Abstract
Ryanodine receptor type 3 (RyR3) is expressed in myometrial smooth muscle cells (MSMCs). The short isoform of RyR3 is a dominant negative variant (DN-RyR3) and negatively regulates the functions of RyR2 and full-length (FL)-RyR3. DN-RyR3 has been suggested to function as a major RyR3 isoform in non-pregnant (NP) mouse MSMCs, and FL-RyR3 may also be upregulated during pregnancy (P). This increase in the FL-RyR3/DN-RyR3 ratio may contribute to the strong contractions by MSMCs for parturition. In the present study, spontaneous contractions by the myometrium in NP and P mice were highly susceptible to nifedipine but were not affected by ryanodine. Ca2+ image analyses under a voltage clamp revealed that the influx of Ca2+ through voltage-dependent Ca2+ channels did not cause the release of Ca2+ from the sarcoplasmic reticulum (SR). Cytosolic Ca2+ concentrations ([Ca2+]cyt) in MSMCs were not affected by caffeine. Despite the abundant expression of large conductance Ca2+-activated K+ channels in MSMCs, spontaneous transient outward currents were not observed in the resting state because of the substantive lack of Ca2+ sparks. Quantitative PCR and Western blot analyses indicated that DN-RyR3 was strongly expressed in the NP myometrium, while the expression of FL-RyR3 and DN-RyR3 was markedly reduced in the P myometrium. The messenger RNA (mRNA) expression of RyR2 and RyR1 was negligible in the NP and P myometria. Moreover, RyR3 knockout mice may become pregnant and deliver normally. Thus, we concluded that none of the RyR subtypes, including RyR3, play a significant role in the regulation of [Ca2+]cyt in or contractions by mouse MSMCs regardless of pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ryanodine receptor (RyR) is one of the major intracellular Ca2+ release channels and conducts excitation–contraction (E–C) coupling in striated and some smooth muscle cells (SMCs) [31, 36, 42]. Previous studies identified three subtypes of RyR, i.e., RyR1, RyR2, and RyR3, which are coded by distinct genes in vertebrates [26]. RyR1 is abundantly expressed in skeletal muscle cells and physically interacts with voltage-dependent Ca2+ channels (VDCC) [45]. RyR2 is widely expressed in excitable cells, such as cardiomyocytes, neurons, and SMCs [32, 38]. RyR3 is expressed at low levels in a wide range of different cell types [16]. All three isoforms are present in SM tissues, and the relative proportion of the expression of each channel is dependent on the tissue and species [48].
In the SMCs of some visceral tissues as well as in cardiac myocytes, RyR2 is considered to be mainly responsible for the Ca2+-induced Ca2+ release (CICR) mechanism [12, 17, 18]. When SMCs are depolarized by physiological stimuli, the influx of Ca2+ occurs via VDCC opening. This Ca2+ activates loosely coupled RyRs and induces “Ca2+ hotspots”, which in turn evoke global CICR, resulting in muscle contraction in some highly excitable SMCs [31]. On the other hand, RyRs in the sarcoplasmic reticulum (SR) cause local, spontaneous, and transient calcium release, i.e., Ca2+ sparks in the resting state [33]. These Ca2+ events occur in local areas in SMCs in order to couple with large conductance Ca2+-activated K+ channel (BKCa, KCa1.1) activity and contribute to the action potential repolarization phase and occurrence of spontaneous transient outward currents (STOCs) [36]. Membrane repolarization and hyperpolarization by BKCa channel activation reduce VDCC activities and are regarded as the main negative feedback mechanisms for the regulation of [Ca2+]cyt to stabilize tone in various types of SM tissues [19].
RyR3 is ubiquitously expressed in various cell types; however, its physiological impact has not yet been elucidated in detail. A homozygous RyR3 gene deletion (RyR3−/−) does not result in a lethal phenotype, in contrast to RyR1−/− and RyR2−/−. RyR3−/− shows increases in locomotor activity and memory impairment [4, 14, 24, 44]. The knockdown of RyR3 results in a decrease in the frequency of Ca2+ oscillations in Jurkat T cell and duodenal SMCs [9, 25]. A previous study reported the existence of a dominant negative isoform (DN-RyR3) lacking 29 amino acid residues corresponding to exon 97 [21]. This variant is widely expressed in SM tissues such as the uterus and duodenum, but not in the brain or skeletal muscle. This variant channel does not display any channel function and negatively regulates RyR2 and full-length RyR3 (FL-RyR3) by forming heterotetramers [8, 10, 21]. Mironneau et al. suggested that RyR3 is the main RyR expressed in myometrial SMCs (MSMCs) and only functions when Ca2+ is over-loaded to intracellular stores in non-pregnant (NP) cells [30]. Dabertland et al. reported that the expression of FL-RyR3 increases toward term and facilitates contraction ability for complete parturition [8]. On the other hand, the same group has also shown that caffeine, an activator of RyR, does not contract but relaxes the pregnant rat myometrium [40]. In addition, another group showed that Ca2+ sparks in myometrium were absent, although they had found them in vascular preparations [7].
The results obtained in the present study were not consistent with previous findings. In our experimental system, RyR3 was not sufficiently expressed as the functional Ca2+ release channel in NP or pregnant (P) mouse MSMs. Moreover, the messenger RNA (mRNA) expression of FL-RyR3 and DN-RyR3 was significantly weaker in the P uterus than in the NP uterus. The most important result is that RyR3−/− displayed normal pregnancy and parturition. Collectively, these results suggest that RyR3 is not necessary for parturition, whereas Ca2+ influx through L-type VDCC and Ca2+ release from IP3 receptors are required for spontaneous and agonist-induced contractions, respectively.
Methods
Animals
The generation of RyR3 knockout mice on the C57BL/6 background (RyR3−/−) has been reported previously [44]. In order to elucidate the genotypes of mutant mice, a polymerase chain reaction (PCR) was performed using the following primers: forward primer (TCCAGGAATCTCTGGTATACTAGGG) and reverse primer (ATGAAAGTTGTACTCCAGTGCATTGC). Amplified DNA was analyzed on 2% agarose gels. Wild-type female mice (C57BL/6, RyR3+/+) of 8 weeks old were purchased from Japan SLC (Hamamatsu, Japan). Non-pregnant myometrium was obtained from non-parous mice. To obtain pregnant myometrium, three primiparous female mice (8–16 weeks old) were paired with a male mouse (8–25 weeks old) overnight (day 0). The next morning, the male mouse was moved to separate cage. Pregnant myometrium tissues were obtained from mice with day 18 of gestation. In this study, myometrial tissues were obtained from 8 to 20-week-old mice. All experiments were approved by the Ethics Committee of Nagoya City University and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the Japanese Pharmacological Society.
Measurements of contractility from myometrial tissue strips
Myometria from 8 to 20-week-old female mice were removed and placed in Ca2+-free Krebs solution. The myometrium was cut open and isolated as small strips (0.8–1.2 mm wide and 5–7 mm long). Each strip was placed in a tissue bath (5 ml in volume) containing Krebs solution aerated with 95% O2 and 5% CO2 and kept at 37 °C. One end of the strip was pinned to the chamber bottom, and the other end was connected to a force–displacement transducer for the measurement of isometric contractility [17]. Strips were stretched to a tension of 0.2 to 0.3 mN.
Cell isolation
Single MSMCs were enzymatically isolated from NP and P mice as previously reported [15]. In brief, the myometrium was dissected out and freed from other tissues in Ca2+-free physiological salt solution for dissociation (dPSS). This solution was then replaced with Ca2+-free dPSS solution containing 0.1% papain (Sigma-Aldrich, St. Louis, USA) and 0.1% dithiothreitol (Sigma-Aldrich) at 4 °C. After a 30-min treatment, the solution was replaced with 100 μM Ca2+ dPSS solution containing 0.1% collagenase (Wako Pure Chemical Industries, Osaka, Japan) at 37 °C. After a 25-min treatment, the solution was replaced with enzyme-free and Ca2+-free dPSS solution. The tissue was then gently triturated using a glass pipette to isolate cells. Urinary bladder SMCs (UBSMCs) were also isolated as described previously [17]. At the start of each experiment, a few drops of the cell suspension were placed in a recording chamber.
Electrophysiological recording and data analysis
Electrophysiological experiments were performed using the whole-cell patch clamp technique with an EPC7 amplifier (HEKA Electronics, Darmstadt, Germany), analog–digital converter (DIGIDATA 1440A; Axon Instruments, Foster City, USA), and pCLAMP software (version 10.2; Axon Instruments) [42]. Membrane currents and voltage signals were also stored and analyzed using Clampex 10.2 and Clampfit 10.2 software (Axon Instruments).
[Ca2+]cyt measurements
[Ca2+]cyt in the whole cell area was measured using Fura-2/AM (Invitrogen, Carlsbad, USA) and the ARGUS/HiSCA system (Hamamatsu Photonics, Hamamatsu, Japan) as reported previously [17].
Changes in local [Ca2+]cyt mediated by Ca2+ sparks were recorded using a total internal reflection fluorescence (TIRF) imaging system (Nikon, Tokyo, Japan) including an EM-CCD camera (C9100-12; Hamamatsu Photonics) and AQUACOSMOS software (Ver. 2.6; Hamamatsu Photonics) [42]. MSMCs were loaded with 10 μM Fluo-4/AM (Invitrogen) in standard HEPES-buffered solution at room temperature for 10 min (23 ± 1 °C). Fluo-4/AM was excited with a 488-nm argon laser (Spectra-Physics, Santa Clara, USA), and the emission was collected using a filter cube (DM505/BA520; Nikon). Fluorescence intensity (F) in the regions of interest (ROIs) was expressed as average fluorescence within the ROIs (2 μm in diameter). F was normalized to basal fluorescence intensity (F 0). Images were recorded at 37 Hz (512 × 512 pixels). The threshold for the Ca2+ spark was set at the averaged intensity plus 10 times of standard deviation of the average from quiescent areas (2 μm in diameter), in which sparks were not observed during the recording for 1 s. Transient increase in fluorescence above the threshold was defined as “a Ca2+ spark.”
When membrane currents and Ca2+ events were simultaneously recorded, a laser scanning confocal fluorescent microscope (A1R; Nikon) equipped with a fluorescent microscope (ECLIPSE Ti; Nikon) and NIS Elements software (version 3.10; Nikon) was utilized. Cells were loaded with 100 μM Fluo-4 by diffusion from recording electrodes. The excitation wavelength from the multi-argon laser (Melles Griot, Carlsbad, USA) was 488 nm, and the emission light was collected by a band-pass filter (515/30 nm). F in the ROIs was expressed as average fluorescence within the whole cell area.
RNA extraction and quantitative PCR
Total RNAs were extracted from myometrium homogenates by the acid guanidium thiocyanate–phenol method, and reverse transcription (RT) was performed with SUPERSCRIPT II reverse transcriptase and a random hexamer (Invitrogen) [37]. Real-time quantitative PCR was performed using SYBR Green Chemistry (Takara Bio Inc., Kusatsu, Japan) on an ABI 7000 sequence detector (Applied Biosystems, Foster City, USA).
Standard curves (threshold cycle vs. log of DNA dilution) were constructed by amplifying the complementary DNA (cDNA) of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). It was confirmed that GAPDH level judging from Ct value does not change between pregnant and non-pregnant state by real-time PCR. Each cDNA sample was tested in triplicate. Quantitative PCR experiments were performed using the following specific primers: total RyR3 (NM_177652.2) (forward: AACCTGAGTTCACGACAAGCTACA and reverse: CTTCGTGCACAAAAGCCAAGT), FL-RyR3 (NM_177652.2) (forward: CCTGAGGTTCCTTGCTCTGTTT and reverse: CATCCTCTGTCTCTTCCTCTAAAGGT), DN-RyR3 (NM_177652.2) (forward: TGAGAAGCCAGAAGCCTTTATG and reverse: CAAGTTATTGGTCACTGAAGAACC), RyR1 (NM_009109.2) (forward: ATTACAGAGCAGCCCGAGGAT and reverse: AGAACCTTCCGCTTGACAAACT), RyR2 (NM_023868.2) (forward: CTTCGATGTTGGCCTTCAAGAG and reverse: AGAACCTTCCGCTTGACAAACT), and GAPDH (NM_001289726.1) (forward: CATGGCCTTCCGTGTTCCT and reverse: CCTGCTTCACCACCTTCTTGA).
Western blot
The SR membrane fraction was prepared from the mouse myometrium. Briefly, tissues were suspended in homogenizing buffer containing 4 mM HEPES, 320 mM sucrose, and protease inhibitor cocktail (Sigma-Aldrich), mechanically homogenized, and then centrifuged for 10 min at 8000×g. The supernatants were retrieved and centrifuged for 1 h at 100,000×g. The pellets containing the SR membrane fraction were suspended in sample buffer (60 mM Tris-HCl (pH 6.8), 15% glycerol, 2% SDS, and 0.001% bromophenol blue). Equal protein samples were subjected to SDS-PAGE on 7.5% polyacrylamide gels, and the proteins were then transferred to PVDF membranes (Amersham Biosciences, Piscataway, USA). Membranes were blocked with 2% BSA and 0.1% Tween 20 in PBS. The blots were incubated with anti-RyR (34C) (GeneTex Inc., Irvine, USA) and then incubated with anti-mouse horseradish peroxidase-conjugated IgG (Chemicon International Inc., Temecula, USA). An enhanced chemiluminescence detection system (GE Healthcare Life Sciences, Marlborough, USA) was used to detect the bound antibody. The resulting images were analyzed using a LAS 3000 device (Fujifilm, Tokyo, Japan).
Solutions
The compositions of solutions used in this study were shown in Table 1.
Materials
The sources of pharmacological agents were as follows: CdCl2, TEA-Cl, and ryanodine from Wako Pure Chemical Industries; nifedipine and paxilline (Pax) from Sigma-Aldrich; EGTA, BAPTA, and HEPES from Dojin (Kumamoto, Japan); and oxytocin (Oxy) from the Peptide Institute (Osaka, Japan).
Statistical analysis
Data are expressed as the mean ± SEM. “n” means the number of myometrial strips or isolated cells. The significance of differences between two or among multiple groups was examined using the Student’s t test or Tukey’s test after an F test or one-way ANOVA, respectively. Significance was expressed in the figures by asterisks: ∗p < 0.05, ∗∗p < 0.01.
A concentration–response relationship was examined by measuring the relative amplitude of a contraction versus the control and fitting a curve to each set of data based on the Hill equation: y = 1 / [1 + (D / [EC50])n], where D is the drug concentration, EC50 is the concentration giving a half-maximal response, and n is the Hill coefficient. A concentration–inhibition relationship was also investigated by fitting a curve to each set of data based on the Hill equation: y = 1 − {1 / [1 + (D / [IC50])n]}, where IC50 is half-maximal inhibition.
Results
RyR3 does not contribute to Ca2+ release from the SR or spontaneous contractions
The effects of ryanodine, an inhibitor of RyR, on spontaneous contractions were examined using strip preparations from the NP myometrium and P myometrium. The treatment with 50 μM ryanodine did not change the relative amplitude of spontaneous contractions in NP (0.97 ± 0.04, n = 4 from three mice) or P (1.28 ± 0.07, n = 4 from two mice) from that in the control to which vehicle was added (DMSO) (NP 0.93 ± 0.05, n = 4 from three mice; P 1.29 ± 0.27, n = 4 from two mice; p > 0.05, respectively) (Fig. 1a, b). Similarly, the relative frequency of contractions was not significantly changed by ryanodine in NP (1.00 ± 0.06) or P (1.05 ± 0.09) from that in the control (DMSO) (NP 0.87 ± 0.05, P 1.14 ± 0.10; p > 0.05) (Fig. 1a, c). Thus, ryanodine did not significantly affect spontaneous contractions in the NP myometrium or P myometrium.
No contribution of RyR to spontaneous contractions. a Typical traces of spontaneous contractions in myometrial strips from non-pregnant (NP) and pregnant (P) mice. DMSO-containing solution was used for the control group. b, c Summarized data on the amplitude (b) and frequency (c) of spontaneous contractions. Relative values after the exposure to 10 μM ryanodine or only DMSO (used as vehicle) for 10 min were normalized to those recorded 10 min prior to the addition of ryanodine. d NP-MSMCs and P-MSMCs exhibiting spontaneous Ca2+ oscillations were treated with 10 μM tetracaine. e The effects of 10 μM tetracaine on the amplitude of Ca2+ oscillations were summarized. No significant difference was observed between control and test group (b, c, e). f The response to 10 mM caffeine was examined in NP-MSMCs and P-MSMCs. UBSMCs (Uri) were used as the positive control
In Fig. 1d, effects of tetracaine, another RyR inhibitor, on spontaneous Ca2+ oscillations were examined by measuring changes in [Ca2+]cyt with Fura-2/AM in single myocytes freshly isolated from the NP myometrium or P myometrium. As shown in Fig. 1d, 10 μM tetracaine had no effect on Ca2+ oscillations in NP-MSMCs or P-MSMCs (relative amplitudes were 1.03 ± 0.26 and 1.06 ± 0.12, n = 3 from two mice, respectively, Fig. 1e).
We then investigated whether caffeine, an activator of RyR, evokes Ca2+ release from the SR in MSMCs. UBSMCs (Uri in Fig. 1f) were used as a positive control. The application of 10 mM caffeine caused a transient increase in [Ca2+]cyt, presumably via its release from the SR in UBSMCs (90% of 140 mM KCl-positive cells, n = 10 from three mice) (Fig. 1f). On the other hand, 10 mM caffeine did not induce [Ca2+]cyt increase in NP-MSMCs or P-MSMCs (NP 3%, n = 38 from three mice; P 0%, n = 18 from five mice), whereas 140 mM KCl markedly elevated [Ca2+]cyt.
These results indicate that RyR is not functional as a Ca2+ release channel and, thus, does not contribute to spontaneous contractions regardless of pregnancy in mouse MSMCs.
Spontaneous contractions are highly dependent on Ca2+ influx through L-type Ca2+ channels
The effects of nifedipine, an L-type Ca2+ channel blocker, on spontaneous contractions were examined in NP and P myometrial tissue strips (Fig. 2a). The amplitudes of contractions were reduced by nifedipine in a concentration-dependent manner (1–100 nM). Figure 2b shows the dose–response relationship for nifedipine-induced decreases in the relative amplitudes of contractions. The IC50 values obtained for nifedipine were not significantly different between NP (20.0 ± 2.8 nM, n = 3) and P (25.0 ± 9.8 nM, n = 3, p > 0.05). The Hill coefficient was 1.20 ± 0.36 in NP-MSMs and 1.34 ± 0.35 in P-MSMs (p > 0.05).
High dependency of spontaneous contractions on Ca2+ influx through L-type Ca2+ channels. a Typical traces of spontaneous contractions in NP and P myometrial strips. Nifedipine was added cumulatively at the concentrations indicated under the traces. b The dose-dependent inhibition of spontaneous contractions by nifedipine was calculated based on a decrease in contraction amplitude. Amplitude was normalized to the value obtained 5 min prior to the addition of nifedipine. c Typical traces of VDCC currents in NP-MSMCs and P-MSMCs. Extracellular 2.2 mM Ca2+ was replaced with 30 mM Ba2+, and VDCC currents were obtained as 100 μM Cd2+-sensitive current components. Cells were depolarized for 150 ms from a holding potential of −60 to +20 mV. Note that the amplitude of VDCC currents in a P-MSMC was sixfold larger than that in an NP-MSMC. d Current–voltage relationships for VDCC currents were plotted. e Summarized data of the VDCC current density at +20 mV and cell capacitance. **p < 0.01 vs. NP
Membrane currents were measured from freshly isolated MSMCs by whole-cell patch clamp recording. VDCC currents were measured in NP-MSMCs and P-MSMCs under the block of K+ currents by Cs+ and TEA-Cl in the pipette solution, and extracellular Ca2+ was replaced with 30 mM Ba2+ (Fig. 2c). Figure 2d shows current–voltage relationships from NP-MSMCs and P-MSMCs. The peak VDCC current density at +20 mV and cell capacitance were summarized in Fig. 2e. No significant difference was observed in current densities between NP-MSMCs (−13.2 ± 1.4 pA/pF, n = 3 from three mice) and P-MSMCs (−13.3 ± 1.9 pA/pF, n = 5 from three mice; p > 0.05), while cell capacitance was significantly larger in P-MSMCs (68.7 ± 6.6 pF) than in NP-MSMCs (13.1 ± 3.2 pF; p < 0.01) (Fig. 2e).
The lack of CICR through RyR and decreases in BKCa current density after pregnancy
In order to measure the Ca2+ entry by membrane depolarization and subsequent CICR in MSMCs, membrane currents and [Ca2+]cyt were simultaneously recorded (Fig. 3a), as has been performed in mouse UBSMCs [31]. The relative increase in [Ca2+]cyt upon depolarization to +10 mV was not significantly changed by pregnancy (NP control 1.28 ± 0.16, n = 5 from three mice; P control 2.41 ± 0.72, n = 4 from three mice; p > 0.05) (Fig. 3b). Although the shapes and amplitudes of membrane currents appeared to be changed by pregnancy (Fig. 3a), the density of the peak outward current was not affected (NP control 6.79 ± 0.10 pA/pF, n = 5 from three mice; P control 4.00 ± 0.39 pA/pF, n = 4 from three mice; p > 0.05). [Ca2+]cyt was not significantly affected by the application of 10 μM ryanodine in NP-MSMCs (ryanodine 0.99 ± 0.08, n = 3 from three mice, Fig. 3b) or P-MSMCs (ryanodine 2.21 ± 0.25, n = 3 from two mice; Fig. 3b). Accordingly, membrane current density was not affected by ryanodine in NP-MSMCs (ryanodine 9.06 ± 3.74 pA/pF, n = 3 from three mice; Fig. 3c) or P-MSMCs (ryanodine 4.36 ± 0.83 pA/pF, n = 3 from two mice; Fig. 3c). These results suggest that membrane depolarization does not cause CICR in MSMCs.
Substantive lack of CICR through RyR3. a Ca2+ events and membrane currents were simultaneously recorded under a voltage clamp in single MSMCs isolated from NP and P mice. Typical changes upon depolarization to +10 mV in [Ca2+]cyt (F/F 0) in the whole cell area were measured by confocal microscopy in the absence and presence of 10 μM ryanodine. Fluo-4 was added to cells through electrodes. Data were acquired at 120 frame/s. b Summarized data of increases in [Ca2+]cyt (F/F 0) from whole cell areas. c Summarized data of peak outward current density. d Typical traces of total outward, Pax-insensitive, and Pax-sensitive (BKCa) currents in NP-MSMCs and P-MSMCs are shown in the left, middle, and right panels, respectively. These traces were obtained at membrane potentials ranging between −70 and +60 mV and summarized as I–V relationships in the bottom panels. The Ca2+ concentration in the pipette filling solution was pCa 6.0. The Ca2+ current was blocked by 0.1 mM Cd2+. e Summarized data of the outward current density at +10 mV in NP-MSMCs and P-MSMCs. *p < 0.05. f Summarized data of the Pax-sensitive (BKCa) current density at +60 mV in NP-MSMCs and P-MSMCs. **p < 0.01
In order to examine changes in BKCa channel currents after pregnancy, outward currents were recorded in MSMCs. Figure 3d shows outward currents and I–V relationships before and after the treatment with 1 μM paxilline (Pax), a specific BKCa channel blocker (NP n = 4 from four mice; P n = 3 from three mice). The I–V relationships revealed the following: (1) The density of outward currents upon depolarization was markedly reduced by pregnancy (at +10 mV, NP 6.8 ± 0.8 pA/pF; P 3.7 ± 0.8 pA/pF, p < 0.05) (Fig. 3d (left panel), e). (2) The density of Pax-insensitive outward currents, presumably voltage-gated K+ currents, was significantly decreased by pregnancy (at +10 mV, Pax-insensitive current density, NP 5.3 ± 0.7 pA/pF; P 2.0 ± 0.3 pA/pF, p < 0.05) (Fig. 3d (middle panel), e). (3) The Pax-sensitive (BKCa channel) current density at +60 mV was significantly decreased by pregnancy (NP 49.0 ± 10.9 pA/pF; P 6.7 ± 0.9 pA/pF, p < 0.01; Fig. 3d, (right panel), f), while the density at +10 mV was small and not significantly changed by pregnancy (NP 1.5 ± 0.5 pA/pF; P 1.7 ± 0.6 pA/pF, p > 0.05; Fig. 3d (right panel), e).
These results indicate that significant BKCa channel activation by CICR upon depolarization does not occur in MSMCs regardless of pregnancy and also that outward currents upon depolarization, particularly the BKCa channel current component, were significantly downregulated by pregnancy, as has been reported previously [5].
STOCs and Ca2+ sparks were not detected in MSMCs
STOCs at holding potentials of −10 mV were measured in MSMCs and UBSMCs (Fig. 4a). STOCs were detected in UBSMCs (average amplitude 109.3 ± 28.6 pA; frequency 1.83 ± 0.87 Hz, n = 4 from four mice), which is consistent with previous findings [17, 31]. In contrast, clear STOCs with amplitudes of greater than 5 pA were not recorded in NP-MSMCs or P-MSMCs. As shown in Fig. 4b, integrated STOCs for 10 s from the baseline at −10 mV in NP-MSMCs and P-MSMCs were significantly smaller than those in UBSMCs (Uri 79.4 ± 14.4 pC, n = 4 from four mice; NP 15.2 ± 9.5 pC, n = 4 from three mice; P 7.7 ± 2.6 pC, n = 5 from three mice). Thus, STOCs were clearly detected in UBSMCs, but not in NP-MSMCs or P-MSMCs. Since BKCa channels were functional in NP-MSMCs and P-MSMCs (Fig. 3f), we hypothesized that spontaneous Ca2+ release through RyR, i.e., Ca2+ sparks, are absent or small/rare in MSMCs.
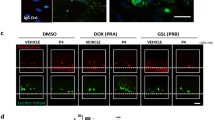
STOCs were not detected in MSMCs due to the substantive lack of Ca2+ sparks. a STOCs were recorded at −10 mV in UBSMCs (Uri), NP-MSMCs (NP), and P-MSMCs (P). b Outward currents at −10 mV for 10 s were integrated (pC). Note that almost no STOCs were detected in NP-MSMCs or P-MSMCs. c Typical Ca2+ images in UBSMCs (Uri), NP-MSMCs (NP), and P-MCMCs (P) at 110-ms intervals are shown. Images were obtained at 37 Hz by a TIRF microscope. d Changes in [Ca2+]cyt (F/F 0) in Ca2+ spark sites. F means the average fluorescence intensity within ROIs (2.0 μm in diameter). F 0 is the basal fluorescence intensity of the ROIs. e Summarized data of changes in F/F 0 (calculated as the area under the curve) are shown. **p < 0.01. f The [Ca2+]cyt elevation by 100 nM Oxy was confirmed in each cell used in “c–e.” Note that Oxy-induced [Ca2+]cyt increases in NP-MSMCs were similar to those in P-MSMCs
Fluo-4/AM was used in UBSMCs and MSMCs to measure Ca2+ sparks. Ca2+ sparks were detected in UBSMCs (n = 11 from six mice), but not in NP-MSMCs or P-MSMCs under the same recording conditions (n = 5 from three mice and n = 9 from four mice, respectively; Fig. 4c, d). Integrated intracellular Ca2+ elevations for 10 s in NP-MSMCs and P-MSMCs were significantly smaller than those in UBSMCs (Uri 53.4 ± 5.3, n = 11 from six mice; NP 13.7 ± 4.4, n = 5 from three mice; P 11.6 ± 1.7, n = 9 from four mice; p < 0.01 vs. UBSMCs, Fig. 4e). Only MSMCs in which [Ca2+]i elevations were observed in response to 100 nM Oxy were used in analyses of Ca2+ sparks (Fig. 4f). The treatment with 100 nM Oxy caused similar [Ca2+]cyt elevations in NP-MSMCs and P-MSMCs (p > 0.05). Therefore, the substantive lack of STOCs is attributable to the absence of clear Ca2+ sparks in NP-MSMCs and P-MSMCs.
Changes in mRNA and protein levels of RyRs in the NP myometrium and P myometrium
RyR1, RyR2, and RyR3 mRNA levels in the myometrium from NP and P mice were quantified using real-time PCR (Fig. 5a). The relative expression levels of RyR1 and RyR2 were low in the NP myometrium (RyR1 0.0013 ± 0.0005; RyR2 0.0008 ± 0.0002, n = 4 from four mice) and P myometrium (RyR1 0.0005 ± 0.0001; RyR2 0.0008 ± 0.0002, n = 4 from four mice). The transcriptional level of total RyR3 (i.e., FL-RyR3 + DN-RyR3) was significantly lower in the P myometrium than in the NP myometrium (NP 0.013 ± 0.003, n = 4 from four mice; P 0.003 ± 0.001, n = 4 from four mice; p < 0.05). By using specific primer pairs, we also measured the mRNA levels of FL-RyR3 and DN-RyR3. FL-RyR3 and DN-RyR3 transcripts were both abundant in the NP myometrium (FL-RyR3 0.004 ± 0.001; DN-RyR3 0.008 ± 0.002, n = 4 from four mice) but were significantly reduced in the P myometrium (FL-RyR3 0.0009 ± 0.0001; DN-RyR3 0.0013 ± 0.0004, n = 4 from four mice; p < 0.05 vs. NP). A Western blotting analysis using an anti-RyR3 antibody suggested that the relative expression of RyR3 was reduced in the P myometrium (NP 0.028 ± 0.009, n = 3 from three mice; P 0.001 ± 0.002, n = 3 from three mice; p < 0.05; Fig. 5b). These results indicate that the expression of RyR3 is downregulated toward the term of pregnancy.
RyR3 was not significantly functional in labor. a Quantitative real-time PCR analyses of RyR1–3 in the NP myometrium and P myometrium were performed. As for RyR3, the mRNA levels of total RyR3 (measured by the detection of the RyR3 transcript sequence common to FL-RyR3 and DN-RyR3), FL-RyR3, and DN-RyR3 were separately evaluated. *p < 0.05. b Western blot analyses of RyR3 in the NP myometrium and P myometrium were performed. Proteins extracted from the mouse brain were used as a positive control (left panels). RyR3 protein levels were normalized by that of β-actin (right panel). *p < 0.05. c The expression of RyR3 and its absence were confirmed by Western blotting in RyR3+/+ and RyR3−/− mice, respectively. d The number of pups per parturition of primiparous RyR3+/+ and RyR3−/− mice were counted. Note that RyR3−/− mice exhibited normal pregnancies and parturition
Deletion of the RyR3 gene had no effect on pregnancy/labor, myometrial contractility, or expression levels of other proteins that contribute to Ca2+ regulation in MSMCs
Experiments were performed using RyR3−/− mice. The NP myometrium from RyR3−/− mice lacked the RyR3 protein (Fig. 5c). The number of pups per parturition was counted from primiparous mice younger than 20 weeks of age. The number of pups was not significantly different between RyR3+/+ and RyR3−/− mice; parity was 8.3 ± 0.4 in RyR3+/+ mice (nine mice) and 8.1 ± 0.5 in RyR3−/− mice (nine mice; p > 0.05; Fig. 5d). The deletion of RyR3 did not affect pregnancy or parturition.
Spontaneous contractions were measured in myometrial strips from RyR3−/− mice (Fig. 6a). No significant difference was observed between RyR3+/+ and RyR3−/− mice in the amplitude (RyR3+/+ 0.31 ± 0.04 mN, n = 4 from four mice; RyR3−/− 0.44 ± 0.08 mN, n = 4 from four mice; p > 0.05) or frequency (RyR3+/+ 10.5 ± 3.7/min; RyR3−/− 11.3 ± 3.0/min; p > 0.05) of spontaneous contractions (Fig. 6b). The transcript levels of Ca2+ handling proteins such as IP3 receptor type 1 (IP3R), VDCC, Na+-Ca2+ exchanger type 1 (NCX), and SR-Ca2+-ATPase type 2A (SERCA2A) in P-MSM were similar between RyR3+/+ (three mice) and RyR3−/− (three mice) mice (Fig. 6c).
RyR3−/− mice exhibited normal myometrial contractions and ion channel expression. a Typical traces of spontaneous contractions by NP-MSMs from RyR3+/+ and RyR3−/− mice. b Summarized data of spontaneous contraction amplitudes and frequencies. No significant difference was observed between RyR3+/+ and RyR3−/− mice. c The expression of Ca2+ handling proteins in P-MSMs was analyzed by quantitative real-time PCR. d Typical traces of VDCC currents in NP-MSMCs and P-MSMCs from RyR3−/− mice. In this experiment, 2.2 mM of extracellular Ca2+ was replaced with 30 mM Ba2+. Cells were depolarized for 150 ms from a holding potential of −60 to +20 mV. e Current–voltage relationships for VDCC currents were plotted. f Summarized data of the current density of VDCC at +20 mV and cell capacitance. **p < 0.01 vs. NP
Ca2+ currents were also measured in the same manner as in Fig. 2c–e (Fig. 6d–f). The peak VDCC current density at +20 mV in NP-MSMCs and P-MSMCs from RyR3−/− mice and cell capacitance was summarized (Fig. 6f). Current density was similar between NP-MSMCs (−11.7 ± 0.7 pA/pF, n = 3 from three mice) and P-MSMCs (−13.2 ± 1.0 pA/pF, n = 5 from three mice; p > 0.05), while cell capacitance was significantly larger in P-MSMCs (73.8 ± 5.8 pF) than in NP-MSMCs (16.1 ± 0.2 pF; p < 0.01). These values were not significantly different from those obtained in RyR3+/+ mice (p > 0.05 vs. Fig. 2).
The sensitivity of the myometrium in RyR3−/− mice to Oxy was similar to that in RyR3+/+ mice
The dose-dependent contractions induced by Oxy were compared among four groups in the presence of nifedipine (Fig. 7a). EC50 values were reduced by pregnancy in RyR3+/+ (EC50 4.76 ± 0.97 nM in NP, n = 3 from two mice and 1.28 ± 0.64 nM in P, n = 5 from four mice, p < 0.01) and RyR3−/− mice (3.2 ± 0.24 nM in NP, n = 6 from four mice and 0.79 ± 0.02 nM in P, n = 5 from two mice, p < 0.01) (Fig. 7b). However, EC50 was not significantly affected by a RyR3 deficiency in the NP myometrium or P myometrium (p > 0.05). The Hill coefficient of Oxy was 1.02 ± 0.10 in NP-MSMs and 1.00 ± 0.04 in P-MSMs from RyR3+/+ mice and 1.06 ± 0.03 in NP-MSMs and 1.11 ± 0.10 in P-MSMs from RyR3−/− mice (p > 0.05 among four groups). The force of contractions by P myometrial strips induced by the 100 nM Oxy stimulation was also similar between RyR3+/+ and RyR3−/− mice (5.66 ± 0.77 mN, n = 5 from four mice in NP and 4.99 ± 0.51 mN, n = 5 from two mice in P; p > 0.05; Fig. 7c).
The sensitivity of the myometrium to Oxy was not changed by RyR3 deficiency. a The sensitivity of the myometrium to Oxy was assessed by means of muscular contraction recordings. b The EC50 values obtained are summarized. No significant difference in NP-MSMs and P-MSM between RyR3+/+ and RyR3−/− mice. **p < 0.01. c Peak contractions by P-MSM from RyR3+/+ and RyR3−/− mice in response to 100 nM Oxy were compared
Discussion
The results of the present study clearly demonstrated that RyRs made almost no functional contribution to spontaneous contractions by MSMCs, regardless of pregnancy. Moreover, the expression of RyR3 was decreased by pregnancy. These results are not consistent with previous findings, which suggested that an increase in the ratio of FL-RyR3 versus DN-RyR3 at the late stage of pregnancy markedly contributed to strong contractions during labor [8].
Ryanodine and tetracaine had no effect on spontaneous contractions in NP or P myometrial strips (Fig. 1). On the other hand, nifedipine (Fig. 2) or the removal of external Ca2+ (data not shown) completely suppressed spontaneous contractions. Thus, spontaneous contractions in MSMCs are essentially dependent on the Ca2+ entry though VDCC but are not mediated by Ca2+ release from RyR (CICR). The direct recording of [Ca2+]cyt demonstrated that depolarization-induced Ca2+ entry through VDCC weakly induced CICR in NP-MSMCs and P-MSMCs (Fig. 3, see also [29]). Previous studies have shown that RyR activators have weak or negligible effects on spontaneous contractions and calcium oscillations in NP-MSMs from humans and rats [41, 43]. Our results suggest that spontaneous myometrial contractions in mice at the late stage of pregnancy are also insensitive to ryanodine. In contrast, ∼60% of MSMs from pregnant rats are susceptible to ryanodine [28, 30, 43]. Thus, inconsistencies, including possible species differences, exist between previous studies on the involvement of RyR in myometrial spontaneous contractions.
In the many kinds of SMCs, such as those in the urinary bladder, vas deferens, gastrointestinal tract, trachea, and blood vessels, local spontaneous Ca2+ release through RyR, Ca2+ sparks, stimulates BKCa channels and induces STOCs [33, 36]. STOCs cause membrane hyperpolarization and reduce the open probability of VDCC, leading to the attenuation of smooth muscle tone [20, 27]. The expression of RyR3 has been reported to increase at the late stage of pregnancy in MSMCs [8]. Therefore, we expected the upregulation of RyR3 to stabilize resting tone through the generation of STOCs as well as promotion of muscle contractions for labor. However, STOCs were not detected under the whole-cell voltage clamp mode (Fig. 4a), while Pax-sensitive BKCa currents were observed upon depolarization in MSMCs (Fig. 3f). The density of the whole-cell BKCa current in MSMCs was significantly reduced by pregnancy, and this result was consistent with previous findings from another group [5]. The lack of STOCs in MSMCs is attributable to the absence of Ca2+ sparks (Fig. 4c). Ca2+ sparks have not been clearly detected in isolated SMCs from the myometria of other species [7, 35].
In human myometrial strips, BKCa channel blockers potentiate spontaneous contractions and Ca2+ elevations [3], while openers inhibit spontaneous contractions [11] and relax contracting muscles [22]. Therefore, BKCa channels in MSMs may be preferentially activated by [Ca2+]cyt elevations caused by depolarization-induced Ca2+ influx through VDCC and/or agonist-induced Ca2+ release from the SR, leading to a negative feedback mechanism for VDCC activity. The decrease in BKCa channel expression at the late stage of pregnancy (Fig. 3f) may be involved in the enhancement of muscle excitability for labor. On the other hand, other groups suggested that BKCa weakly contributed to the control of myometrial excitability in the NP myometrium and P myometrium from rats [1, 34]. Thus, further studies are required in order to elucidate the functional roles of BKCa channels in MSM, including species-dependent diversity.
The functional expression of RyR3 in the NP-MSMCs of mice has been reported under the condition of increased SR Ca2+ loading [30]. Dabertland et al. demonstrated that the expression of DN-RyR3 was higher than that of FL-RyR3 in NP-MSMCs, whereas only FL-RyR3 was upregulated at the late stage of pregnancy [8]. They suggested that these changes increased the FL-RyR3/DN-RyR3 ratio and total protein level of functional RyR3, which promotes stronger contractions for labor. However, our real-time PCR analyses demonstrated that both transcripts decreased in the late stage of pregnancy (Fig. 5a). The results of the Western blot analysis also indicated that total RyR3 expression was markedly decreased at the late stage of pregnancy (Fig. 5b). We did not detect the functions of RyR in the present study, such as Ca2+ sparks, increases in [Ca2+]cyt in response to caffeine, and the effects of ryanodine/tetracaine on spontaneous contractions, regardless of pregnancy. The functions of FL-RyR3 in MSMCs from non-pregnant mice may be strongly suppressed by the formation of a heterotetramer with DN-RyR3 [8]. In MSMCs from mice at the late stage of pregnancy, the expression levels of both RyR3 isoforms may be too low for them to function as Ca2+ release channels. The most important result of the present study is that RyR3−/− mice, which lacked RyR3 due to the gene targeting technique [44], had normal pregnancies and deliveries (Fig. 5d). This result strongly suggests that RyR3 is not essential for reproductive function in female mice. The reason for the inconsistencies between the results of the present study and previous findings currently remains unclear but may be due to the different conditions used for experiments performed. In the present study, freshly isolated myometria and myocytes were utilized, whereas primary cultured myometrial cells were used in a previous study [8]. Changes in the expression levels of RyR2 and RyR3 in cultured arterial smooth muscles have also been reported [6, 46].
The results of the present study suggest that the strong contractile force for parturition in the MSMs of mice is generated by (i) depolarization-induced Ca2+ entry through VDCC and (ii) agonist (e.g., Oxy and prostaglandins)-induced Ca2+ release from the SR. The results suggest that both VDCC current amplitude and cell surface area (capacitance) were markedly increased in P-MSMCs. VDCC current density was similar between NP-MSMCs and P-MSMCs. However, this change may not be advantageous for strengthening muscle contractions because a decrease in the ratio of the cell volume versus surface area prevents increases in [Ca2+]cyt in the central region of the cell and subsequent force production. On the other hand, the Ca2+ sensitivity of the contractile system in myocytes [2, 39] and electrical junctions between myocytes [13] are extensively enhanced at the late stage of pregnancy. Some contractile proteins [13, 47] are also known to be upregulated in P-MSMs. These changes appear to support stronger contractions for labor in spite of VDCC current density remaining unchanged. Regarding agonist-induced Ca2+ elevations and contractions, MSMCs exhibit marked changes with the progression of pregnancy. The sensitivity of MSMs in RyR3+/+ and RyR3−/− mice to Oxy was elevated by pregnancy to a similar extent. This change may be induced by the upregulation of receptors [23] and increases in Ca2+ release through IP3Rs [43]. RyR3 may not be involved in IP3-induced Ca2+ release or contractions because the maximum contraction induced by Oxy in P-MSMs of RyR3−/− mice was comparable to that of RyR3+/+ mice (Figs. 6 and 7).
In conclusion, the present study revealed that RyR3 is not significantly involved in spontaneous contractions in MSMs during the non-pregnant period, pregnancy, or parturition. Spontaneous contractions in NP-MSMs and P-MSMs as well as strong contractions for labor are mainly conducted by (i) Ca2+ entry through VDCCs and (ii) agonist-induced Ca2+ release from intracellular Ca2+ stores through IP3Rs. The upregulation of Ca2+ sensitivity, gap junctions, contractile proteins, and G protein-coupled receptor signaling (Gq-phospholipase C (PLC)-IP3R) may play important roles in normal delivery.
References
Aaronson PI, Sarwar U, Gin S, Rockenbauch U, Connolly M, Tillet A, Watson S, Liu B, Tribe RM (2006) A role for voltage-gated, but not Ca2+-activated, K+ channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharmacol 147:815–824
Aguilar HN, Mitchell BF (2010) Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update 16:725–744
Anwer K, Oberti C, Perez GJ, Perez-Reyes N, McDougall JK, Monga M, Sanborn BM, Stefani E, Toro L (1993) Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol Cell Physiol 265:C976–C985
Balschun D, Wolfer DP, Bertocchini F, Barone V, Conti A, Zuschratter W, Missiaen L, Lipp HP, Frey JU, Sorrentino V (1999) Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J 18:5264–5273
Benkusky NA, Fergus DJ, Zucchero TM, England SK (2000) Regulation of the Ca2+-sensitive domains of the maxi-K channel in the mouse myometrium during gestation. J Biol Chem 275:27712–27719
Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA (2008) Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 295:C779–C790
Burdyga T, Wray S, Noble K (2007) In situ calcium signaling: no calcium sparks detected in rat myometrium. Ann N Y Acad Sci 1101:85–96
Dabertrand F, Fritz N, Mironneau J, Macrez N, Morel JL (2007) Role of RYR3 splice variants in calcium signaling in mouse nonpregnant and pregnant myometrium. Am J Physiol Cell Physiol 293:C848–C854
Dabertrand F, Mironneau J, Macrez N, Morel JL (2008) Full length ryanodine receptor subtype 3 encodes spontaneous calcium oscillations in native duodenal smooth muscle cells. Cell Calcium 44:180–189
Dabertrand F, Morel JL, Sorrentino V, Mironneau J, Mironneau C, Macrez N (2006) Modulation of calcium signalling by dominant negative splice variant of ryanodine receptor subtype 3 in native smooth muscle cells. Cell Calcium 40:11–21
Doheny HC, Houlihan DD, Ravikumar N, Smith TJ, Morrison JJ (2003) Human chorionic gonadotrophin relaxation of human pregnant myometrium and activation of the BKCa channel. J Clin Endocrinol Metab 88:4310–4315
Fabiato A (1985) Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol 85:291–320
Fetalvero KM, Zhang P, Shyu M, Young BT, Hwa J, Young RC, Martin KA (2008) Prostacyclin primes pregnant human myometrium for an enhanced contractile response in parturition. J Clin Invest 118:3966–3979
Futatsugi A, Kato K, Ogura H, Li ST, Nagata E, Kuwajima G, Tanaka K, Itohara S, Mikoshiba K (1999) Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron 24:701–713
Greenwood IA, Yeung SY, Tribe RM, Ohya S (2009) Loss of functional K+ channels encoded by ether-a-go-go-related genes in mouse myometrium prior to labour onset. J Physiol 587:2313–2326
Hakamata Y, Nakai J, Takeshima H, Imoto K (1992) Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett 312:229–235
Hotta S, Morimura K, Ohya S, Muraki K, Takeshima H, Imaizumi Y (2007) Ryanodine receptor type 2 deficiency changes excitation-contraction coupling and membrane potential in urinary bladder smooth muscle. J Physiol 582:489–506
Iino M (1989) Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol 94:363–383
Imaizumi Y, Ohi Y, Yamamura H, Ohya S, Muraki K, Watanabe M (1999) Ca2+ spark as a regulator of ion channel activity. Jpn J Pharmacol 80:1–8
Jaggar JH, Porter VA, Lederer WJ, Nelson MT (2000) Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278:C235–C256
Jiang D, Xiao B, Li X, Chen SR (2003) Smooth muscle tissues express a major dominant negative splice variant of the type 3 Ca2+ release channel (ryanodine receptor). J Biol Chem 278:4763–4769
Khan RN, Smith SK, Ashford ML (1998) Contribution of calcium-sensitive potassium channels to NS1619-induced relaxation in human pregnant myometrium. Hum Reprod 13:208–213
Kimura T, Takemura M, Nomura S, Nobunaga T, Kubota Y, Inoue T, Hashimoto K, Kumazawa I, Ito Y, Ohashi K, Koyama M, Azuma C, Kitamura Y, Saji F (1996) Expression of oxytocin receptor in human pregnant myometrium. Endocrinology 137:780–785
Kouzu Y, Moriya T, Takeshima H, Yoshioka T, Shibata S (2000) Mutant mice lacking ryanodine receptor type 3 exhibit deficits of contextual fear conditioning and activation of calcium/calmodulin-dependent protein kinase II in the hippocampus. Brain Res Mol Brain Res 76:142–150
Kunerth S, Langhorst MF, Schwarzmann N, Gu X, Huang L, Yang Z, Zhang L, Mills SJ, Zhang LH, Potter BV, Guse AH (2004) Amplification and propagation of pacemaker Ca2+ signals by cyclic ADP-ribose and the type 3 ryanodine receptor in T cells. J Cell Sci 117:2141–2149
Laporte R, Hui A, Laher I (2004) Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev 56:439–513
Ledoux J, Werner ME, Brayden JE, Nelson MT (2006) Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21:69–78
Martin C, Hyvelin JM, Chapman KE, Marthan R, Ashley RH, Savineau JP (1999) Pregnant rat myometrial cells show heterogeneous ryanodine- and caffeine-sensitive calcium stores. Am J Physiol Cell Physiol 277:C243–C252
Matthew A, Kupittayanant S, Burdyga T, Wray S (2004) Characterization of contractile activity and intracellular Ca2+ signalling in mouse myometrium. J Soc Gynecol Investig 11:207–212
Mironneau J, Macrez N, Morel JL, Sorrentino V, Mironneau C (2002) Identification and function of ryanodine receptor subtype 3 in non-pregnant mouse myometrial cells. J Physiol 538:707–716
Morimura K, Ohi Y, Yamamura H, Ohya S, Muraki K, Imaizumi Y (2006) Two-step Ca2+ intracellular release underlies excitation-contraction coupling in mouse urinary bladder myocytes. Am J Physiol Cell Physiol 290:C388–C403
Nakai J, Imagawa T, Hakamat Y, Shigekawa M, Takeshima H, Numa S (1990) Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett 271:169–177
Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ (1995) Relaxation of arterial smooth muscle by calcium sparks. Science 270:633–637
Noble K, Floyd R, Shmygol A, Mobasheri A, Wray S (2010) Distribution, expression and functional effects of small conductance Ca-activated potassium (SK) channels in rat myometrium. Cell Calcium 47:47–54
Noble K, Matthew A, Burdyga T, Wray S (2009) A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. Eur J Obstet Gynecol Reprod Biol 144(Suppl 1):S11–S19
Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y (2001) Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol 534:313–326
Ohya S, Niwa S, Yanagi A, Fukuyo Y, Yamamura H, Imaizumi Y (2011) Involvement of dominant-negative spliced variants of the intermediate conductance Ca2+-activated K+ channel, KCa3.1, in immune function of lymphoid cells. J Biol Chem 286:16940–16952
Otsu K, Willard HF, Khanna VK, Zorzato F, Green NM, MacLennan DH (1990) Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem 265:13472–13483
Ozaki H, Yasuda K, Kim YS, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H (2003) Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol 140:1303–1312
Savineau JP, Mironneau J (1990) Caffeine acting on pregnant rat myometrium: analysis of its relaxant action and its failure to release Ca2+ from intracellular stores. Br J Pharmacol 99:261–266
Shmigol AV, Eisner DA, Wray S (1998) Properties of voltage-activated [Ca2+]i transients in single smooth muscle cells isolated from pregnant rat uterus. J Physiol 511:803–811
Suzuki Y, Yamamura H, Ohya S, Imaizumi Y (2013) Caveolin-1 facilitates the direct coupling between large conductance Ca2+-activated K+ (BKCa) and Cav1.2 Ca2+ channels and their clustering to regulate membrane excitability in vascular myocytes. J Biol Chem 288:36750–36761
Taggart MJ, Wray S (1998) Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational dependence in isolated rat uterus. J Physiol 511:133–144
Takeshima H, Ikemoto T, Nishi M, Nishiyama N, Shimuta M, Sugitani Y, Kuno J, Saito I, Saito H, Endo M, Iino M, Noda T (1996) Generation and characterization of mutant mice lacking ryanodine receptor type 3. J Biol Chem 271:19649–19652
Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T et al (1989) Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339:439–445
Thorne GD, Paul RJ (2003) Effects of organ culture on arterial gene expression and hypoxic relaxation: role of the ryanodine receptor. Am J Physiol Cell Physiol 284:C999–C1005
Word RA, Stull JT, Casey ML, Kamm KE (1993) Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. J Clin Invest 92:29–37
Wray S, Burdyga T (2010) Sarcoplasmic reticulum function in smooth muscle. Physiol Rev 90:113–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuki, K., Takemoto, M., Suzuki, Y. et al. Ryanodine receptor type 3 does not contribute to contractions in the mouse myometrium regardless of pregnancy. Pflugers Arch - Eur J Physiol 469, 313–326 (2017). https://doi.org/10.1007/s00424-016-1900-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1900-z