of MCC950 (Fig. 2b). These results suggest that the production of inflammatory cytokines in the PVN is involved in the signal transduction in capsaicin-induced ERR.
Effects of PVN microinjection of PBS, three different doses of MCC950 (1, 10, 100 pmol). a Baseline RSNA and MAP changes induced by the PVN microinjection of MCC950. b Effects of PVN microinjection of MCC950 on excitatory renal reflex. The reflex was induced by renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min. The PVN microinjection was carried out 10 min before renal infusion. * P < 0.05 vs PBS. Values are mean ± SE. n = 6 per group
Representative recordings showing capsaicin-induced excitatory renal reflex. a Renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min causes reflex changes of arterial blood pressure (ABP), mean arterial pressure (MAP), and contralateral renal sympathetic nerve activity (RSNA). b Effect of pretreatment with PVN microinjection of MCC950 (100 pmol) or IL-1β (0.5 pmol) on capsaicin-induced excitatory renal reflex. The pretreatment was carried out 10 min before renal infusion of vehicle (Veh) or capsaicin
Roles of IL-1β in ERR
IL-1β is an important inflammatory cytokine. The roles of IL-1β in the PVN were evaluated by the effects of the PVN microinjection of IL-1 receptor antagonist IL-1Ra, specific IL-1β antibody, and IL-1β on the ERR. The renal infusion of vehicle or capsaicin was performed 10 min after the pretreatment with bilateral PVN microinjection of PBS, IL-1 receptor antagonist (IL-1Ra, 10 pmol), IL-1β (0.5 pmol), control antibody (50 ng), or IL-1β antibody (50 ng). The PVN microinjection of IL-1Ra, IL-1β antibody had no significant effects on baseline RSNA and MAP, but IL-1β increased baseline RSNA and MAP (Fig. 3a, Fig. S2). The capsaicin-induced ERR was abolished by the PVN microinjection of IL-1Ra or IL-1β antibody, but enhanced by IL-1β (Fig. 3b). These results indicate that IL-1β in the PVN enhances sympathetic activity and ERR, and endogenous IL-1β in the PVN is responsible for mediating the ERR.
Effects of PVN microinjection of IL-1Ra, IL-1β, and IL-1β antibody on excitatory renal reflex. a Baseline RSNA and MAP changes caused by PVN microinjection of IL-1Ra (10 pmol), IL-1β (0.5 pmol), or IL-1β antibody (Ab, 50 ng). *P < 0.05 vs PBS. b Effects of PVN microinjection of IL-1Ra (10 pmol), IL-1β (0.5 pmol), or IL-1β Ab (50 ng) on excitatory renal reflex. The reflex was induced by renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min. The PVN microinjection was carried out 10 min before renal infusion. *P < 0.05 vs PBS + Veh; †P < 0.05 vs PBS + Cap or Ctrl Ab + Cap. Values are mean ± SE. n = 6 per group
Effects of losartan and captopril on capsaicin-induced IL-1β and TNF-α changes in PVN
Recent studies in our lab have shown that PVN microinjection of losartan, an AT1R antagonist, or captopril, an angiotensin converting enzyme (ACE) inhibitor, prevented the capsaicin-induced ERR, indicating that Ang II and AT1 receptors in the PVN mediating the ERR [28]. It is interesting to know whether capsaicin-induced ERR could increase the IL-1β and TNF-α levels in the PVN, and whether losartan and captopril could inhibit the ERR-related IL-1β and TNF-α changes in the PVN. Bilateral PVN microinjection of PBS, 1% DMSO, losartan (10 nmol), or captopril (10 nmol) was carried out 10 min before the renal infusion of vehicle or capsaicin for 20 min. The measurements were carried out at the end of the infusion. Renal infusion of capsaicin increased IL-1β level, but not TNF-α level in the PVN. The capsaicin-induced IL-1β production was prevented by the PVN microinjection of losartan or captopril (Fig. 4a and b). These results indicate that IL-1β is a downstream signal molecule of Ang II in mediating the capsaicin-induced ERR.
Effects of PVN microinjection of losartan and captopril on capsaicin-induced IL-1β and TNF-α changes in the PVN. a Representative images showing IL-1β and TNF-α expression. b Bar graphs showing IL-1β and TNF-α expression in the PVN. Renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min was used to induce excitatory renal reflex. PVN microinjection of PBS, 1% DMSO, losartan (10 nmol), or captopril (10 nmol) was carried out 10 min before the renal infusion. The measurements were carried out at the end of the renal infusion. *P < 0.05 vs Veh; †P < 0.05 vs PBS or DMSO. Values are mean ± SE. n = 5 per group
Effects of tempol and apocynin on capsaicin-induced IL-1β and TNF-α changes in PVN
We have shown that PVN microinjection of tempol, a superoxide anion scavenger, or apocynin, an NADPH oxidase inhibitor, prevented the capsaicin-induced ERR. Ang II mediates the ERR via the AT1R-NADPH oxidase-superoxide anions signal pathway [28]. We wonder whether NADPH oxidase activation and the superoxide anion production might be responsible for the capsaicin-induced IL-1β production in the PVN. Bilateral PVN microinjection of PBS, 1% DMSO, tempol (20 nmol), or apocynin (1 nmol) was carried out 10 min before the renal infusion of vehicle or capsaicin for 20 min. The measurements were carried out at the end of the infusion. The capsaicin-induced IL-1β production was prevented by the PVN microinjection of tempol or apocynin (Fig. 5a and b). These results indicate that IL-1β is a downstream signal molecule of Ang II-AT1R-NADPH oxidase-superoxide anions signal pathway in the ERR.
Effects of PVN microinjection of tempol and apocynin on capsaicin-induced IL-1β and TNF-α changes in the PVN. a Representative images showing IL-1β and TNF-α expression. b Bar graphs showing IL-1β and TNF-α expression in the PVN. Renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min was used to induce excitatory renal reflex. PVN microinjection of PBS, 1% DMSO, tempol (20 nmol), or apocynin (1 nmol) was carried out 10 min before the renal infusion. The measurements were carried out at the end of the renal infusion. *P < 0.05 vs Veh; †P < 0.05 vs PBS or DMSO. Values are mean ± SE. n = 5 per group
Effects of BMS-345541 on ERR
It is well known that ROS is an important factor to activate NFκB [13, 25, 45], while the activation of the NFκB contributes to inflammation and autoimmune in various inflammatory diseases [12, 26, 43]. We suspect that NFκB in the PVN may be involved in the ERR. Renal infusion of vehicle or capsaicin was conducted 10 min after the pretreatment with bilateral PVN microinjection of PBS or BMS-345541 (100 pmol), an NFκB inhibitor. The PVN microinjection of BMS-345541 had no significant effects on the baseline RSNA and MAP (Fig. 6a, Fig. S3), but almost abolished the capsaicin-induced sympathetic activation and pressor responses (Fig. 6B). These results suggest that NFκB in the PVN is involved in the ERR.
Effects of PVN microinjection of BMS-345541 on excitatory renal reflex. a Baseline RSNA and MAP changes caused by PVN microinjection of BMS-345541 (100 pmol). b Effects of PVN microinjection of BMS-345541 on excitatory renal reflex. The reflex was induced by renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min. The PVN microinjection was carried out 10 min before renal infusion. *P < 0.05 vs PBS + Veh; †P < 0.05 vs PBS + Cap. Values are mean ± SE. n = 6 per group
Effects of BMS-345541 on ROS and inflammatory cytokine production
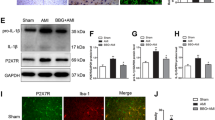
BMS-345541 was used to determine the upstream and downstream signals of the NFκB in the PVN in capsaicin-induced ERR. Renal infusion of vehicle or capsaicin was performed 10 min after pretreatment with bilateral PVN microinjection of PBS or BMS-345541 (100 pmol). ROS and inflammatory cytokine production were examined immediately after finishing the renal infusion. Bilateral PVN microinjection of BMS-345541 had no significant effects on NADPH oxidase activity and superoxide anion production in the rats with renal infusion of vehicle or capsaicin (Fig. 7a). However, BMS-345541 prevented capsaicin-induced increase in the IL-1β production (Fig. 7b). These data suggest that ROS is the upstream signal of NFκB, while IL-1β is the downstream signal of NFκB.
Effects of PVN microinjection of BMS-345541 on capsaicin-induced oxidative stress and inflammatory cytokine production in the PVN. a superoxide anion production and NADPH oxidase activity in the PVN. b IL-1β and TNF-α levels in the PVN. Renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min was used to induce excitatory renal reflex. PVN microinjection of PBS or BMS-345541 (100 pmol) was carried out 10 min before the renal infusion. The measurements were carried out at the end of the renal infusion. *P < 0.05 vs Veh; †P < 0.05 vs PBS. Values are mean ± SE. n = 5 per group
Effects of losartan, captopril, tempol, and apocynin on p65 phosphorylation
In order to further confirm the upstream signals of NFκB in the PVN, bilateral PVN microinjections of PBS, 1% DMSO, losartan (10 nmol), captopril (10 nmol), tempol (20 nmol), or apocynin (1 nmol) were carried out 10 min before the renal infusion of capsaicin. The phosphorylated p65 (P-p65) level, an index for NFκB activation, was examined immediately after finishing the renal infusion. The renal infusion of capsaicin promoted the phosphorylation of p65 in the PVN, which were prevented not only by the PVN pretreatment with losartan or captopril (Fig. 8a) but also by the pretreatment with tempol or apocynin (Fig. 8b).
Effects of PVN microinjection of losartan, captopril, tempol, and apocynin on capsaicin-induced phosphorylation in the PVN. a Effects of PVN microinjection of losartan and captopril. b Effects of PVN microinjection of tempol and apocynin. Renal infusion of capsaicin (Cap) at 1 nmol/min for 20 min was used to induce excitatory renal reflex. PVN microinjection of PBS, 1% DMSO, losartan (10 nmol), captopril (10 nmol), tempol (20 nmol), or apocynin (1 nmol) was carried out 10 min before the renal infusion. The measurements were carried out at the end of the renal infusion. *P < 0.05 vs Veh; †P < 0.05 vs PBS or DMSO. Values are mean ± SE. n = 5 per group
Discussion
Chemical stimulation of the kidney induced an ERR, which causes sympathetic activation and pressor responses, and the PVN of hypothalamus is an important central area for the integration of the ERR [40]. Ang II in the PVN mediated the reflex via AT1 receptor-dependent NADPH oxidase activation and subsequent superoxide anion production [28]. The primary novel findings in this study are that IL-1β in the PVN mediates the capsaicin-induced ERR and the IL-1β production within the PVN is caused by AT1R-dependent superoxide anion generation and subsequent NFκB activation.
Inflammatory cytokines in the PVN are involved in regulating sympathetic activity and blood pressure [1, 23, 39]. IL-1β in the PVN enhances Ang II-induced pressor response [21]. MCC950 is a specific and potent small-molecule NLRP3 inhibitor, and is a potential therapeutic for inflammatory and autoimmune diseases [8, 9]. A variety of studies showed that MCC950 inhibits the production of active IL-1β [15, 24, 46]. In the present study, The PVN microinjection of MCC950, IL-1Ra, or specific IL-1β antibody abolished the capsaicin-induced ERR, while IL-1β enhanced the ERR. Renal infusion of capsaicin increased IL-1β content in the PVN. The capsaicin-induced increase in IL-1β content in the PVN was abolished by the PVN microinjection of the AT1 receptor antagonist, ACE inhibitor, NADPH oxidase inhibitor, or ROS scavenger. These results indicate that the IL-1β in the PVN mediates the ERR. The IL-1β production induced by capsaicin is dependent on the AT1R-superoxide anion signal pathway in the PVN, which is supported by the fact that blockade of AT1R or scavenge of superoxide anions in the PVN abolished the capsaicin-induced ERR [28]. It is noted that renal infusion of capsaicin had no significant effects on TNF-α content in the PVN. Blockage of Ang II, scavenge of ROS, or inhibition of NFκB reduced IL-1β production without significant effect on TNF-α production. The results suggest that capsaicin-induced IL-1β production in the PVN may serve as a signal molecule to cause sympathetic activation and pressor responses, while TNF-α is not involved in mediating the ERR. It is noted that moderate or high dose of MCC950 had no significant effects on the baseline RSNA and MAP, but lowest dose of MCC950 caused a slight sympathetic activation and pressor response. The mechanism is not known. There is a possibility that MCC950 in the PVN might have a mild direct role in activating presympathetic neurons in the PVN, which is independent of the IL-1β inhibition. Moderate or high dose of MCC950 had a strong inhibitory effect on the IL-1β production, which blocked the sympatho-excitatory roles of peripheral inputs, and thereby counteracted its direct sympathetic activation. This hypothesis needs further experimental verification.
NFκB activation contributes to the ROS-induced IL-1β production [3, 35]. The activated NFκB promotes NLRP3 inflammasome activation and subsequent IL-1β production [17, 31]. The NFκB dimer p50/p65 phosphorylation leads to NFκB translocate to the nucleus and the subsequent IL-1β production [34]. Blockade of NFκB in the PVN inhibits peripheral Ang II-induced increased in blood pressure and IL-1β mRNA level [4]. In the present study, inhibition of NFκB with BMS-345541 prevents capsaicin-induced ERR, sympathetic activation and pressor responses, and the increase in IL-1β content in the PVN. However, BMS-345541 had no significant effects on the capsaicin-induced increases in the superoxide anion content and NADPH oxidase activity. Furthermore, the PVN pretreatment with losartan, captopril, tempol, or apocynin abolished the capsaicin-induced phosphorylation of p65-NFκB. These results indicate that capsaicin-induced ERR is mediated by the ROS-NFκB-IL-1β signal pathway in the PVN.
Capsaicin is well known to stimulate afferents and induces excitation at low concentration, but capsaicin causes transient excitation followed by denervation at high concentration. The concentration of capsaicin (1 nmol/μL) in this study was much lower than the concentration of capsaicin (33 nmol/μL) used for denervation in the previous studies [10, 11]. There may be a possibility that the actual capsaicin concentration in the local renal tissue may be greatly diluted because of abundant blood flow in the kidney. The effects of capsaicin used in the present study was caused by the stimulation of renal afferents rather than injury of renal afferents, which was evidenced by our recent study that renal infusion of the same dose of capsaicin increased ipsilateral renal afferent activity within the 20 min of infusion, and the capsaicin-induced ERR was abolished by ipsilateral renal denervation [40]. It is noted that renal infusion of capsaicin only stimulates some renal afferents near the infusion site, but not all the afferent nerve fibers in the kidney. Our recent study showed that renal infusion of capsaicin to the medulla, cortico-medullary border, or cortex of the kidney caused similar sympathetic activation and pressor responses. Infusion of capsaicin into the upper, lateral, or lower parts of the kidney also showed similar sympathetic activation and pressor effects. The ERR can be induced by renal infusion of some other chemicals such as bradykinin, adenosine, or angiotensin II [40]. Although direct infusion of capsaicin into the renal tissue is an unphysiological way of stimulation, local renal infusion was selected to induced the ERR instead of renal artery infusion in the present study based on the following reasons. (1) The afferent and efferent fibers of the kidney must be kept intact in examining the ERR. Any damage of the renal nerves will affect the effectiveness in the evaluation of the reflex. However, the surgical procedure of renal artery infusion via catheter is hard to avoid the damage of the renal fibers in a certain extent. (2) The kidney is very sensitive to blood flow. Infusion in renal artery or its branch will reduce the renal blood flow or the local blood flow in the kidney. It is difficult to determine whether the effects were caused by the capsaicin or by the reduced blood flow due to the artery infusion. (3) Renal artery infusion will cause more capsaicin circulating in the blood of the whole body rather than localizing in the kidney. It is known that much higher dose is needed for the infusion in artery compared with local infusion. It is difficult to exclude the possible effects of the capsaicin in the circulation completely. Therefore, infusion of capsaicin into the renal tissue may be better than infusion of capsaicin in the renal artery in examine the ERR.
In conclusion, capsaicin-induced ERR is mediated by IL-1β in the PVN where Ang II acts on AT1R, and in turn activates NADPH oxidase, leading to the superoxide anion production and subsequent NFκB activation in the PVN. The activated NFκB promotes IL-1β production in the PVN and then causes sympathetic activation and pressor responses.
References
Bai J, Yu XJ, Liu KL, Wang FF, Li HB, Shi XL, Zhang Y, Huo CJ, Li X, Gao HL, Qi J, Liu JJ, Zhu GQ, Chen WS, Cui W, Kang YM (2017) Tert-butylhydroquinone attenuates oxidative stress and inflammation in hypothalamic paraventricular nucleus in high salt-induced hypertension. Toxicol Lett 281:1–9
Bie P, Evans RG (2017) Normotension, hypertension and body fluid regulation: brain and kidney. Acta Physiol (Oxf) 219:288–304
Brigelius-Flohe R, Banning A, Kny M, Bol GF (2004) Redox events in interleukin-1 signaling. Arch Biochem Biophys 423:66–73
Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J (2012) Angiotensin II-induced hypertension is modulated by nuclear factor-κB in the paraventricular nucleus. Hypertension 59:113–121
Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM, Zhu GQ (2015) Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf) 213:778–794
Cheng ZJ, Wang R, Chen QH (2019) Autonomic rregulation of the cardiovascular system: diseases, treatments, and novel approaches. Neurosci Bull 35:1–3
Ciarcia R, Damiano S, Florio A, Spagnuolo M, Zacchia E, Squillacioti C, Mirabella N, Florio S, Pagnini U, Garofano T, Polito MS, Capasso G, Giordano A (2015) The protective effect of apocynin on cyclosporine A-induced hypertension and nephrotoxicity in rats. J Cell Biochem 116:1848–1856
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255
Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB, Schroder K (2019) MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol 15:556–559
Fitzgerald M (1983) Capsaicin and sensory neurones--a review. Pain 15:109–130
Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW (2015) A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308:R112–R122
Ghosh S, Hayden MS (2008) New regulators of NF-kB in inflammation. Nat Rev Immunol 8:837–848
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505
Grassi G, Ram VS (2016) Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens 10:457–466
Guo C, Fu R, Wang S, Huang Y, Li X, Zhou M, Zhao J, Yang N (2018) NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin Exp Immunol 194:231–243
Harms JE, Kuczmarski JM, Kim JS, Thomas GD, Kaufman MP (2017) The role played by oxidative stress in evoking the exercise pressor reflex in health and simulated peripheral artery disease. J Physiol 595:4365–4378
Huang Z, Zhuang X, Xie C, Hu X, Dong X, Guo Y, Li S, Liao X (2016) Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell Physiol Biochem 40:1578–1590
Kopp UC (2015) Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol 308:R79–R95
Li Y, Zhu H, Kuppusamy P, Zweier JL, Trush MA (2016) Mitochondrial electron transport chain-derived superoxide exits macrophages: implications for mononuclear cell-mediated pathophysiological processes. React Oxyg Species 1:81–98
Lopes NR, Milanez MIO, Martins BS, Veiga AC, Ferreira GR, Gomes GN, Girardi AC, Carvalho PM, Nogueira FN, Campos RR, Bergamaschi CT, Nishi EE (2020) Afferent innervation of the ischemic kidney contributes to renal dysfunction in renovascular hypertensive rats. Pflugers Arch 472:325–334
Lu Y, Chen J, Yin X, Zhao H (2009) Angiotensin II receptor 1 involved in the central pressor response induced by interleukin-1 beta in the paraventricular nucleus. Neurol Res 31:420–424
Lu P, Jiang SJ, Pan H, Xu AL, Wang GH, Ma CL, Shi Z (2018) Short hairpin RNA interference targeting interleukin 1 receptor type I in the paraventricular nucleus attenuates hypertension in rats. Pflugers Arch 470:439–448
Lu P, Liang LW, Xu AL, Sun YY, Jiang SJ, Shi Z (2020) Pro-inflammatory cytokines in the paraventricular nucleus mediate the adipose afferent reflex in rats. Pflugers Arch 472:343–354
Luo Y, Lu J, Ruan W, Guo X, Chen S (2019) MCC950 attenuated early brain injury by suppressing NLRP3 inflammasome after experimental SAH in rats. Brain Res Bull 146:320–326
Nakajima S, Kitamura M (2013) Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med 65:162–174
Park MH, Hong JT (2016) Roles of NF-kB in cancer and inflammatory diseases and their therapeutic approaches. Cells 5:E15
Pereira SC, Parente JM, Belo VA, Mendes AS, Gonzaga NA, do Vale GT, Ceron CS, Tanus-Santos JE, Tirapelli CR, Castro MM (2018) Quercetin decreases the activity of matrix metalloproteinase-2 and ameliorates vascular remodeling in renovascular hypertension. Atherosclerosis 270:146–153
Qiu Y, Zheng F, Ye C, Chen AD, Wang JJ, Chen Q, Li YH, Kang YM, Zhu GQ (2020) Angiotensin type 1 receptors and superoxide anion production in hypothalamic paraventricular nucleus contribute to capsaicin-induced excitatory renal reflex and sympathetic activation. Neurosci Bull 36:463–474
Sheu ML, Shen CC, Chen YS, Chiang CK (2017) Ochratoxin A induces ER stress and apoptosis in mesangial cells via a NADPH oxidase-derived reactive oxygen species-mediated calpain activation pathway. Oncotarget 8:19376–19388
Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ (2011) Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 203:289–297
Shimogaki S, Ito S, Komatsu S, Koike R, Miyasaka N, Umezawa K, Kubota T (2014) Inhibition of the NF-κB pathway as a candidate therapeutic strategy for cryopyrin-associated periodic syndrome. Mod Rheumatol 24:517–524
Sho T, Xu J (2019) Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol Appl Biochem 66:4–13
Sun J, Ren XS, Kang Y, Dai HB, Ding L, Tong N, Zhu GQ, Zhou YB (2019) Intermedin in paraventricular nucleus attenuates sympathoexcitation and decreases TLR4-mediated sympathetic activation via adrenomedullin receptors in rats with obesity-related hypertension. Neurosci Bull 35:34–46
Viatour P, Merville MP, Bours V, Chariot A (2005) Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci 30:43–52
Wu N, Ye C, Zheng F, Wan GW, Wu LL, Chen Q, Li YH, Kang YM, Zhu GQ (2020) MiR155-5p inhibits cell migration and oxidative stress in vascular smooth muscle cells of spontaneously hypertensive rats. Antioxidants 9:E204
Xiong XQ, Chen WW, Zhu GQ (2014) Adipose afferent reflex: sympathetic activation and obesity hypertension. Acta Physiol (Oxf) 210:468–478
Xu B, Zheng H, Liu X, Patel KP (2015) Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol 308:H1103–H1111
Xu S, Zhao Y, Jin C, Yu L, Ding F, Fu G, Zhu J (2017) PKC/NADPH oxidase are involved in the protective effect of pioglitazone in high homocysteine-induced paracrine dyfunction in endothelial progenitor cells. Am J Transl Res 9:1037–1048
Yang Q, Yu XJ, Su Q, Yi QY, Song XA, Shi XL, Li HB, Qi J, Zhu GQ, Kang YM (2020) Blockade of c-Src within the paraventricular nucleus attenuates inflammatory cytokines and oxidative stress in the mechanism of the TLR4 signal pathway in salt-induced hypertension. Neurosci Bull 36:385–395
Ye C, Qiu Y, Zhang F, Chen AD, Zhou H, Wang JJ, Chen Q, Li YH, Kang YM, Zhu GQ (2020) Chemical stimulation of renal tissue induces sympathetic activation and pressor response via hypothalamic paraventricular nucleus. Neurosci Bull 36:143–152
Yu XJ, Miao YW, Li HB, Su Q, Liu KL, Fu LY, Hou YK, Shi XL, Li Y, Mu JJ, Chen WS, Cui W, Zhu GQ, Ebenezer PJ, Francis J, Kang YM (2019) Blockade of endogenous angiotensin-(1-7) in hypothalamic paraventricular nucleus attenuates high salt-induced sympathoexcitation and hypertension. Neurosci Bull 35:47–56
Yuan N, Zhang F, Zhang LL, Gao J, Zhou YB, Han Y, Zhu GQ (2013) SOD1 gene transfer into paraventricular nucleus attenuates hypertension and sympathetic activity in spontaneously hypertensive rats. Pflugers Arch 465:261–270
Zand H, Morshedzadeh N, Naghashian F (2017) Signaling pathways linking inflammation to insulin resistance. Diabetes Metab Syndr 11:S307–S309
Zhang LL, Ding L, Zhang F, Gao R, Chen Q, Li YH, Kang YM, Zhu GQ (2014) Salusin-beta in rostral ventrolateral medulla increases sympathetic outflow and blood pressure via superoxide anions in hypertensive rats. J Hypertens 32:1059–1067
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W (2016) ROS and ROS-mediated cellular signaling. Oxidative Med Cell Longev 2016:4350965
Zhang C, Zhu X, Li L, Ma T, Shi M, Yang Y, Fan Q (2019) A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. Diabetes Metab Syndr Obes 12:1297–1309
Zheng H, Patel KP (2017) Integration of renal sensory afferents at the level of the paraventricular nucleus dictating sympathetic outflow. Auton Neurosci 204:57–64
Zhou JJ, Ma HJ, Shao JY, Pan HL, Li DP (2019) Impaired hypothalamic regulation of sympathetic outflow in primary hypertension. Neurosci Bull 35:124–132
Funding
This study was supported by National Natural Science Foundation of China (31871148, 91639105, 31571167 & 81770426).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A Commentary to this article is available online at https://doi.org/10.1007/s00424-020-02462-6
Electronic supplementary material
ESM 1
(PDF 214 kb)
Rights and permissions
About this article
Cite this article
Zheng, F., Ye, C., Wan, GW. et al. Interleukin-1β in hypothalamic paraventricular nucleus mediates excitatory renal reflex. Pflugers Arch - Eur J Physiol 472, 1577–1586 (2020). https://doi.org/10.1007/s00424-020-02461-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02461-7