Abstract
The G protein-regulated inducer of neurite growth (GRIN) family has three isoforms (GRIN1-3), which bind to the Gαi/o subfamily of G protein that mediate signal processing via G protein-coupled receptors (GPCRs). Here, we show that GRIN3 is involved in regulation of dopamine-dependent behaviors and is essential for activation of the dopamine receptors (DAR)-β-arrestin signaling cascade. Analysis of functional regions of GRIN3 showed that a di-cysteine motif (Cys751/752) is required for plasma membrane localization. GRIN3 was co-immunoprecipitated with GPCR kinases 2/6 and β-arrestins 1/2. Among GRINs, only GRIN3, which is highly expressed in striatum, strongly interacted with β-arrestin 2. We also generated GRIN3-knockout mice (GRIN3KO). GRIN3KO exhibited reduced locomotor activity and increased anxiety-like behavior in the elevated maze test, as well as a reduced locomoter response to dopamine stimulation. We also examined the phosphorylation of Akt at threonine 308 (phospho308-Akt), which is dephosphorylated via a β-arrestin 2-mediated pathway. Dephosphorylation of phospho308-Akt via the D2R-β-arrestin 2 signaling pathway was completely abolished in striatum of GRIN3KO. Our results suggest that GRIN3 has a role in recruitment and assembly of proteins involved in β-arrestin-dependent, G protein-independent signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Signaling pathways involving G protein-coupled receptors (GPCRs) regulate physiological processes in mammals and are the primary target of many pharmaceutical therapies. Activation of GPCRs induces dissociation of G protein subunits, generating GTP-bound α subunits and free βγ subunits, which regulate various downstream effector molecules. For example, the Gαs subunit stimulates adenylyl cyclase (AC), leading to the formation of cyclic AMP (cAMP) and subsequent phosphorylation of intracellular targets by protein kinase A. Gαi/o subunit, on the other hand, inhibits AC and reduces the concentration of cAMP [20, 21]. Hydrolysis of GTP to GDP on the α-subunit leads to reassociation of α and βγ subunits to form an inactive heterotrimer.
GPCR can be desensitized by phosphorylation or internalization mediated by G protein-coupled receptor kinases (GRKs) [10, 17]. Phosphorylated or internalized receptors bind to β-arrestins, which block further stimulation of G protein-dependent signaling. Recently, it was reported that β-arrestins also function as signal transducers in G protein-independent or noncanonical signal transduction, and a rapidly growing number of signaling pathways has been found to be stimulated by β-arrestin-mediated signaling, including phosphorylation of p44/42 MAPK on threonine 202/tyrosine 204 and dephosphorylation of Akt on threonine 308 [3, 14]. However, the mechanisms linking G protein-dependent signaling to GRK/β-arrestin-mediated signaling and their crosstalk remain poorly understood.
G protein-regulated inducer of neurite growth 1 and 2 (GRIN1 and GRIN2, respectively) are known to have a Gαo-binding region at the C-terminal region and are proposed to work as downstream effectors for Gαo in addition to AC [11, 15, 16]. The GRIN family also contains a third member, GRIN3, which was found to be vertebrate-specific by BLAST search. However, the molecular functions of the GRIN family remain unclear, partly because of the extremely low amino acid homology among the family numbers (Fig. S1). In the present study, we identified functional regions of GRIN3 by expressing a series of fragments or deletion mutants in HEK293FT cells. Co-immunoprecipitation studies showed that GRIN3 interacts with GRKs 2/6 and β-arrestins 1/2. We further prepared GRIN3-knockout mice (GRIN3KO) and found that they exhibit dopamine-dependent behavioral abnormalities. Our results indicate that GRIN3 has an important role in the noncanonical dopamine receptors (DAR) signaling cascade, i.e., DAR-β-arrestin signaling.
Materials and methods
Reagents and antibodies
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) or TOCRIS Bioscience (Bristol, UK). All antibodies were commercial products as follows:
Dopamine D1 receptor (D1R) (Lifespan), D2R (Santa Cruz), D3R (Abcam), muscarinic acetylcholine receptor type 4 (M4R) (Lifespan), Gαo (Santa Cruz), GRK2 (Santa Cruz), GRK6 (Santa Cruz), rabbit HA (Santa Cruz), Flag (Sigma), mouse GFP (Invitrogen), rabbit GFP (Invitrogen), rabbit Phospho-Akt (Thr308) (Cell Signaling), Akt (Cell Signaling), lacZ (Promega), and β-actin (Sigma). GRIN1 and GRIN3 antibodies were generated using the following peptides, coupled to adjuvant via cysteine residue at their amino terminus (indicated by brackets).
GRIN1: (C)14TRKEEAGSLRNEESMLKG31.
GRIN3: (C)422SQNTETEEDLRLSASKE438.
Western blot analysis
Western blot analysis was conducted using a total of 10–20 μg protein as described previously [19].
Generation of GRIN3KO
The targeting vector for GRIN3KO was constructed according to the BAC engineering system (Fig. 1b). Most of the GRIN3 coding region except for the first 17 amino acids residues was replaced with Escherichia coli (E. coli) -derived lacZ sequence. The resulting targeting construct was linearized and electroporated into ES cells derived from 129sv mouse strain. The cells were selected with geneticin, and the colonies were picked up and screened by PCR with KOD FX (TOYOBO, Tokyo, Japan). Positive clones were confirmed by Southern blot analysis with 3′ external probe. Finally, positive ES cells were microinjected into blasocysts derived from C57BL/6 mice and the blastocysts were transferred into uterus of foster mother mice. Germ-line transmission was confirmed by mating with C57BL/6 females. To remove the neomycin cassette, GRIN3 heterozygous mice were crossed with CAG-FLPe transgenic mice. Finally, GRIN3 heterozygous mice were backcrossed for seven generations onto C57BL/6 females and then interbred to obtain GRIN3 homozygous mice (GRIN3KO). Wild-type (WT) littermates were used as controls. All mice were housed in air-conditioned animal rooms at an ambient air temperature of 22 ± 2 °C and relative humidity of 50 ± 15%, under specific pathogen-free conditions with a 12-h light/dark cycle. Animals were treated in accordance with institutional guidelines. The experimental protocol was approved by the Animal Care and Use Committee of the National Center for Global Health and Medicine.
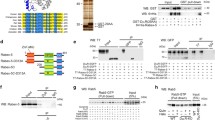
Generation of GRIN3 KO mice. a Expression levels of endogenous GRIN3 (upper) and GRIN1 (middle) in the total lysates prepared from striatum, whole brain, heart, liver, thymus, lung, kidney, and intestine by western blot analysis. b Targeted disruption of GRIN3 gene. The partial structure of the GRIN3 gene (WT allele), targeting vector construct (targeting vector), and the resultant targeted allele are shown. The positions of the lacZ-SV40 poly A tail (lacZ-SV40 polyA) followed by phosphoglycerate kinase promoter neo cassette (PGK-neo) are also shown. The 3′ external probe and PCR primers for genotyping are indicated. Black triangles, frt sequence; E, EcoRI. c Southern blotting analysis of genomic DNA from the targeted embryonic stem (ES) cell clones. Genomic DNA from control embryonic stem cells and homologous targeted clones was digested with EcoRI and hybridized with the 3′-external probe as indicated in (b). d Mice were genotyped by PCR are shown using the PCR primers as indicated in (b). e Western blotting analyses of striatum from homozygous (Homo) and heterozygous (Hetero) mice compared with WT using GRIN3-specific antibody. GRIN3 appears as a blurred band of approximately 100 kDa (calculated molecular weight, 82.5 kDa) in WT and heterozygous mice. The amount of GRIN3 was greatly decreased in heterozygous mice, and GRIN3 was completely lost in homozygous (KO) mice. Sharp bands seen in homo (KO) lanes appear to be non-specific. f Representative pictures of LacZ-stained coronal brain sections derived from GRIN3 heterozygous mice. LacZ-positive cell numbers were moderate in prefrontal cortex (a), and hippocampus (c). Strong expression was recognized in nucleus accumbens (a, arrows) and dorsal striatum (b). Scale bars, 100 μm. g Expression levels of various GPCRs in the striatum of GRIN3KO. Total lysates were examined by western blot analysis using specific antibodies. The expression levels of these GPCRs were not altered in GRIN3KO, compared with WT
β-Galactosidase (LacZ) staining
Brain tissues were immersed into freshly prepared 4% PFA at 4 °C for 2 h after perfusion with 4% PFA. Tissues were cryoprotected in 30% sucrose, embedded in OCT, and sectioned at 40 μm on a cryostat. The section were washed and permeabilized in TBS containing 0.01% Tween 20, 2 mM MgCl2 for 30 min, and then stained with 1 mg/ml X-gal in TBS containing 0.01% Tween 20, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, and 2 mM MgCl2 at 37 °C for about 6 h. Sections were then rinsed with TBS, refixed in 4% PFA for about 4 h, and coverslipped.
Quantitative real-time PCR
Total RNA was isolated from HEK293FT cells at 24 h after transfection (Fig. S5) or from WT mice (Figs. S2 and S3) using Isogen (Nippon Gene, Tokyo, Japan). The RNA concentrations and quality were determined by spectrophotometry. To remove contaminating genomic DNA, samples were treated with DNase I. Total RNA was reverse-transcribed with ReverTra Ace (Toyobo, Osaka, Japan) and random primers.
mRNAs of full-length GRIN3(1-763) and GRIN3(1-763/668-686del) were quantified by quantitative real-time PCR (q-PCR) using a 7900HT Fast Real-Time PCR system (Applied Biosystems, CA, USA) with the FastStart Universal SYBR Green Master (Roche Diagnostics, Mannheim, Germany). The primer pair for full-length GRIN3 and GRIN3 mutant was as follows.
Forward, 5′-TCTCACCACAACCAGCTCAG-3′.
Reverse, 5′-ACTGGCTCTCCCTCACTGAA-3′.
The relative amount (172 base pairs) of full-length GRIN3(1-763) and GRIN3(1-763/668-686del) mRNA was normalized to β-actin or GAPDH.
Cloning
Rat GRK6-, rat wild-type Gαo subcloned into pCMV5, and mouse GRIN1 and mouse GRIN2 subcloned into pEGFP-C1 (GFP-GRIN1 and GFP-GRIN2) (Clontech, Mountain View, CA, USA) were kindly provided from Dr. Tohru Kozasa (Yokohama University of Pharmacy). GαoQ205L mutant was generated with a mutagenesis kit (Takara, Tokyo, Japan). Flag-tagged mouse GRIN3 and mouse GRIN3 were cloned into pRK5 and pEGFP-C1, respectively. Hemagglutinin (HA)-tagged mouse GRK2, HA-tagged mouse β-arrestin 1, and HA-tagged mouse β-arrestin 2 were cloned into pEGFP-C1.
Cell culture and transfection
HEK293FT cells were maintained with DMEM plus 10% fetal calf serum and transfected with Lipofectamine 2000 (Invitrogen, Carisbad, CA, USA) according to the manufacturer’s protocol. Cells were harvested at 24 h after transfection.
Co-immunoprecipitation assay
Total lysates of HEK293FT cells were used for co-immunoprecipitation assay. Briefly, 1 ml of immunoprecipitation buffer (20 mM Tris-HCl, pH 7.4, with 150 mM NaCl, 1 mM MgCl2, 1 mM GDP, 1 mM Na3VO4, 10 mM sodium pyrophosphate, 50 mM NaF, and 1% Triton X-100 containing protease inhibitors) was added to the cells, and the mixture were homogenized and centrifuged at 4 °C. Next, 0.5–1 μg of the appropriate antibody was added to 25–30 μl of protein G-dynabeads (Invitrogen) and mixed with 600 μl of samples in immunoprecipitation buffer. The protein beads were washed three times with immunoprecipitation buffer. Protein was eluted with SDS sample buffer for 5 min at 95 °C or for 15 min at 70 °C. Proteins were eluted for 10 min at 37 °C or at room temperature.
Immunocytochemistry
HEK293FT cells were seeded onto glass coverslips coated with poly-l-lysine (Sigma) and Collagen Type I (Collagen Corporation, Palo Alto, CA, USA).
Cells were fixed with 4% paraformaldehyde in PBS (pH 7.4) at 4 °C for 10 min, rinsed with PBS three times, and permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. For nuclear staining, the cells were incubated with 2 mg/ml Hoechast 33258 (1:2000, Dojindo, Kumamoto, Japan) in PBS for 30 min at room temperature. After washing, cells were mounted on an adhesive slide glass and coverslipped with PermaFluor Aqueous Mounting Medium (Thermo Shandon Immunon, Waltham, MA, USA). The cells for immunocytochemistry were excited at the wavelength of 488 nm for EGFP and 405 nm for Hoechst 33258, respectively. The images were obtained with a confocal microscopy (TCS SP5, Leica, Wetzer, Germany) using a × 40, 1.25 numerical aperture (NA) oil-immersion objective.
Behavioral testing
Locomotor activity was measured by scoring the number of photobeam breaks in an open field test chamber (40 cm × 40 cm × 35 cm) (Panlab S. I., Barcelona, Spain). Four-month-old WT and KO male mice were used for the open field test. For measurement of locomotor activity in a novel environment, each mouse was placed in the center of the chamber, and total distance covered (in cm) and the time spent in the center were recorded for 120 min.
To test the effect of GBR12909 (Tocris) on locomotor activity, mice were habituated in the chamber for 150 min, and 10 mg/kg of GBR12909 was administered via intraperitoneal injection (ip) After administration, the total distance covered and the rearing activity were recorded for 210 min.
Elevated plus maze test
The experimental apparatus was a plus-shaped maze with two open arms (35 cm length, 5 cm width) and two closed arms (35 cm length, 5 cm width, 35 cm height). The entire apparatus was elevated 60 cm from the ground and testing was performed under normal lighting (250–300 lx). Mice were placed in the center of the maze facing an open arm. The number of entries and the time spent in each arm during the 20-min session were recorded by a researcher blinded as to genotype.
Statistical analysis
Statistical analysis was conducted by two-tailed Student’s t test or two-way analysis of variance (ANOVA) with Bonferroni’s post hoc test as appropriate. The criterion of significance was taken as P < 0.05. The statistical tests were performed using the GraphPad Prism 5.0 software (San Diego, CA, USA). Values in graphs were expressed as mean ± SEM.
Results
Generation of GRIN3 knockout mice
Endogenous GRIN3 was expressed predominantly in the striatum of the brain, in which GRIN1 was hardly detectable (Fig. 1a). We also examined endogenous GRIN3 and found that its expression at both the mRNA and protein levels was similar in each organ (Fig. 1a; Fig. S2). These data support the specificity of the anti-GRIN3 antibody, and thus the reliability of the immunodetection using this antibody.
In order to investigate GRIN3 function in vivo, the GRIN3 gene was disrupted in mice by means of homologous recombination [19, 20, 22] (Fig. 1b). The GRIN3 coding sequence is included in a single exon on mouse genome. In our GRIN3KO mice, GRIN3 coding sequence was replaced by an E. coli-derived lacZ sequence. Targeted embryonic stem cell clones were genotyped by southern blotting (Fig. 1c) and mice were genotyped by PCR using genomic DNA from tail biopsies (Fig. 1d). We performed western blotting of striatum from homozygous (Homo) and heterozygous (Hetero) mice compared with WT using anti-GRIN3 antibody. The amount of GRIN3 was decreased in heterozygous mice by approximately 60% (Fig. S3b), and GRIN3 was hardly detectable in homozygous (KO) mice (Fig. 1e). We also extracted total RNA of cerebrum, striatum, hippocampus, and liver from homozygous (KO), heterozygous and WT. We performed PCR using GRIN3 primer pairs, but amplified PCR products were not detectable in KO mice (Fig. S3a). We further confirmed that GRIN3 mRNA was significantly decreased by approximately 60% in heterozygous mice by means of q-PCR (n = 3 each, Student’s t test, vs. WT, P < 0.01) (Fig. S3c–f).
GRIN3KO were viable and fertile, and appeared similar to WT mice in their postnatal growth, at least up to 4 months. Histological analysis by LacZ staining confirmed that GRIN3 was highly expressed in the nucleus accumbens and dorsal striatum (Fig. 1f (a (arrows) and b). In addition, GRIN3 was observed in CA1 and CA3 regions of hippocampus and II, III, and V layers of prefrontal cortex (Fig. 1f (a and c)).
The expression levels of dopamine receptors (D1R-D3R) as well as M4R (the major isoform in adult striatum) were similar in WT and GRIN3KO [2, 12] (Fig. 1g).
GRIN3 interacts with Gαo at its C-terminal region
To identify the Gαo-binding region of GRIN3, we generated two constructs with green fluorescent protein (GFP) fused at the N-terminus of full-length murine GRIN3(1-763) and GRIN3(1-763/668-686del) (Fig. 2a (#1 and #2)). Of the two, GRIN3(1-763/668-686del) did not contain a region highly homologous with the Gαo-binding region identified in GRIN1 and GRIN2 (Fig. 2b; Fig. S1). We thus performed co-IP assay using full-length GRIN3(1-763) and GRIN3(1-763/668-686del) in HEK293FT cells coexpressing wild-type Gαo (Gαowt) or constitutively active mutant GαoQ205L, followed by homogenization and immunoprecipitation using an anti-GFP antibody. Washed immunoprecipitates were analyzed by western blotting with an anti-Gαo antibody and an anti-GFP antibody, confirming that equal amounts of GFP-tagged protein were used for this co-IP assay, and thus that the association of GRIN3 with Gαo was decreased by deletion of residues 668-686 (Fig. 2c) [16]. Therefore, residues 668-686 appear to include the Gαo-binding site of GRIN3.
GRIN3 interacts with Gαo at the C-terminal region and subcellular localization of GRIN3 in HEK293FT cells. a Full-length GRIN3 (#1) and GRIN3 mutants with (#3) or without (#2 and #4) the Go-binding region (residues 668-686, black square) or substitutions of C751A/C752A (#3 and #4, red line) are schematically represented. Gαo-binding region (residues 668-686) is shown as black. Amino acid residue is abbreviated as aa. b Comparison of deduced Gαo-binding site of GRIN3 at the C-terminal region with those of mouse GRIN1 and mouse GRIN2. c Flag-tagged full-length GRIN3 (#1) and GRIN3 mutants (#2) were expressed in HEK293FT cells coexpressing Gowt or GoQ205L. Co-IP assay showed that the association of full-length GRIN3(1-763) (#1) with Gαo was decreased by deletion of residues 668-686 (#2). d GFP alone (pEGFP-C1), GFP-fused full-length GRIN3 (#1) and GRIN3 mutant (#2-#4) were expressed in HEK293FT cells. At 24 h after transfection, immunocytochemistry showed that GFP protein (green) was expressed abundantly in cytosol in pEGFP-C1-transfected cells (right upper). Full-length GRIN3(1-763) (#1) and GRIN3 mutant (#2) showed plasma membrane distribution, but GRIN3 mutants with C751A/C752A (#3 and #4) did not. The nuclei were labeled in blue. Scale bar, 10 μm. e Western blot analysis showed that protein level of GRIN3(1-763/668-686del) (#2) was reduced, compared with that of full-length GRIN3(1-763) (#1) (upper), indicating that the Gαo-binding region is required to maintain the GRIN3 level at the plasma membrane (**P < 0.01, Student’s t test, n = 4). Representative immunoblotting results indicate in GFP-GRIN3 (#1 and #2) and β-actin as control (lower)
However, we anticipated that GRIN3 mutant(1-763/668-686del) might have another Gαo-binding region because western blotting with anti-Gαo antibody still showed a weak association of GRIN3 with Gαo (Fig. 2c (upper)).
We thus generated GRIN3 mutant(1-763/668-686del/735-747del) (Fig. S4a) and performed co-IP-assay in HEK293FT cells co-expressing wild-type Gαo (Gαowt) or constitutively active mutant GαoQ205L, as in the experiments shown in Fig. 2c. We found that the association of GRIN3 with Gαo was totally abolished, indicating that residues 735-747 of GRIN3 includes a second Gαo-binding site of GRIN3 (Fig. S4b).
Role of two conserved cysteine residues and Gαo-binding region of GRIN3
It was reported that the membrane localization of GRIN1 is determined by two cysteine residues (Cys818 and Cys819) at the C-terminal region, and these are conserved in GRIN2 and GRIN3 (Fig. S1) [16]. Thus, we examined the role of these residues in the subcellular localization of GRIN3.
We first transfected GFP alone (pEGFP-C1) into HEK293FT cells and confirmed that transfection was successfully achieved in HEK293FT cells and that GFP protein was expressed abundantly in the cytosol (Fig. 2d (right upper)).
As shown in Fig. 2a, we next generated two more constructs with GFP fused at the N-terminus of GRIN3 mutants, i.e., GRIN3(1-763/C751A/C752A) [23] in which both Cys751 and Cys752 were mutated to alanine (#3), and GRIN3(1-763/668-686del/C751A/C752A) (#4). These mutants were expressed in HEK293FT cells, and the subcellular localization of GRIN3 was examined by immunocytochemistry (Fig. 2d). Full-length GRIN3 and GRIN3(1-763/668-686del) were localized to the plasma membrane (Fig. 2d (#1 and #2)), indicating that the Gαo-binding site did not influence the subcellular localization.
However, GRIN3(1-763/C751A/C752A) and GRIN3(1-763/668-686del/C751A/C752A) showed cytoplasmic distribution (Fig. 2d (#3 and #4)), indicating that the di-cysteine motif (Cys751 and Cys752) is important for membrane localization, as in the case of GRIN1 [16].
There is growing evidence that G protein signaling components are regulated by post-translational modification [4]. We thus examined the protein level of full-length GRIN3(1-763) and GRIN3(1-763/668-686del) in transfected HEK293FT cells by western blotting using an anti-specific GFP antibody and corrected for uneven loading based on the expression of reference protein β-actin (Fig. 2e). We also conducted quantitative real-time PCR (Fig. S5).
The expression of GRIN3(1-763/668-686del) was significantly decreased by 65%, compared with that of full-length GRIN3(1-763) at the protein level (Fig. 2e), but the mRNA expression levels were similar (Fig. S5), suggesting that the decreased expression of GRIN3(1-763/668-686del) was mediated via post-translational modification. These data indicate that the Gαo-binding region is required to maintain the GRIN3 protein level at the membrane.
GRIN family interacts with GRK2/6
GPCR can be desensitized by phosphorylation or internalization mediated by GRKs, which block further stimulation of G protein-dependent signaling [10, 17]. Therefore, we examined whether GRIN family members interact with GRK2 or GRK6, which are the major GRK subtypes expressed in striatum and might phosphorylate DAR [8, 9] (Fig. 3). We expressed GFP-fused full-length GRIN1(1-827), GRIN2(1-455), or GRIN3(1-763) in HEK293FT cells co-expressing GRK2 or GRK6 and found that GRIN1–3 were all similarly co-immunoprecipitaed with GRK2 (Fig. 3a), as well as GRK6 (Fig. 3b).
GRIN family interacts with GRK2/6 and β-arrestin1/2. a, b GFP-fused full-length GRIN1(1-827), GRIN2(1-455), or GRIN3(1-763) was expressed together with GRK2 or GRK-6 in HEK293FT cells. Co-IP assay showed that GRIN1(1-827), GRIN2(1-455), and GRIN3(1-763) were immunoprecipitaed with HA-tagged GRK2 (a) as well as GRK6 (b). c GFP-fused GRIN3(668-763) (#1) and GFP-fused GRIN3 (687-763) (#2) are schematically represented. Gαo-binding region (668-686) are shown as black. Amino acid residue is abbreviated as aa (upper). GFP-fused GRIN3(668-763) or GRIN3(687-763) was expressed with GRK6 with or without coexpression of Gαowt or GαoQ205L (lower). Co-IP assay showed that association with GRK6 was augmented in cells expressing GRIN3(668-763) and Gαowt or GαoQ205L (lanes 2–4), but not in cells expressing GRIN3(687-763) (lanes 5–7). d GFP-fused full-length GRIN1(1-827), GRIN2(1-455), or GRIN3(1-763) was expressed together with HA-tagged β-arrestin 1 or β-arrestin 2 in HEK293FT cells. Co-IP assay showed that GRIN1(1-827), GRIN2(1-455), or GRIN3(1-763) were similarly immunoprecipitated with β-arrestin 1 (left). In contrast, GRIN3 was preferentially immunoprecipitated with β-arrestin 2 (right)
Gαo-binding site is important for the association with GRK6
In order to examine the role of the Gαo-binding sites within the C-terminal region of GRIN3 in the binding to GRK6, which is known to localize in the striatum receiving dopaminergic input and that DAR are physiological target of this kinase [8, 9]. GRK6 was expressed concomitantly with GFP-fused GRIN3(668-763) or GRIN3(687-763) and myc-tagged D2R in HEK293FT cells co-expressing or not co-expressing Gαowt or GαoQ205L (Fig. 3c (upper)). The results of co-IP assay showed that association of GRK6 with GRIN3 was increased in cells expressing GFP-fused GRIN3(668-763) as well as Gαowt or GαoQ205L (Fig. 3c (lanes 2–4)) but not in cells expressing GFP-tagged GRIN3(687-763) (Fig. 3c (lanes 5–7)), indicating that the Gαo-binding site (residues 668-686) of GRIN3 might play an important role in the association of GRIN3 with GRK6 through Gαo.
All GRIN family members interacts with β-arrestin 1 but only GRIN3 binds tightly to β-arrestin 2
GRK regulate cell signaling by phosphorylation or internalization, and this results in recruitment of multifunctional scaffolding proteins termed β-arrestin 1 and β-arrestin 2, also known as arrestin-2 and arrestin-3, respectively, in order to desensitize phosphorylated or internalized GPCR [10, 13, 17, 24]. More importantly, only GRK2 and GRK6, regulates β-arrestin 2 recruitments to the dopamine receptor [23]. We thus investigated whether GRIN family members interact with β-arrestin 1/2.
GFP-fused full-length GRIN1(1-827), GRIN2(1-455), or GRIN3(1-763) was expressed together with HA-tagged β-arrestin 1 or β-arrestin 2 in HEK293FT cells (Fig. 3d). Co-IP assay showed that GRIN1, GRIN2, and GRIN3 were similarly immunoprecipitated with β-arrestin 1 (Fig. 3d (left)). They were also immunoprecipitated with β-arrestin 2, but the association of GRIN3 with β-arrestin 2 was stronger than that of GRIN1 or GRIN2 (Fig. 3d (right)).
Locomotor activity and anxiety-like behavior in GRIN3KO mice
Since GRIN3 was localized in the striatum [25] (Fig. 1a), we examined the influence of GRIN3 on dopamine-dependent behaviors. Mice were placed in an open-field test apparatus and the overall distance covered and the time spent in the center of the open field were measured to assess locomotor activity and anxiety-like behavior (Fig. 4a–c). The overall distance traveled by GRIN3KO mice in each 30 min interval was significantly smaller than that by WT mice for 90 min after the start of the open-field test (Fig. 4a) and total distances covered up to 120 min was also significantly smaller in GRIN3KO than those in WT (WT (n = 9) vs. KO (n = 12) 24819 ± 1566 vs. 16338 ± 1076 cm, P < 0.01) (Fig. 4b).
Locomotor activity and anxiety-like behavior in GRIN3KO. a, b The overall distance covered in each 30 min interval by the WT mice was significantly greater than that by GRIN3KO for 120 min after the start of the open-field test (*P < 0.05, **P < 0.01, two-way ANOVA, vs. WT, n = 9–12) (a) and the total overall distance covered in 120 min were significantly greater in WT than that in GRIN3KO (**P < 0.01, Student’s t test, vs. WT, n = 9–12) (b). c The percent time in the center of the open field by GRIN3KO was significantly longer than that in WT (**P < 0.01, Student’s t test, vs. WT, n = 9–12). d, e The time in the open bars (d) as well as the entered number of open bars or closed bar (e) were significantly smaller in GRIN3KO than that in WT (**P < 0.01, Student’s t test, vs. WT, n = 8). Also, the time spent in closed bars and the entered number of closed bars were significantly greater in GRIN3KO than that in WT (**P < 0.01, Student’s t test, vs. WT, n = 8). f, g The rearing number (f) and overall distance covered (g) were not different between WT and GRIN3KO at baseline, but they gradually increased and reached the maximum at 1 h after GBR12909 injection (10 mg/kg ip). The magnitudes of the increase in every 10 min interval were significantly smaller in GRIN3KO than that in WT for 210 min (*P < 0.05, **P < 0.01, two-way ANOVA, vs. WT, n = 11–12)
The proportion of time spent in the center of the open-field was also significantly smaller in GRIN3KO than in WT (WT (n = 9) vs. KO (n = 12) 15 ± 2.1 vs. 6 ± 1.5%, P < 0.01) (Fig. 4c).
We also examined anxiety-like behavior in GRIN3KO using the elevated plus-maze test. The time spent in the open bars (WT (n = 8) vs. KO (n = 8) 29 ± 3.5 vs. 2 ± 0.6%) (Fig. 4d (left)) as well as the number of entries into the open bars (WT (n = 8) vs. KO (n = 8) 42 ± 1.9 vs. 6 ± 2.0%) (Fig. 4e (left)) were significantly smaller in GRIN3KO than in WT (P < 0.01). Also, the time spent in closed bars (WT (n = 8) vs. KO (n = 8) 71 ± 3.5 vs. 98 ± 0.7%) (Fig. 4d (right)) and the number of entries into the closed bars (WT (n = 8) vs. KO (n = 8) 57 ± 1.9% vs. 94 ± 1.9%) (Fig. 4e (right)) were significantly greater in GRIN3KO than in WT (P < 0.01). Thus, locomotor activity in a novel environment was decreased, and anxiety-like behavior was augmented in GRIN3KO.
Effect of dopamine reuptake inhibitor on rearing number and distance covered in GRIN3KO
We next examined the effect of GBR12909 1-(2-(bis(4-fluorophenyl)methoxy)ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride, a specific dopamine reuptake inhibitor, on the rearing number and distance covered. GRIN3KO and WT were left to habituate in open-field chambers for 150 min prior to GBR12909 administration (10 mg/kg ip), and then changes of locomotor activity following GBR12909 injection were monitored over 10-min intervals (Fig. 4f, g). Locomotor activity was measured by counting the rearing number (Fig. 4f) and total horizontal movement distance (Fig. 4g). Basal locomotor activities were not different between WT and GRIN3KO for 10 min prior to administration of GBR12909 (Fig. 4f, g). As shown in Fig. 4f, g, the rearing number and total distance covered in WT mice gradually increased and reached the maximum at approximately 20–30 min after administration of GBR12909. GRIN3KO showed a similar pattern, but the magnitudes of the increase were significantly smaller than in WT mice (P < 0.05 or 0.01, WT (n = 12), KO (n = 11)). Rearing number and total distance covered during 30–210 min after injection of GBR12909 were also significantly reduced in GRIN3KO mice compared with WT mice (rearing number, WT vs. KO, 4374 ± 405 vs. 2231 ± 388, P < 0.01; total distance, WT vs. KO, 71902 ± 10307 cm vs. 32338 ± 4257 cm, P < 0.01). These results indicated that the response to GBR12909 was attenuated in GRIN3KO compared with WT, suggesting that GRIN3KO mice exhibited abnormality of dopamine-mediated intracellular signaling, because the expression levels of D1R-D3R in GRIN3KO were not altered (Fig. 1g).
Phosphorylation of Akt on threonine 308 in response to GBR12909 was blunted in GRIN3KO mice
In order to examine the mechanism leading to attenuated locomotor activity and increased anxiety-like behavior in GRIN3KO, we examined the phosphorylation of Akt on threonine 308 (phospho308-Akt) in the striatum of GRIN3KO, because dopamine is known to promote dephosphorylation of phospho308-Akt in striatum via the D2R/β-arrestin 2 signaling cascade [1], and GRIN3 strongly interacts with GRK 2/6, in addition to β-arrestin 1/2 as shown in Fig. 3. More importantly, GRK2/6, the major GRK subtypes expressed in striatum, were chiefly responsible for D2R phosphorylation and the subsequent β-arrestin 2 recruitment to the dopamine receptor [9, 18, 23].
Phospho308-Akt expression level was examined by western blot analysis using total lysates prepared from striatum. In WT, the phospho308-Akt level gradually decreased from 10 min after administration of GBR12909 (Fig. 5b). In contrast, the phospho308-Akt level in GRIN3KO level was sustained up to 30–45 min after administration of GBR12909. These results suggested that GRIN3 might play an important role to induce activation of DAR/β-arrestin 2-Akt signaling.
Effect of GBR12909 on phosphorylation of Akt and ERK phosphorylation in GRIN3KO. a, b Phospho308-Akt level gradually decreased after the injection of GBR12909 (10 mg/kg ip) in striatum of WT, but no decrease was observed in striatum of GRIN3KO (**P < 0.01, two-way ANOVA, vs. WT, n = 4–9) (b). Representative immunoblotting results show Akt phosphorylated at threonine 308 and total Akt at 30 min after GBR12909 injection (a). c, d Phospho202/204-ERK level gradually increased after the injection of GBR12909 (10 mg/kg ip) in striatum of both WT and GRIN3KO, but the magnitude of the increase was much greater in striatum of GRIN3KO (**P < 0.01, two-way ANOVA, vs. WT, n = 4–9) (d). Representative immunoblotting results show ERK phosphorylated at threonine 202/tyrosine 204 and total ERK at 10 min after GBR12909 injection (c). e, f Phospho473-Akt level gradually increased after the injection of GBR12909 (10 mg/kg ip) in striatum of both WT and GRIN3KO, but the magnitude of the increase was much greater in striatum of GRIN3KO (**P < 0.01, two-way ANOVA, vs. WT, n = 4–9) (f). Representative immunoblotting results show Akt-phosphorylated at serine 473 and total Akt at 20 min after GBR12909 injection (e). The ratio of phosphorylated/total protein expression at baseline (0 min) was taken as onefold in WT
Phosphorylation of ERK and phosphorylation of Akt on serine 473 in response to GBR12909 were increased in GRIN3KO mice
We also examined phosphorylation of ERK on threonine 202/tyrosine 204 (phospho202/204-ERK) and phosphorylation of Akt on serine 473 (phospho473-Akt) in the striatum of GRIN3KO and found that both phosphorylation levels were increased in GRIN3KO, compared with WT (Fig. 5c–f).
It was reported that the levels of phospho202/204-ERK and phospho473-Akt in response to dopaminergic stimulation in β-arrestin 2 KO were similar to those in WT [3], indicating that GRIN3 may regulate phosphorylation of ERK as well as Akt on serine 473 independently of DAR/β-arrestin 2 signaling.
Discussion
Multifunctional proteins, i.e., β-arrestin 1, β-arrestin 2, and GRK, are involved in the uncoupling of GPCR from G protein, leading to termination or attenuation of G protein-mediated signaling. This is known as desensitization. More importantly, they recruit a broad spectrum of signaling molecules and assemble them at receptors, inducing β-arrestin-dependent, G protein-independent signaling [14]. The mechanisms involved remain to be fully established. Here, we have investigated the role of the vertebrate-specific Gαo-binding protein GRIN3, an isoform of the GRIN family [11], and our results indicate that it might play an important role to prevent further G protein activation and initiate a β-arrestin-dependent, G protein-independent signaling.
First, we identified a Gαo-binding region (residues 668-686) within the C-terminal region of GRIN3. The Gαo-binding region is required to maintain GRIN3 level at the plasma membrane. We also established that GRIN3 interacts with GRK2 and GRK6, and GRK6, which is the major GRK expressed in striatum, might associate with GRIN3 [3]. Importantly, the Gαo-binding region might play an important role in the association of GRIN3 with GRK6 through Gαo. GRIN3 also interacted with both β-arrestin 1 and β-arrestin 2. These data strongly indicate that GRIN3 serves to recruit and assemble signaling molecules at DAR to initiate β-arrestin-dependent, G protein-independent signaling.
We thus examined the role of GRIN3 in β-arrestin-dependent, G protein-independent signaling in vivo using GRIN3KO mice. In WT mice, GRIN1 is widely expressed in the CNS and GRIN2 is expressed predominantly in the brain stem [5, 15], whereas GRIN3 is expressed in striatum, indicating that GRIN3 may play a role in dopamine-dependent behavior. We found that Akt phosphorylation on threonine 308 was not attenuated in the striatum of GRIN3KO after injection of dopamine reuptake inhibitor GBR12909 and this is consistent with the decrease of locomotor activity and increase of anxiety-like behavior in GRIN3KO mice [3, 6].
Recently, β-arrestin 2 was reported to be essential for the dephosphorylation/inactivation of Akt at threonine 308 in response to dopamine stimulation [3]. However, the regulatory mechanism of the transition from G protein-dependent signaling to β-arrestin-dependent, G protein-independent signaling upon stimulation of DAR remains poorly understood [7]. Our results show that association of GRIN3 to DAR might play an important role to prevent further G protein activation therefore ensuring receptor desensitization.
Abbreviations
- GPCR:
-
G protein-coupled receptor
- AC:
-
Adenylyl cyclase
- cAMP:
-
Cyclic adenosine 3′,5′-cyclic monophosphate
- GRK:
-
G protein-coupled receptor kinase
- GRIN:
-
G protein-regulated inducer of neurite growth
- GRIN3KO:
-
GRIN3-knockout mice
- q-PCR:
-
Quantitative real-time PCR
- DAR:
-
Dopaminr receptor
- lacZ:
-
β-Galactosidase
- co-IP:
-
Co-immunoprecipitation
- ip:
-
Intraperitoneal injection
- WT:
-
Wild-type
- D1R:
-
Dopamine D1 receptor
- D2R:
-
Dopamine D2 receptor
- D3R:
-
Dopamine D3 receptor
- M4R:
-
Muscarinic acetylcholine receptor M4
- HA:
-
Hemagglutinin
- GFP:
-
Green fluorescent protein
- HA:
-
Numerical aperture
- Gαowt:
-
Wild-type Gαο
- GRR12909:
-
1-(2-(bis(4-fluorophenyl)methoxy)ethyl)-4-(3-phenylpropyl)piperazine dihydrochloride
References
Beaulieu JM, Del’guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR (2011) Beyond cAMP: the regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci 4:38
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG (2005) An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261–273
Chen CA, Manning DR (1999) Regulation of G proteins by covalent modification. Oncogene 20:1643–1652
Chen LT, Gilman AG, Kozasa T (1999) A candidate target for G protein action in brain. J Biol Chem 274:26931–26938
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493
Del'guidice T, Lemasson M, Beaulieu JM (2011) Role of β-arrestin 2 downstream of dopamine receptors in the basal ganglia. Front Neuroanat 5:58
Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Hollt V (2001) Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain Res Mol Brain Res 95:129–137
Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT (2003) Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38:291–303
Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27:107–144
Iida N, Kozasa T (2004) Identification and biochemical analysis of GRIN1 and GRIN2. Methods Enzymol 390:475–483
Iwamoto T, Okumura S, Iwatsubo K, Kawabe J, Ohtsu K, Sakai I, Hashimoto Y, Izumitani A, Sango K, Ajiki K, Toya Y, Umemura S, Goshima Y, Arai N, Vatner SF, Ishikawa Y (2003) Motor dysfunction in type 5 adenylyl cyclase-null mice. J Biol Chem 278:16936–16940
Lefkowitz RJ (2007) Seven transmembrane receptors: a brief personal retrospective. Biochim Biophys Acta 1768:748–755
Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by β-arrestins. Science 308:512–517
Masuho I, Mototani Y, Sahara Y, Asami J, Nakamura S, Kozasa T, Inoue T (2008) Dynamic expression patterns of G protein-regulated inducer of neurite outgrowth 1 (GRIN1) and its colocalization with Gαo implicate significant roles of Gαo-GRIN1 signaling in nervous system. Dev Dyn 237:2415–2429
Nakata H, Kozasa T (2005) Functional characterization of Gαo signaling through G protein-regulated inducer of neurite outgrowth 1. Mol Pharmacol 67:695–702
Namkung Y, Dipace C, Javitch JA, Sibley DR (2009) G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem 284:15038–15051
Namkung Y, Dipace C, Urizar E, Javitch JA, Sibley DR (2009) G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J Biol Chem 284:34103–34115
Okumura S, Fujita T, Cai W, Jin M, Namekata I, Mototani Y, Jin H, Ohnuki Y, Tsuneoka Y, Kurotani R, Suita K, Kawakami Y, Hamaguchi S, Abe T, Kiyonari H, Tsunematsu T, Bai Y, Suzuki S, Hidaka Y, Umemura M, Ichikawa Y, Yokoyama U, Sato M, Ishikawa F, Izumi-Nakaseko H, Adachi-Akahane S, Tanaka H, Ishikawa Y (2014) Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. J Clin Invest 124:2785–2801
Okumura S, Kawabe J, Yatani A, Takagi G, Lee MC, Hong C, Liu J, Takagi I, Sadoshima J, Vatner DE, Vatner SF, Ishikawa Y (2003) Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ Res 93:364–371
Okumura S, Suzuki S, Ishikawa Y (2009) New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: effects of targeted disruption of the type 5 adenylyl cyclase gene. J Pharmacol Sci 109:354–359
Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, Ishikawa Y (2003) Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A 100:9986–9990
Violin JD, Ren XR, Lefkowitz RJ (2006) G-protein-coupled receptor kinase specificity for β-arrestin recruitment to the β2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem 281:20577–20588
Wettschureck N, Offermanns S (2005) Mammalian G proteins and their cell type specific functions. Physiol Rev 85:1159–1204
Zhou QY, Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83:1197–1209
Funding
This study was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (22791147, 20790860 to Y.M., 15K18973, 24970219 to K. Suita, 17K12067 to Y.O., 17K11977, 26463127 to M.N., 17K17342, 26861803 to D.U., 15K12330 to K. Shiozawa, 16H05300, 23591087 to S.O.), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1511018 to S.O.), Yokohama Academic Foundation (Y.O.), the Naito Foundation (2015-119 to S.O.), Senshin Medical Research Foundation (S.O.), an Academic Contribution from Pfizer Japan (AC160910, AC1500818, AC170780 to S.O.), the Research Foundation for Community Medicine (S.O.), Mitsui Life Social Welfare Foundation (S.O.), and Research Promotion Grant from the Society for Tsurumi University School of Dental Medicine (29002, 27010 to I.A., 28006 to N.K., 28006 to Y.Y., 29007 to K.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures and protocols for the experiments were approved by the Committee for Animal Research of Tsurumi University and National Center for Global Health and Medicine.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 738 kb)
Rights and permissions
About this article
Cite this article
Mototani, Y., Okamura, T., Goto, M. et al. Role of G protein-regulated inducer of neurite outgrowth 3 (GRIN3) in β-arrestin 2-Akt signaling and dopaminergic behaviors. Pflugers Arch - Eur J Physiol 470, 937–947 (2018). https://doi.org/10.1007/s00424-018-2124-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2124-1