Abstract
Complex regional pain syndrome (CRPS) is a disorder that involves abnormal inflammation and nerve dysfunction frequently resistant to a broad range of treatments. Peripheral nerve stimulation with electroacupuncture (EA) has been widely used in different clinical conditions to control pain and inflammation; however, the use of EA in the treatment of CRPS is under investigation. In this study, we explore the effects of EA on hyperalgesia and edema induced in an animal model of chronic post-ischemia pain (CPIP model) and the possible involvement of endothelin receptor type B (ETB) in this effect. Female Swiss mice were subjected to 3 h hind paw ischemia/reperfusion CPIP model. EA treatment produced time-dependent inhibition of mechanical and cold hyperalgesia, as well as edema in CPIP mice. Peripheral administration (i.pl.) of BQ-788 (10 nmol), an ETB antagonist, prevented EA-induced antihyperalgesia while intrathecal administration prolonged EA’s effect. Additionally, peripheral pre-treatment with sarafotoxin (SRTX S6c, 30 pmol, ETB agonist) increased EA anti-hyperalgesic effect. Furthermore, the expression of peripheral ETB receptors was increased after EA treatments, as measured by western blot. These results may suggest that EA’s analgesic effect is synergic with ETB receptor activation in the periphery, as well as central (spinal cord) ETB receptor blockade. These data support the use of EA as a nonpharmacological approach for the management of CRPS-I, in an adjuvant manner to ETB receptor targeting drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex regional pain syndrome of type-I (CRPS-I) is a multifactorial nervous dysfunction originated from an ischemic lesion [10]. Clinical manifestations include regional pain, allodynia, hyperalgesia, edema, changes of skin temperature, and vasomotor dysfunctions [43]. As CRPS-I responds in a refractory way to several treatments [30]. there is continuous search for a better understanding of the pathophysiology underlying its clinical manifestations [4, 39].
As researchers seek to establish more effective approaches to reduce the signs and symptoms of CRPS-I [34], an animal model has been developed and is being widely used: the so-called chronic post-ischemic pain (CPIP) model which mimics CRPS-I by inducing ischemia followed by a rapid reperfusion period [24].
Involvement of the endothelinergic system in the pathophysiology of CRPS-I has been previously suggested, as microvascular lesions in endothelial cells [31] are often implicated in ischemia and reperfusion (IR). Additionally, preclinical and clinical studies have highlighted the participation of the endothelinergic system in the signaling of pain [9, 18].
It is well established that endothelins (ETs) exert their effects through two subtype receptors: ETA and ETB and that ET-1 binds equally to either receptor [20]. Peripheral ETB receptors are expressed in endothelial cells [11], smooth muscle cells [38], in macrophages [37], and in keratinocytes [19]. In the nervous system, ETB receptors are expressed in structures such as the sciatic nerve, the dorsal root ganglion (DRG), and the hypothalamus, among others [3, 13]. Activation of ETB receptors at the periphery induces analgesia [31], while the spinal blockage of this receptor by administration of Bq-788 (ETB receptor antagonist) reduces nociception [22].
In the spinal cord, the concentration of ET-1 increases, following peripheral nerve injury in rats, as is the case of the spinal nerve ligation [22]. In addition, spinal cord administration of ET-1 induces antihyperalgesia in animal models of neuropathic and inflammatory pain [16, 17]. Moreover, an increase in spinal ET-1 has been shown to induce mechanical hyperalgesia in an animal model of CRPS-I [22].
Neuronal stimulation is emerging in modern medicine as an interesting approach to help control organ dysfunction and re-establish physiological homeostasis [42]. While manual acupuncture uses the mechanical stimulation of needles inserted at specific reactive sites in the body (the so-called acupoints) to control pain among other conditions [47], electroacupuncture (EA) consists of the stimulation of the same acupoints with low amperage electric current [47]. EA in acupoints ST36 (stomach 36) and SP6 (spleen 6) has been shown to reduce mechanical hyperalgesia in animal models of neuropathy [6, 12, 25] and inflammation induced by complete Freund’s adjuvant (CFA) [15]. However, the effect of EA on the signs and symptoms of CRPS-1 has not yet been investigated, nor has the mechanism of action adjacent to this effect been yet fully elucidated.
In both patients and animal models, CRPS induces an acute (inflammatory) and posteriorly a chronic (neuropathic) phase [6]. Despite extensive literature on ischemic lesions, the chronic phase of the disease is not well studied, since the majority of the research has focused on the nociceptive response during the acute phase of CRPS-I.
In this context, the present study was carried out to determine the involvement of peripheral and central (spinal cord) ETB receptors in the antihyperalgesic effect of EA in an animal model of CPIP/CRPS-I, in the chronic phase of the disease.
Material and methods
Animals
All experiments were conducted using female Swiss mice (25–35 g), housed at 22 ± 2 °C under a 12-h light/dark cycle (lights on at 6:00 am) and with free access to food and water. The animals were acclimated to the laboratory for at least 1 h before the tests that were carried out between 8:00 and 12:00 am. All animal care and experimental procedures were carried out in accordance with the National Institutes of Health Animal Care Guidelines (NIH publications number 80-23) and were previously approved by the Ethics Committee of the Federal University of Santa Catarina (protocol number 15.045.2.07. IV).
Chemical reagents
The following substances were used: acetone (Audaz Brazil, SP, Brazil), anti-endothelin B receptor antibody (Abcam, Cambridge, MA, USA); ETB receptor antagonist Bq-788 (Sigma-Aldrich, St Louis, MO, USA), Anti-beta Actin antibody (HRP) (Sigma-Aldrich, St. Louis, MO, USA), isoflurane (Biochimico, Itatiaia, RJ, Brazil); pentobarbital sodic (Intervet, SP, Brazil); ETB receptor agonist sarafotoxin 6c (SRTX S6c)—sarafotoxins are a family of peptides identified from the venom of a snake Atractaspis engaddensis that exhibits high selectivity for the ETB receptor subtype over the ETA subtype [20] (Sigma-Aldrich Co., St. Louis, MO, USA).
Chronic post-ischemic pain (CPIP)/complex regional pain syndrome-type I (CRPS-I)
CPIP induction was performed as previously described [31]. Mice were anesthetized with sodium pentobarbital (55 mg/kg, i.p.) and supplemented with up to 27.5 mg/kg (i.p.), when necessary. After induction of anesthesia, an elastic O-ring for braces (Elástico Ligadura 000–1237, Uniden) with 1.2 mm internal diameter was placed around the animals’ right hind limb, proximal to the ankle joint. The O-rings were selected to provide a tight-fit that produced ischemia and were left on the limb for 3 h as initially described. The O-ring was always positioned at a point on the limb, just proximal to the medial malleolus by sliding it off the outside of a 100-mL pipette tip after the right hind paw was inserted into the pipette as far as possible. Naive animals were anesthetized but not subjected to the IR procedure.
Electroacupuncture treatment
EA treatment was performed according to the procedures described in previous studies [23, 32]. Mice were slightly sedated with 1–2% isoflurane delivered via a nose cone to minimize restraint-induced stress, then stainless acupuncture needles (Dong Bang, 0.18 mm/diameter and 8 mm/length) with electrodes soldered to their handles were inserted with 0.3 mm of depth into the acupoints SP6 and ST36 ipsilateral to the injured paw [44]. The needles were positioned with approximately 3 mm of depth in each acupoint and the intensity of the electrostimulation was increased until a mild twitch was observed at the tibialis anterior muscle (ST36) and flexor digitorum muscle (SP6), usually obtain with the intensity of 2 to 3 mA. In total, this needling procedure typically lasted than 20 s. The acupoints used in this study were selected because they stimulate the tibial nerve (SP6) and the fibular nerve (ST36) which are related to the segmental innervation the hind paw of the animal [41], moreover, several studies have demonstrated the effects of these acupoints to control pain and inflammation in different animal models [28, 45]. The parameters for electrostimulation included a dense-disperse asymmetric balanced wave (F1 = 2 Hz, 0.7 ms pulse with, 5 s of stimulation; F2 = 10 Hz, 0.2 ms pulse with, 5 s of stimulation) with alternating polarities using the NKL EL-608 electrostimulator (NKL produtos eletronicos, Brusque, SC). These selected EA parameters were used based on previous studies, which revealed that 2 to 10 Hz frequencies are effective to inhibit inflammatory and neuropathic pain [28, 45]. To investigate the most effective treatment duration, 5, 10, and 20 min of electrical stimulation were tested in different groups of animals.
To determine if the electrostimulation and the needle position were relevant to the treatment, two different needling control groups were included. In control group 1, the same procedures of the active EA group were performed but no electrical current was delivered to the ST36 and SP6 acupoints. In control group 2, needles were inserted bilaterally with 3 mm of depth into a nonacupoint located at the gluteal region, at the height of the iliac crest 5 mm from the midline and electrical current was delivered with the same parameters of the active EA group [29]. The rationale for selecting this nonacupoint was to observe the effect of the electrical stimulation into a region of the hind limb (gluteus inferior nerve) that was not directly related to the innervation of the paw.
Study outline
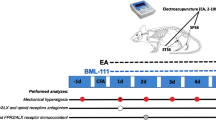
One day prior to the IR procedure, baseline hyperalgesia, edema, and temperature were recorded. Mechanical hyperalgesia was assessed by the von Frey test after daily EA treatments for seven consecutive days (7 to 14 or 14 to 21 post-IR). Additionally, on days 3, 7, 14, and 21, the time course of the effect of EA was assessed. Different EA durations (5, 10, and 20 min long continuous stimulation) were performed only on the third and seventh day and the time course of the effect was evaluated. After determining the treatment duration that produced the best antihyperalgesic effect (20 min), cold hyperalgesia was also evaluated, and an analysis of the time course of the antihyperalgesic effect of EA on the third and seventh day was performed. The time course of the effect was evaluated and then the daily effect of EA for seven consecutive days (from 7 to 14) was recorded. Analysis of the effect of EA on paw edema and temperature was performed from the first to the third day after IR. After characterizing the antihyperalgesic effect of EA, the next step was to analyze the involvement of peripheral and central (spinal cord) ETB receptors in EA effect during the chronic phase of CPIP. To this end, on the 14th day after IR, the mice were pre-administered intraplantarly (i.pl.) or intrathecally (i.t.) with saline or ETB receptor antagonist (Bq-788) and treated with EA, as well as with the peripheral ETB receptor agonist, SRTX S6c and treated with EA, mechanical hyperalgesia was then evaluated. Groups for the respective controls were also used. Finally, on fourteenth day after IR (chronic phase of CPIP), we analyzed the expression of peripheral and central (spinal cord) ETB receptors in the paw and spinal cord tissues of EA-treated animals (Fig. 1).
Behavioral measurement: mechanical and thermal hyperalgesia
Mechanical hyperalgesia was assessed with a von Frey monofilament (0.6 g, VFH, Stoelting, Chicago, IL, USA). The animals were individually placed in a 9 × 7 × 11 cm, bottomless observation acrylic chamber positioned on a 6 mm (70 × 6 mm) wire mesh platform 40 cm. Paw withdrawal frequency obtained in 10 right hind paw stimulations with the von Frey monofilament was recorded as indicative of mechanical hyperalgesia.
To characterize baseline response, the animals were evaluated on day-1 (1 day previous to IR). Only the animals that responded 20% or less to the von Frey stimuli were selected for the study. The filament test was applied perpendicularly to the plantar surface with sufficient pressure to provide the curvature of the filament, thereby obtaining total pressure. In addition, the animals were evaluated when all four paws were accommodated on the mesh, and the withdrawal response was recorded only when the animal completely removed the paw from the support mesh [27].
Mechanical hyperalgesia was evaluated 0.5, 1, and 2 h after the treatment to verify the time course of its anti-hyperalgesic effect. To investigate the effects of repeated treatments, EA was performed once a day. Mechanical hyperalgesia was evaluated 0.5 h after each treatment (time with maximal inhibition observed in the acute treatment) for seven consecutive days (7 to 14 and 14 to 21 post-IR).
To assess hyperalgesia to cold stimulus, the acetone drop method [4] with minor modifications was used. The amount of time the animals spent flicking/stamping or licking the plantar aspect of the hind paw during a 20-min observation period was recorded with a chronometer and considered to be indicative of cold hyperalgesia. The evaluations were performed before (0) IR and on days 3, 7, 14, and 21 post-IR.
Paw temperature
The temperature of the ventral and dorsal surface of the right and left hind paws was evaluated using a Thermal Image Camera (Testo 880®) with an accuracy of ± 0.1 °C and an infrared spectrum range of 7.5 to 13 m [33]. The cold/hot color pallet was used with temperature variation between 20 and 40 °C. The result was obtained through the difference of the right paw in relation to the left. Evaluations were conducted on days 1, 2, and 3 after IR, 30 min after the treatments.
Paw edema
Edema evaluation was performed by measuring the thickness of the right hind paw with a digital micrometer (Insize®, SP, Brazil) [8]. The results were expressed as the difference between the measure obtained and the baseline (before IR) evaluation of the same paw. Evaluations were conducted on days 1, 2, and 3 after IR, 30 min, 1, 2, and 24 h after the treatments.
Open field test
In this test, a wooden apparatus (box) measuring 40 × 60 × 50 cm was used, and the floor of the box was divided into 12 equal squares. Locomotor activity was evaluated by counting the number of squares the animals crossed with all paws over a 6-min evaluation period [36].
Involvement of peripheral ETB receptors in the anti-hyperalgesic effect of EA
To test the hypothesis that peripheral ETB receptors are involved in the anti-hyperalgesic effect of EA, different groups of mice subjected to IR were also treated with an ETB receptor agonist, SRTX S6c alone (30 pmol/i.pl.) [35] or in combination with EA (20 min). Mechanical hyperalgesia was evaluated 15, 30, 60, 120, and 150 min after the treatments. IR + Control group 1 was also evaluated in parallel.
Since it has been shown that activation of ETB peripheral receptors produces analgesia [31], we evaluated the involvement of these receptors in the anti-hyperalgesic effect of EA, analyzing whether the effect of SRTX S6c (30 pmol/i.pl.), EA or the combination of the two could be prevented by peripheral administration of Bq-788 (10 nmol/i.pl.). To this end, mice were subjected to IR, pre-treated with Bq-788 (i.e., fixed volume of 10 μl), and after 15 min, were treated with SRTX S6c (30 pmol/i.pl.), with EA or the combination of the two. Mechanical hyperalgesia was evaluated 30 min after the treatments and control groups were evaluated in parallel.
Involvement of central (spinal cord) ETB receptors in the anti-hyperalgesic effect of EA
Initially, we evaluated the effect of the i.t. administration of the ETB receptor antagonist upon mechanical hyperalgesia induced by IR. Different groups of animals previously subjected to IR were treated with Bq-788 (3–30 nmol/i.t.). Mechanical hyperalgesia was evaluated 15, 30, 60, 120, 180, and 210 min after the treatments. IR + control group 1 was also evaluated in parallel.
Since it has been shown that blocking ETB receptors produces analgesia [16, 17, 22], we evaluated the involvement of these receptors in the anti-hyperalgesic effect of EA directly by blocking ETB receptors and by combining EA with Bq-788 administration (ETB receptor antagonist). To this end, different groups of mice subjected to IR were treated with Bq-788 (3 nmol, i.t.) or with 20-min EA alone and in association. Mechanical hyperalgesia was evaluated 15, 30, 60, 120, 180, and 210 min after the treatments. The IR + control group 1 was also evaluated in parallel.
Western blotting
Quantification of ETB receptor in muscle and spinal cord
The samples were collected on the 14th day after IR, 30 min after the daily treatment (the animals had been treated once a day for seven consecutive days). Samples of plantar flexor tissue of the right hind paw and spinal cord of the lumbar region (segment L4-L6) were collected and stored in the freezer (− 80 °C).
Spinal cord tissue samples were manually homogenized with micropistils in ice-cold RIPA buffer containing 1% protease inhibitor (Sigma-Aldrich, St Louis, MO), while the paw muscles were immersed in liquid nitrogen, pulverized and immediately placed in tubes containing RIPA buffer, and then incubated on ice for 30 min. The tubes containing the lysates were centrifuged at 10.000 rpm for 20 min at 4 °C, and the supernatants were collected. Protein concentration was determined using the Bradford method.
The electrophoretic separation was conducted using 30 μg of protein per well in 10% polyacrylamide gel electrophoresis (SDS-PAGE), running in a Mini-PROTEAN® Tetra cell apparatus under a PowerPac TM HC power supply (both from Bio-Rad, CA, USA). The proteins were transferred onto a PVDF membrane (Bio-Rad Laboratories Inc., Hercules, CA, USA), blocked in 5% BSA (prepared in TBS-T buffer, pH 7.4; concentration in mmol/L: 20 Tris-HCl, 137 NaCl, 0.1% Tween 20) and incubated overnight at 4 °C with primary antibodies to ETB receptor (dilution 1:1000; Abcam, Cambridge, MA, USA). Peroxidase-conjugated monoclonal antibody against β-actin (dilution 1:45000) was used as a loading control for all samples tested. After incubation with primary antibodies, the membranes were washed three times (10 min each) with TBS-T solution and incubated with the specific secondary antibody conjugated to horseradish peroxidase (HRP) at room temperature for 1 h. The membranes were washed another three times (10 min each) with TBS-T solution and exposed to HRP substrate (Pierce Biotechnology, Rockford, IL, USA), and immune complexes were visualized by chemiluminescence using Chemidoc MP System (Bio-Rad Laboratories). Bands were quantified by densitometry using the software from the manufacturer (Image Lab; version 4.1; Bio-Rad Laboratories, Hercules, CA, USA). Values were normalized using the data obtained for β-actin and expressed as arbitrary units. In these experiments, the following groups (n = 8) were analyzed: Naive, IR + control 1, and IR + EA 20 min.
Statistical analysis
The results were analyzed with the Graph Pad Prism program (version 6.0 - La Jolla, California, USA). Initially, the Shapiro-Wilk normality test was applied to evaluate the normality of the data. The results are expressed as means ± standard deviation (SD) for continuous variables. The data was analyzed using both one-way analysis of variance (ANOVA) with the Student Newman-Keuls test and two-way ANOVA with repeated measures. Differences with a value of P < 0.05 were considered significant.
Results
EA reduces mechanical hyperalgesia in mice subjected to IR
The results illustrated in Fig. 2a–d demonstrate that the experimental model of CPIP induced mechanical hyperalgesia, when compared to the baseline results, i.e., before the animals were submitted to IR (− 1 day). Figure 2 shows the time course effect of EA treatment on the 3rd (panel a), 7th (panel a), and 14th day (panel d) after IR; as well as he results of EA daily treatments from the 7th to 14th day (panel c). In the evaluations performed on the 3rd, 7th, and 14th day after IR (Fig. 2a–d), no statistically significant difference was observed between the IR + control 1 group in relation to the IR + control 2 group, for this reason, in the subsequent evaluations, only IR + control 1 group was used.
Antihyperalgesic effect of EA after IR. Time course evaluation of the treatment with EA on the 3rd (a), 7th (b), and 14th (d) day after IR. Evaluation of hyperalgesia after 30 min of daily EA for 7 consecutive days (c). Data were expressed as mean ± standard deviation (SD) (n = 8 animals). * P < 0.05, ** P < 0.01 and *** P < 0.001 when compared with the IR + control group. ### P < 0.001 when compared to baseline before IR (− 1 day). Two-way ANOVA followed by the Bonferroni test. EA electroacupuncture; IR ischemia and reperfusion; d days; h hours
On the 3rd day after IR (Fig. 2a), 20-min EA treatment significantly (P < 0.001) reduced mechanical hyperalgesia up to 1 h after the treatment. 10-min EA was effective for only 0.5 h (P = 0.007) after treatment, and 5-min EA had no analgesic effect. Figure 2b shows that on the 7th day after IR, 20-min EA reduced mechanical hyperalgesia for up to 1 h [0.5 h (P < 0.001) and 1 h (P = 0.005)]; 10-min EA was effective for up to 30 min [0.5 h (P = 0.046)] and 5-min EA did not affect paw withdrawal threshold. Confirming our previous observations, 20-min EA was more effective than 10 and 5-min EA, with the highest efficacy obtained 0.5 h after treatment.
Given the results, daily 20-min EA was conducted from day 7 to 14 post-IR and the evaluations were conducted 0.5 h after treatment. In these parameters, EA reduced mechanical hyperalgesia from the 7th to the 14th day (P < 0.001) post-IR when compared to the IR + control 1 group (panel c). On the 14th day after IR (panel d), daily 20-min EA continued to present the same profile, reducing mechanical hyperalgesia 0.5 h (P < 0.001) and 1 h (P = 0.002) after treatment, when compared to the IR + control group 1. Thus, no cumulative antihyperalgesic effect was observed with the daily EA treatment.
The results shown in Fig. 3a–c demonstrate that IR increased paw withdrawal frequency when compared to baseline evaluations, i.e., before IR (− 1 day). The results of EA performed at different times after IR on day 14 are shown on panel a, daily treatment from day 14 to day 21 on panel b, and on day 21 on panel c. The results presented on the 14th day after IR (panel a) demonstrate that 20-min EA reduced mechanical hyperalgesia 0.5 h (P < 0.001) and 1 h (P < 0.001) after the treatment, when compared to the group IR + control 1. On the 21st day after IR (panel B) after daily 20-min EA sessions, for 7 consecutive days, mechanical hyperalgesia reduction was only observed 0.5 h (P < 0.001) after treatment, when compared to the IR + control 1 group, which indicates there is no cumulative effect. With daily 20-min EA, from the 14th to the 21st day, it was possible to observe that EA reduced mechanical hyperalgesia on a daily basis 0.5 h after treatment, until the 21st (P = 0.002) day post-IR.
Antihyperalgesic effect of EA from 14th to 21st day after IR. Time course evaluation of the treatment with EA on the 14th (a) and 21st (b) day after IR. Evaluation of hyperalgesia after 30 min of daily EA treatment for 7 consecutive days (c). Data were expressed as mean ± standard deviation (SD) (n = 8 animals). * P < 0.05, ** P < 0.01 when compared to the IR + control group. ### P < 0.001 when compared to baseline before IR (− 1 day). Two-way ANOVA followed by the Bonferroni test. EA electroacupuncture; IR ischemia and reperfusion; d days; h hours
EA reduces cold hyperalgesia in CPIP mice
The results shown in Fig. 4a–c demonstrate the development of cold allodynia after IR when compared to baseline evaluations, i.e., before IR (− 1 day). Figure 4 shows the effect of 20-min EA on day 3 (panel a), 7 (panel b), and after daily treatments from day 14 to day 21 (panel c). Panel a of Fig. 4 represents the acute phase of the CPIP model. 20-min EA reduced cold allodynia 0.5 h after treatment (P < 0.001) when compared to the IR group + control 1 (Fig. 4a), on the 3rd day after IR. However, in the chronic phase of the CPIP model, i.e., on the 7th day after IR (panel b), 20-min EA reduced allodynia to cold 0.5 h (P < 0.001), 1 h (P = 0.007), and 2 h (P = 0.015) after treatment, whereas 10-min EA effect lasted for only 0.5 h (P < 0.001), demonstrating thus that 20-min EA is more effective. Twenty-minute EA reduced cold allodynia on all treatment days until the 19th day (P = 0.003), when compared to the IR + control group 1 (Fig. 4c). Cold hyperalgesia thresholds were also drastically reduced in IR + control group 1 on the 20th and 21st days post-IR.
EA reduces cold hyperalgesia in mice subjected to IR. Time course evaluation of the treatment with EA on the 3rd day (a) and 7th (b) day after IR. Evaluation of cold hyperalgesia after 30 min of daily EA treatment for 7 consecutive days (c). Data were expressed as mean ± standard deviation (SD) (n = 8 animals). * P < 0.05, ** P < 0.01, and *** P < 0.001 when compared with the IR + control group. ### P < 0.001 when compared to baseline before IR (− 1 day). Two-way ANOVA followed by the Bonferroni test. EA electroacupuncture; IR ischemia and reperfusion; d days; h hours
EA decreases edema, but does not affect paw temperature
Figure 5, panels a and b, demonstrates data regarding the surface temperature of the paw after IR. Neither the IR procedure nor the daily treatment for 3 days with EA altered paw temperature (neither on the ventral surface nor on the dorsal surface of the paw). In panels c and d, it was observed that on the 1st, 2nd, and 3rd days after IR, there was an increase (P < 0.001) in paw edema IR + control 1 group, when compared to the naive group. There was a significant reduction of paw edema (P = 0.03) induced by 20-min EA on the 1st day, 24 h after the treatment, when compared to the IR + control 1 group.
Effect of EA on paw temperature and edema in mice subjected to IR. Quantification of the ventral surface temperature (a), dorsal surface (b) and paw edema (c–e). Data were expressed as mean ± standard deviation (SD) (n = 8 animals). * P < 0.05, when compared with the IR + control 1 group, # P < 0.05 and ### P < 0.001 when compared to naive. Two-way ANOVA followed by the Bonferroni test. EA electroacupuncture; IR ischemia and reperfusion; d days; Δ delta; °C degrees centigrade; mm millimeters
EA does not affect locomotive activity
In the open-field test conducted after 7 days of daily treatment with EA (14th day after IR), it was verified that 30 min after EA, the animals’ locomotor activity was not affected, when compared to the group IR + control 1. The mean number of crosses over 6 min for the groups was 120.6 crossings for the naive, 118.3 crossings for the IR + control 1 group, and 118.3 crossings for the IR + EA 20 min group (Supplementary Fig. S1).
Peripheral ETB receptors are involved in the anti-hyperalgesic effect of EA in the chronic phase of CPIP/CRPS-I
In Fig. 6a, the administration of SRTX S6c (30 pmol/i.pl.) was shown to reduce mechanical hyperalgesia 15 and 30 min after the treatment (P < 0.001), as well as after 20-min EA, in the later, for up to 1 h (P < 0.001). When both treatments were associated, i.e., SRTX S6c (30 pmol/i.pl.) and 20-min EA, the effect lasted for up to 2 h, suggesting an added effect (P < 0.001).
Peripheral ETB receptors participate in the antihyperalgesic effect of EA. Effect of peripheral ETB receptor on mechanical hyperalgesia with administration of SRTX S6c (i.pl.) and EA on the 14th day after IR (a) and Bq-788 (i.pl.), EA and SRTX S6c (i.pl.) on the 14th day after IR (b). Data were expressed as mean ± standard deviation (SD) (n = 8 animals). *** P < 0.001 when compared to the IR + control group 1. ### P < 0.001, when compared to the saline group (20 μl/i.pl.) + SRTX S6c (30 pmol/i.pl.) (b) or when compared to baseline before IR (− 1 day, a). ††† P < 0.001, when compared to the saline group (20 μl/i.pl.) + IR EA 20 min (b). P = 0.001 when compared to the saline group (20 μl/i.pl.) + (SRTX S6c 30 pmol/i.pl.) + IR EA 20 min) (b). Data were expressed as mean ± standard deviation (SD) (n = 8 animals). One-way or two-way ANOVA followed by Bonferroni tests (a) or Student Newman-Keuls test (b). EA electroacupuncture; min minutes; h hours; d days; SRTX S6c sarafotoxin S6c (ETB receptor agonist); Bq-788 ETB receptor antagonist; i.pl. intraplantar
Figure 6b shows that i.pl. administration of Bq-788 (10 nmol/i.pl.) on the 14th day after IR prevented the antihyperalgesic effect induced by SRTX S6c (P < 0.001), EA (P < 0.001), or the association of the treatments (P < 0.001). These data suggest the involvement of the peripheral ETB receptor in EA induced antihyperalgesia.
EA enhances expression of peripheral ETB receptors in the chronic phase of CPIP/CRPS-I
Figure 7 demonstrates that naive animals constitutively express the ETB receptor in the flexor leg muscle. Fourteen days after paw IR, there was no significant increase in the expression of this receptor. However, in the animals subjected to IR and treated with 20-min EA, there was an increase (P = 0.03) in ETB receptors expression in the flexor muscle of the paw, when compared to the IR + control group 1. Therefore, 20-min EA increases the expression of ETB receptors in the periphery on the 14th day after IR, after 7-day consecutive treatments.
EA induces increased expression of peripheral ETB receptors in mice subjected to IR. Effect of cumulative treatment with EA from day 7 to 14 after IR on the expression of the ETB receptor in the paw muscle. Data were expressed as mean ± standard deviation (SD) (n = 8 animals). * P < 0.005 when compared to the IR + group control 1. One-way ANOVA followed by Student Newman-Keuls test. EA electroacupuncture; IR ischemia and reperfusion; ETB receptor for endothelin B; UA arbitrary units
Spinal ETB receptor contributes to the anti-hyperalgesic effect of EA in the chronic phase of CPIP/CRPS-I
The data presented in Fig. 8 demonstrate that the experimental model induced (P < 0.001) mechanical hyperalgesia, when compared to the baseline evaluation, i.e., before the animals were submitted to IR (− 1 day). In panel A, it can be seen that i.t. administration of Bq-788 (3 nmol) reduced mechanical hyperalgesia 15 (P < 0.001), 30 (P < 0.001) and 60 min (P = 0.002) after treatment. However, the dose of 10 nmol, Bq-788 (i.t.) induced a significantly statistical effect only 15 min after treatment (P = 0.014). Treatment with Bq-788 (30 nmol/i.t.) did not reduce mechanical hyperalgesia.
The antihyperalgesic effect of EA is mediated by central ETB receptors. Dose response curve of the effect of the ETB receptor antagonist (Bq-788, i.t., a). Effect of the association of EA with the administration of the ETB receptor Bq-788 i.t. antagonist upon mechanical hyperalgesia (b). * P < 0.05 (a), ** P < 0.01 (a) and *** P < 0.001 when compared to the IR + control group 1. It is considered ### P < 0.001 when compared to baseline before RI. Data were expressed as mean ± standard deviation (SD) (n = 8 animals). Two-way ANOVA followed by the Bonferroni test. EA electroacupuncture; min minutes; h hours; d days; Bq-788 ETB receptor antagonist; i.t. intrathecal
In Fig. 8b, the antihyperalgesic effect of the most effective dose of Bq-788 (3 nmol/i.t) was demonstrated on the 14th day after IR. Bq-788 and 20-min EA induced antihyperalgesia for up to 1 h after the treatments. However, when Bq-788 was associated with EA, a reduction (P < 0.001) in mechanical hyperalgesia was achieved for up to 3 h after treatment. The results suggest the central (spinal cord) involvement of the ETB receptors in the antihyperalgesic effect of EA.
EA did not affect the expression of spinal ETB receptors in the chronic phase of CPIP/CRPS-I
In Fig. 9, it is possible to observe that naive animals constitutively express the ETB receptor in the spinal cord; however, 14 days after IR, there was no significant increase in the expression of this receptor. Additionally, no changes in ETB receptor expression were evidenced in the animals subjected to IR and treated with 20-min EA. Therefore, 20-min EA did not influence the expression of ETB receptors in the spinal cord after 7 consecutive daily treatments.
EA does not alter the expression of central ETB receptors in mice subjected to IR. Effect of cumulative treatment with EA from day 7 to 14 after IR upon ETB receptor expression in the spinal cord. Data were expressed as mean ± standard deviation (SD) (n = 8 animals). * P < 0.005 when compared to the IR + group control 1. One-way ANOVA followed by Student Newman-Keuls test. EA electroacupuncture; IR ischemia and reperfusion; ETB receptor for endothelin B; UA arbitrary units
Discussion
Endothelins are peptides that have their effects mediated by ETA and ETB receptors, which induce vasoconstriction and vasodilation, respectively [31, 48]. The expression of these receptors on pain pathways suggests their participation in pain modulation. ETB receptors expressed in paw muscles during CPIP, could be an interesting target for the treatment of CRPS-I [31]. Furthermore, central (spinal cord) ETB receptors are known to reduce mechanical hyperalgesia in the CRPS-I animal model [22].
Despite extensive literature on ischemic lesions, the chronic phase of CRPS-I is not well studied, as most of the research has focused on its acute phase. Therefore, this study sought to determine the involvement of peripheral and central (spinal cord) ETB receptors in the antihyperalgesic effect of EA in the chronic phase of an animal model of CPIP/CRPS-I (14 days post-IR).
Our results demonstrate for the first time that IR induces hyperalgesia (to mechanical and cold stimuli) in both the acute (inflammatory) and chronic (neuropathic) phases of the disease, and that even a single treatment with EA effectively reduces it. These results agree with the literature that demonstrated that ST36 and SP6 EA reduces mechanical hyperalgesia in an animal model of neuropathy [6, 12, 25] as well as in the model of persistent inflammatory pain [15].
In regard to cold hyperalgesia, this has been shown that paw IR induces hyperalgesia to cold stimuli which is prevented by peripheral administration of Bq-788, 2 days post-IR [31]. Thus, the results of the present study confirm and extend literature data by demonstrating that EA reduces cold hyperalgesia in the neuropathic phase of CPIP/CRPS-I.
To evaluate the clinical potential of EA, the mice were treated in the two phases. EA was effective both in the inflammatory and in the neuropathic phase without affecting locomotor activity. Additionally, EA did not produce a cumulative effect. On the contrary, on the 14th day, EA had a shorter-lived effect when compared to other evaluation days.
Tolerance to repeated treatment with EA has been associated with the nociceptin/orphanin fq system. It has been shown that tolerance observed with EA is prevented by intracerebroventricular administration of the antibody against orphanin fq in rats [15, 40]. The authors concluded that EA may enhance the endogenous release of orphanin fq in the central nervous system (CNS) which acts as an antagonist to the antihyperalgesic effect of EA in the brain.
In addition, it is known that electrical stimulation itself can induce tolerance, as with TENS. It has been previously demonstrated that cholecystokinin B receptor blockade (CCK-B) prevented the tolerance induced by repeated transcutaneous electrical nerve stimulation (TENS) in rats with kaolin/carrageenan-induced knee inflammation [7]. Since octapeptide-cholecystokinin (CCK-B receptor agonist) plays an important role in tolerance induced by both morphine and EA, the blockade of this receptor potentiated the effect of EA and prevented tolerance. Thus, these peptidergic mediators may mediate tolerance observed in repeated treatment with EA [14].
Another finding of the present study was the demonstration that EA reduced edema but not the increase in paw temperature in the inflammatory phase of CPIP/CRPS-I. These findings corroborate previous observations that EA reduces inflammation and edema in the CFA [26] and carrageenan models [21], with potential to treat chronic inflammatory conditions [45].
Studies have highlighted the implication of the endothelinergic system in pain processing [20], by activating its receptors with distinct functions at both peripheral and central (spinal cord) sites, for example, activation of the ETB receptor in the periphery produces analgesia [31, 35], whereas centrally (spinal cord), produces nociception [22].
As expected, peripheral administration of SRTX S6c or EA reduced mechanical hyperalgesia in the chronic phase of CPIP/CRPS-I, although a more significant effect was evidenced with the association of the two therapies. The findings suggest that these therapies could share the same mechanism of action, i.e., activation of peripheral ETB receptors. Therefore, we suggest that the observed effects are related to the activity of SRTX S6c on the ETB receptor expressed (1) in the vessel, which induces vasodilatation that is in opposition to the state of chronic hypoperfusion present in the CPIP model; and (2) in the primary nociceptive neuron, which induces the release of opioids causing analgesia. The same has been observed in other studies [31] who administered compound IRL-1620, another ETB receptor agonist, in the CPIP model in mice, but on the second day after IR. A study has shown that the manual stimulation of ST36 and SP6 acupoints may also be effective in increasing blood flow in the lower limb [46], pointing to a possible involvement of the ETB receptor in this effect, as similar results are obtained with the activation of the ETB receptor by SRTX 6c.
The results show that the pharmacological blockade of peripheral ETB receptors in the neuropathic phase of CRPS-I prevented both the effect of SRTX 6c and EA. It also prevented the antihyperalgesic effect induced by the combination of the two therapies. Furthermore, the expression of peripheral ETB receptors on the 14th day post-IR was increased when compared to naive animals, although the results were not statistically significant. However, daily treatment with EA induced a significant increase in ETB receptor expression in the paw muscle of these animals. Taken together, the results suggest the involvement of peripheral ETB receptors in the antihyperalgesic effect of EA.
ET-1 produces pain by the activation of ETB receptors in the spinal cord. It has been demonstrated in an animal model of neuropathic pain induced by spinal root ligation that the administration of Bq-788 reduces mechanical hyperalgesia [22]. Data from the present study showed that i.t. administration of Bq-788 on the fourteenth day after IR also reduced mechanical hyperalgesia, in a dose-dependent manner, in mice subjected to CPIP/CRPS-I. However, IR did not affect ETB receptor expression in the spinal cord. These findings corroborate previous studies that have also shown that blocking the ETB receptor in the spinal cord by Bq-788 produces a dose-dependent antihyperalgesic effect, however in rats, and over a period of 21 days [22].
After determining the antihyperalgesic effect of the ETB receptor antagonist administered in the spinal cord, the next step of the present study was to analyze the involvement of ETB receptors on EA’s effect. The findings of the present study also demonstrated that although daily EA did not affect ETB receptor expression in the spinal cord, i.t. treatment with an ETB receptor antagonist potentiated the antihyperalgesic effect of EA, suggesting the involvement of the central (spinal cord) ETB receptor in the effect of EA.
Since ET-1 is the main endogenous activator of the central (spinal cord) ETB receptor and it has been shown that plasma concentrations of this peptide are increased in the CPIP/CRPS-I model, it is very likely that mechanical hyperalgesia could also be sustained by the action of ET-1 upon central (spinal cord) ETB receptors. In line with this claim, it has been described that the synthesis of ET-1 is modulated by physiological and pathophysiological factors. In addition, factors such as nitric oxide (NO), prostacyclin, and atrial natriuretic hormone have been shown to inhibit the production of ET-1 [5]. A number of studies have shown the effect of EA on NO modulation [1]. Specifically, EA in acupoint ST36 reduced orofacial thermal hyperalgesia in rats, an effect that was prevented by specific inhibitors of neuronal nitric oxide synthase and the induced nitric oxide synthase. In addition, the authors observed that concentrations in spinal brain fluid and plasma were four and three times higher, respectively, after EA [2]. Thus, a plausible explanation for the effect observed here is related to the increase of NO induced by EA that could in turn induce the decrease in the concentrations of ET-1 in the spinal cord and thus reducing ETB receptor activation and hyperalgesia. However, future studies analyzing plasma and tissue concentrations of ET-1 in the CPIP/CRPS-I model on the fourteenth day post-IR and after seven consecutive days of EA are required to confirm this hypothesis.
It can be concluded from these results that daily EA is effective in reducing the signs and symptoms of CPIP/CRPS-I and that peripheral and central ETB receptors participate in the antihyperalgesic effect of EA in the chronic phase of the syndrome, in an opposite manner, in the periphery and the spinal cord (centrally). These results demonstrate EA analgesic effect in yet another chronic painful condition that is rather difficult to treat. In sum, this study deepens our knowledge about EA mechanism of action by demonstrating the involvement of ETB receptors, and adds to the understanding of the pathophysiological events induced by CRPS-I.
Change history
13 September 2018
The original version of this article contains an error. The Author Francisco José Cidral-Filho incorrectly listed as Francisco José Cidra-Filho. The correct spelling is presented above. The original article has been corrected.
References
Almeida RT, Duarte ID (2008) Nitric oxide/cGMP pathway mediates orofacial antinociception induced by electroacupuncture at the ST36 acupoint. Brain Res 10:54–60
Almeida RT, Galdino G, Perez AC, Silva G, Romero TR, Duarte ID (2017) ST36 electroacupuncture activates nNOS, iNOS and ATP-sensitive potassium channels to promote orofacial antinociception in rats. J Physiol Pharmacol 68:27–33
Barrett KE, Barman SM, Boitano S, Heddwen LB (2014) Medical physiology of Ganong, 24th edn. Porto Alegre, AMGH
Bridges D, Thompson SW, Rice AS (2001) Mechanisms of neuropathic pain. Br J Anaesth 87:12–26
Carducci MA, Jimeno A (2006) Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Canc Res 12(Suppl 1):6296–6300
Coderre TJ, Xanthos DN, Francis L, Bennett GJ (2004) Chronic post-ischemia pain (CPIP) - a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain 112:94–105
Desantana JM, Da Silva LF, Sluka KA (2010) Cholecystokinin receptors mediate tolerance to the analgesic effect of TENS in arthritic rats. Pain 148:84–93
Erthal V, Maria-Ferreira D, Werner MF, Baggio CH, Nohama P (2016) Anti-inflammatory effect of laser acupunture in ST36 (Zusanli) acupoint in mouse paw edema. Lasers Med Sci 31:315–322
Ferreira SH, Romitelli M, De Nucci G (1989) Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol 13:220–222
Gay A, Parratte S, Salazard B, Guinard D, Pham T, Legré R, Roll JP (2007) Proprioceptive feedback enhancement induced by vibratory stimulation in complex regional pain syndrome type I - an open comparative pilot study in 11 patients. Joint Bone Spine 74:461–466
Ghoneim MA, Yamamoto T, Hirose S, Nagasawa T, Hagiwara H (1993) Endothelium localization of ETB receptor revealed by immunohistochemistry. J Cardiovasc Pharmacol 22(Suppl 1):111–112
Gim GT, Lee JH, Park E, Sung YH, Kim CJ, Hwang WW, Chu JP, Min BI (2011) Electroacupuncture attenuates mechanical and warm allodynia through suppression of spinal glial activation in a rat model of neuropathic pain. Brain Res Bull 86:403–411
Hans G, Deseure K, Adriaensen H (2008) Endothelin-1-induced pain and hyperalgesia—a review of pathophysiology, clinical manifestations and future therapeutic options. Neuropeptides 42:119–132
Huang C, Hu ZP, Jiang SZ, Li HT, Han JS, Wan Y (2007) CCK(B) receptor antagonist L365, 260 potentiates the efficacy to and reverses chronic tolerance to electroacupuncture-induced analgesia in mice. Brain Res Bull 71:447–451
Huang CP, Chen HN, Su HL, Hsieh CL, Chen WH, Lai ZR, Lin YW (2013) Electroacupuncture reduces carrageenan- and CFA-induced inflammatory pain accompanied by changing the expression of Nav1.7 and Nav1.8, rather than Nav1.9, in mice dorsal root ganglia. Evid based complement alternat med 1-8
Hung VK, Chen SM, Tai LW, Chen AY, Chung SK, Cheung CW (2012) Over- expression of endothelin-1 in astrocytes, but not endothelial cells, ameliorates inflammatory pain response after formalin injection. Life Sci 91:618–622
Hung VK, Tai LW, Qiu Q, Luo X, Wong KL, Chung SK, Cheung CW (2014) Over-expression of astrocytic ET-1 attenuates neuropathic pain by inhibition of ERK1/2 and Akt(s) via activation of ETA receptor. Mol Cell Neurosci 60:26–35
Jr Verri WA, Cunha TM, Parada CA, Wei XQ, Ferreira SH, Liew FY, Cunha FQ (2006) IL-15 mediates immune inflammatory hypernociception by triggering a sequential release of IFN-gamma, endothelin, and prostaglandin. Proc Natl Acad Sci U S A 103:9721–9725
Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G (2003) Endothelin-B receptor activation triggers and endogenous analgesic cascade at sites of peripheral injury. Nat Med 9:1055–1061
Khodorova A, Montmayeur JP, Strichartz G (2009) Endothelin receptors and pain. J Pain 10:2–28
Kim HW, Uh DK, Yoon SY, Roh DH, Kwon YB, Han HJ, Lee HJ, Beitz AJ, Lee JH (2008) Low-frequency electroacupuncture suppresses carrageenan-induced paw inflammation in mice via sympathetic post-ganglionic neurons, while high-frequency EA suppression is mediated by the sympathoadrenal medullary axis. Brain Res Bull 75:698–705
Kim YO, Kim IJ, Yoon MH (2015) Antiallodynic effect through spinal endothelin-B receptor antagonism in rat models of complex regional pain syndrome. Neurosci Lett 584:45–49
Koo ST, Lim KS, Chung K, Ju H, Chung JM (2008) Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain 135:11–19
Laferrière A, Millecamps M, Xanthos DN, Xiao WH, Siau C, de Mos M, Sachot C, Ragavendran JV, Huygen FJ, Bennett GJ, Coderre TJ (2008) Cutaneous tactille allodynia associated with microvascular dysfunction in muscle. Mol Pain 4:1–11
Lau WK, Lau YM, Zhang HQ, Wong SC, Bian ZX (2010) Electroacupuncture versus celecoxib for neuropathic pain in rat SNL model. Neurosc 170:655–661
Liang Y, Fang JQ, Du JY, Fang JF (2012) Effect of electroacupuncture on activation of p38MAPK in spinal dorsal horn in rats with complete Freund’s adjuvant-induced inflammatory pain. Evid based complement Alternat Med 12:1–6
Martins DF, Emer AA, Batisti AP, Donatello N, Carlesso MG, Mazzardo-Martins L, Venzke D, Micke GA, Pizzolatti MG, Piovezan AP, dos Santos AR (2015) Inhalation of Cedrus atlantica essential oil alleviates pain behavior through activation of descending pain modulation pathways in a mouse model of postoperative pain. J Ethnopharmacol 175:30–38
Mayor D (2013) An exploratory review of the electroacupuncture literature: clinical applications and endorphin mechanisms. Acupunct Med 31:409–415
Medeiros MA, Canteras NS, Suchecki D, Mello LE (2003) C-Fos expression induced by electroacupuncture at the Zusanli point in rats submitted to repeated immobilization. Braz J Med Biol Resear 36:1673–1684
Midbari A, Suzan E, Adler T, Melamed E, Norman D, Vulfsons S, Eisenberg E (2016) Amputation in patients with complex regional pain syndrome—a comparative study between amputees and non-amputees with intractable disease. Bone Join J 98:548–554
Millecamps M, Laferrière A, Ragavendran JV, Stone LS, Coderre TJ (2010) Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I). Pain 151:174–183
Moré AO, Cidral-Filho FJ, Mazzardo-Martins L, Martins DF, Nascimento FP, Li SM, Santos AR (2013) Caffeine at moderate doses can inhibit acupuncture-induced analgesia in a mouse model of postoperative pain. J Caff Reser 3:143–148
Moura D (2011) Use of infrared thermography in the analysis of horse thermoregulation in training. Eng Agric 31:23–32
Oerlemans HM, Oostendorp RA, de Boo T, Goris RJ (1999) Pain and reduced mobility in complex regional pain syndrome I—outcome of a prospective randomised controlled clinical trial of adjuvant physical therapy versus occupational therapy. Pain 83:77–83
Piovezan AP, D'Orléans-Juste P, Souza GE, Rae GA (2000) Endothelin-1-induced ET(A) receptor-mediated nociception, hyperalgesia and edema in the mouse hind-paw: modulation by simultaneous ET(B) receptor activation. Br J Pharmacol 129:961–968
Rodrigues AL, da Silva GL, Mateussi AS, Fernandes ES, Miguel OG, Yunes RA, Calixto JB, Santos AR (2002) Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci 70:1347–1358
Sakurai-Yamashita Y, Yamashita K, Yoshida A, Obana M, Takada K, Shibaguchi H, Shigematsu K, Niwa M, Taniyama K (1997) Rat peritoneal macrophages express endothelin ET(B) but not endothelin ET(A) receptors. Eur J Pharmacol 338:199–203
Shetty SS, Okada T, Webb RL, DelGrande D, Lappe RW (1993) Functionally distinct endothelin B receptors in vascular endothelium and smooth muscle. Biochem Biophys Res Commun 191:459–464
Tang C, Li J, Tai WL, Yao W, Zhao B, Hong J, Shi S, Wang S, Xia Z (2017) Sex diferences in complex regional pain syndrome type–I (CRPS-I) in mice. J. Pain Res 10:1811–1819
Tian JH, Zhang W, Fang Y, Xu W, Grandy DK, Han JS (1998) Endogenous orphanin FQ: evidence for a role in the modulation of electroacupuncture analgesia and the development of tolerance to analgesia produced by morphine and electroacupuncture. Br J Pharmacol 124:21–26
Treuting PM, Dintzis S (2011) Comparative anatomy and histology: a mouse and human atlas (expert consult), 1th ed. Academic Press
Uolla L, Quiroz-Gonazalez S, Torres-Rosas R (2017) Nerve stimulation—immunomodulation and control of inflammation. Trends Mol Med 23:1103–1120
Vural SP, Yuzer GFN, Ozcan DS, Ozbudak SD, Ozgirgin N (2016) Effects of mirror therapy in stroke patients with complex regional pain syndrome type 1- a randomized controlled study. Arch Phys Med Rehabil 97:575–581
Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG (2008) A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci 84:159–165
Zhang R, Lao L, Ren K, Berman BM (2014) Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiol 120:482–503
Zhang WP, Onose Y, Fujikawa T (2017) A trial study of moxibustion with a warming needle on edema. J Acupunct Meridian Stud 10:20–25
Zhao ZQ (2008) Neural mechanism underlying acupunture analgesia. Prog Neurobiol 85:355–375
Zhao YD, Springall DR, Wharton J, Polak JM (1991) Autoradiographic localization of endothelin-1 binding sites in porcine skin. J Invest Dermatol 96:152–154
Funding
The present study was supported by grants from Universidade do Sul de Santa Catarina—Curso de Medicina and Programa Unisul de Iniciação Científica (PUIC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - 476454/2013-1), and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC-3414/2012), Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The original version of this article was revised: The Author Francisco José Cidral-Filho incorrectly listed as Francisco José Cidra-Filho. The correct spelling is presented above.
Electronic supplementary material
Figure S1
EA does not affect locomotive activity. Effect of cumulative treatment with EA from day 7 to 14 after IR upon locomotive activity. Data were expressed as mean ± standard deviation (SD) (n = 8 animals). EA: electroacupuncture; IR: ischemia and reperfusion. (JPG 120 kb)
Rights and permissions
About this article
Cite this article
Mazzardo-Martins, L., Salm, D.C., Winkelmann-Duarte, E.C. et al. Electroacupuncture induces antihyperalgesic effect through endothelin-B receptor in the chronic phase of a mouse model of complex regional pain syndrome type I. Pflugers Arch - Eur J Physiol 470, 1815–1827 (2018). https://doi.org/10.1007/s00424-018-2192-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2192-2