Abstract
Electrophysiological properties and molecular background of the zebrafish (Danio rerio) cardiac inward rectifier current (IK1) were examined. Ventricular myocytes of zebrafish have a robust (−6.7 ± 1.2 pA pF−1 at −120 mV) strongly rectifying and Ba2+-sensitive (IC50 = 3.8 μM) IK1. Transcripts of six Kir2 channels (drKir2.1a, drKir2.1b, drKir2.2a, drKir2.2b, drKir2.3, and drKir2.4) were expressed in the zebrafish heart. drKir2.4 and drKir2.2a were the dominant isoforms in both the ventricle (92.9 ± 1.5 and 6.3 ± 1.5 %) and the atrium (28.9 ± 2.9 and 64.7 ± 3.0 %). The remaining four channels comprised together less than 1 and 7 % of the total transcripts in ventricle and atrium, respectively. The four main gene products (drKir2.1a, drKir2.2a, drKir2.2b, drKir2.4) were cloned, sequenced, and expressed in HEK cells for electrophysiological characterization. drKir2.1a was the most weakly rectifying (passed more outward current) and drKir2.2b the most strongly rectifying (passed less outward current) channel, whilst drKir2.2a and drKir2.4 were intermediate between the two. In regard to sensitivity to Ba2+ block, drKir2.4 was the most sensitive (IC50 = 1.8 μM) and drKir2.1a the least sensitive channel (IC50 = 132 μM). These findings indicate that the Kir2 isoform composition of the zebrafish heart markedly differs from that of mammalian hearts. Furthermore orthologous Kir2 channels (Kir2.1 and Kir2.4) of zebrafish and mammals show striking differences in Ba2+-sensitivity. Structural and functional differences needs to be taken into account when zebrafish is used as a model for human cardiac electrophysiology, cardiac diseases, and in screening cardioactive substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zebrafish (Danio rerio), medaka (Oryzias latipes), stickleback (Gasterosteus aculeatus), and other fish species have become popular model species for developmental biology, genetics, physiology, toxicology, evolutionary biology, and human diseases [32]. In particular, the zebrafish is a widely used animal model due to several technical advantages such as transparency in early life stages, a well annotated genome, the relative ease of genetic manipulation, a short generation time, and inexpensive maintenance under laboratory conditions [6]. Understandably, zebrafish have become a popular vertebrate model also for cardiac development and regeneration, congenital and acquired human cardiac diseases, and drug screening [6, 27, 41]. Indeed, the zebrafish heart seems to be, in several respects, a better model than the murine heart for human cardiac electrophysiology. Heart rate (HR) in zebrafish is similar to that of humans (110–130 beats/min at 27 °C), and ventricular action potential (AP) of the zebrafish heart has a clear plateau phase with an AP duration of 270 ms (at 22–24 °C), which is similar to AP duration of the human ventricles (250–260 ms, at 37 °C) [4, 5, 37]. In a strict sense, those functional similarities are, however, only valid when the comparisons are made at temperatures that differ by more than 10 °C. If measured at the common experimental temperature (e.g., 20 °C), AP duration of the human heart would be much longer and HR much lower than in the zebrafish. Fishes are ectotherms, and their body temperature is often markedly lower and more variable than in mammals, and therefore, electrical excitability of the fish heart shows adaptation to function at colder temperatures [45]. Decisive for the use of zebrafish as a cardiac model for humans is whether ion current phenotypes are produced by orthologous genes in human, whether the gene products have similar biophysical properties and sensitivities to medicinal drugs, and whether the ion channel genes are under the same regulatory pathways.
The use of animal models gets its credence from the common descent of animal species, i.e., common genetic and molecular basis of physiological traits over evolution. However, another equally important aspect of evolution is the diversity in form and function of animal species, which is based on the diversity of genomes as adaptation to different environments. Specifically, in teleost fishes, this diversity is considered to be largely based on the whole genome duplication, which occurred about 320–350 million years ago [22]. The duplication event generated genetic material by diversification of duplicated genes for slightly different functions or to solve completely novel physiological problems (neo- and subfunctionalization) [43]. Therefore, it is not granted that ion currents of the heart are produced by orthologous genes in fishes and mammals, and the possibility remains that mammalian and teleost ion channels have different biophysical properties and drug sensitivities, and they are regulated differently under physiological stresses. To this end, the present study tests the hypothesis that the inward rectifier current (I K1) of the heart is produced by orthologous genes in zebrafish and mammals, and therefore, the zebrafish I K1 is functionally similar to its mammalian counterpart. Contrary to this hypothesis, it appears that in the zebrafish heart, the molecular background of I K1 is remarkably different from that of the mammalian heart including significant differences in Ba2+-sensitivity between orthologous Kir2 channels of zebrafish and mammals.
Methods
Animals
Wild type zebrafish (Danio rerio; strain AB/Nott) were reared at +28 °C in the zebrafish facilities at the University of Manchester, UK. Fish were killed by crushing the brains with forceps before the hearts were excised and rapidly frozen in liquid nitrogen for molecular studies or used for isolation of ventricular myocytes. All procedures adhere to the UK Home Office Animals Scientific Procedures Act of 1986.
Isolation of ventricular myocytes
Cardiac myocytes were enzymatically isolated at room temperature by retrograde perfusion of the heart. The heart was gently excised and rinsed in the isolation saline solution (mM, 100 NaCl; 10 KCl; 1.2 KH2PO4; 4 MgSO4; 50 taurine; 20 glucose; and 10 HEPES at pH 6.9 with NaOH). The heart was cannulated to a blunted 35G syringe needle, which was advanced through the bulboventricular valve into the ventricular lumen. Enzyme perfusion with collagenase (Sigma Type 1A, 0.2 mg mL−1), trypsin (Sigma Type VI, 0.12 mg mL−1), and fatty acid-free bovine serum albumin (Sigma, 1 mg mL−1) was continued for 30 min. The digested ventricle was placed in 0.5 mL of isolation solution, minced with scissors and triturated using a Pasteur pipette. Cells were stored at +5 °C and used within 8 h from isolation.
Molecular methods
Cloning of zebrafish Kir2 genes
Excised hearts (n = 5, five atriums or ventricles pooled into one sample) were frozen in liquid nitrogen and stored at −70 °C for later use. Cardiac RNA was extracted from frozen tissue using TriReagent (Thermo Scientific), and genomic DNA (gDNA) was extracted from the myotomal muscle according to the method of Sambrook et al. [31]. Nucleic acids were quantified and qualified by NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and agarose gel electrophoresis, respectively. Total RNA was treated with RQ1 DNase (Promega) according to manufacturer’s instructions and reverse transcribed to complementary DNA (cDNA) using RevertAid Premium Reverse Transcriptase (Thermo Scientific) and random hexamers (Thermo Scientific). Protein coding sequences of intronless drKir2.1a, drKir2.2a, and drKir2.2b were PCR-amplified from zebrafish gDNA and that of drKir2.4 from the cardiac cDNA using Phusion High Fidelity DNA Polymerase (Thermo Scientific) (primers shown in Table 1). The cycling parameters were as follows: initial denaturation at 98 °C for 1 min followed by 35 cycles at 98 °C for 10 s, 63 °C for 30 s, and 72 °C for 45 s and final extension at 72 °C for 5 min. Overhang adenines were added to the 3′-ends of PCR products using Dynazyme II DNA polymerase (Thermo Scientific) and products were ligated to the pGEM-T Easy vector (Promega). Inserts were digested from pGEM-T Easy vector with EcoRI and SpeI and directionally cloned to pcDNA3.1/Zeo(+) digested with EcoRI and XbaI. The resulting plasmids were bidirectionally sequenced. The resulting sequences were assembled and aligned with the corresponding target sequences using Geneious 7.0.4 [23].
Transcript expression
For quantitative RT-PCR (qPCR), five atrial and ventricular samples were prepared by pooling tissues from five fishes. RNA was extracted as described above and treated with DNase to avoid genomic DNA contamination. First-strand cDNA synthesis and qPCR reactions were conducted using DyNAmo™ HS SYBR® Green 2-step qRT-PCR Kit (Thermo Scientific). From every sample, a control cDNA-reaction (-RT-control) containing all other components except RT-enzyme was prepared. Each sample was amplified in triplicates using Chromo4 Continuous Fluorescence Detector (MJ Research, Waltham, Massachusetts, USA) and primers represented in Table 2 under the following cycling parameters: 95 °C for 15 min followed by 40 cycles at 94 °C for 10 s, 61 °C for 20 s and 72 °C for 30 s, then 72 °C for 10 min. After PCR, the specificity of amplification was monitored by melting curve analysis.
To select a stably expressed reference gene for the qPCR experiments, five generally used reference genes: beta-actin (ACTB), DnaJ homologue subfamily A member 2 (DnaJA2), eukaryotic translation elongation factor 1 alpha (EEF1A1), glyceraldehyde phosphate dehydrogenase (GAPDH), and ubiquitin C (UBC) were tested. Each sample was amplified with specific primers for these genes (Table 2), and the results were analyzed with NormFinder [2], Genorm [42], the comparative delta-Ct method [35], and BestKeeper [29] software. Depending on the evaluation method used, DnaJA2, ACTB, or GAPDH was ranked as the most stable control gene, EEF1A1 and UBC showing the most variable expression (data not shown). All four approaches ranked DnaJA2 as the best or second best reference gene, whereas ACTB and GAPDH were ranked as the best or third best reference genes. Thus, DnaJA2 appeared to be the most stably expressed gene in zebrafish cardiac tissues. To minimize the effect of potential differences in reference gene expression on results, the geometric mean of DnaJA2 and ACTB were used for normalization of the drKir2 transcript expression.
Heterologous expression of cardiac drKir2 genes
Human embryonic kidney (HEK293; ECACC) cells were grown in DMEM (EuroClone) supplemented with 10 % fetal bovine serum (FBS; Euroclone) and 100 U/ml penicillin and streptomycin (EuroClone). HEK cells were transiently cotransfected with pEGFP-N1 (Clontech), and either drKir2.1a, drKir2.2a, drKir2.2b, or drKir2.4 were cloned to the pcDNA3.1/Zeo(+) using TurboFect transfection reagent (Thermo Scientific). Whole cell patch-clamp experiments were conducted 24–56 h after transfection.
Electrophysiological experiments
For whole-cell patch-clamp recording of I K1, ventricular myocytes (33.3 ± 2.4 pF) and HEK cells (9.4 ± 0.9 pF) expressing the cloned drKir2 channels were superfused with external saline solution (mM, 150 NaCl; 5.4 KCl; 1.8 CaCl2; 1.2 MgCl2; 10 glucose; and 10 HEPES at pH 7.6 with NaOH) at room temperature (21–23 °C). Tetrodotoxin (1 μM), nifedipine (10 μM), glibenclamide (10 μM) and E-4031 (2 μM) were added into this solution to prevent contamination of the recordings by Na+, Ca2+, ATP-sensitive and delayed rectifier K+ currents (I Kr), respectively. Patch pipettes were filled with intracellular saline solution of the following composition (mM, 140 KCl; 4 MgATP; 1 MgCl2; 5 EGTA; and 10 HEPES at pH 7.2 with KOH). I K1 was elicited from the holding potential of −80 mV by repolarizing voltage ramps (+60–−120 mV for 1 s) for every 10 s. Ba2+-sensitivity of the ventricular I K1 and current generated by each of the four most abundantly expressed drKir2 channels was determined by exposing the cells to cumulatively increasing BaCl2 concentrations (10−9–10−3 M). Cells were exposed to each Ba2+ concentration until the current inhibition leveled out (about 2.5 min). The normalized I K1 was plotted as a function of Ba2+ concentration and fitted to the sigmoidal equation
where I min is the residual I K1 at the highest Ba2+ concentration, I max the maximum IK1 before Ba2+ addition, IC50 the Ba2+ concentration which causes half-maximal inhibition of the I K1, [Ba2+] the molar concentration of Ba2+, and H the Hill slope factor of the line.
Inward rectification of the Ba2+−sensitive I K1 was determined for the ventricular current and each of the four cloned drKir2 channels. The non-rectifying current, obtained from the current-voltage relationship at the negative side of the reversal potential of the I K1, was extrapolated to the voltage area of inward rectification (positive to the reversal potential). The measured (rectifying) I K1 was divided with the linear (non-rectifying) current to obtain inward rectification. The normalized current (relative chord conductance, I/I max) was plotted as a function of membrane voltage, and the Boltzmann equation (below) was fit to the data [11, 34].
In the equation, V is membrane potential, and V 0.5 and k are the midpoint voltage and the slope of the curve, respectively.
Statistics
Results are given as means ± SEM. Differences in drKir2 transcript expressions between atrium and ventricle were tested using Student’s t test. If the data was not normally distributed, Mann-Whitney U test was used. Differences between mean values of the four drKir2 channels were compared using one-way ANOVA with the Tukey’s post hoc test. A P value of 0.05 was regarded as a limit of statistical significance.
Results
Expression pattern of zebrafish Kir2 genes in cardiac tissues
Sequences for zebrafish Kir2 (drKir2) genes were searched from the Ensembl Genome Browser (http://www.ensembl.org/index.html). Altogether, six drKir2 genes were found. Two gene paralogues (a and b) existed for drKir2.1 and drKir2.2, whereas no duplicates were found for drKir2.3 and drKir2.4. In contrast, no orthologues to Kir2.6 were found from the zebrafish genomes. All zebrafish Kir2 genes, with the exception of ENSDARG00000062618, were already annotated and named. ENSDARG00000062618 showed higher homology with zebrafish (72.1 %) and human (71.5 %) Kir2.2 than other Kir2 genes (51.1–65.9 %) and was therefore named as drKir2.2b. This is consistent with the annotation of Leong et al. (2014) who regarded it as a Kir2.2 paralogue. drKir2.2b is also highly homologous (71.5 %) to human Kir2.6, which in turn is nearly identical to human Kir2.2 and possibly a duplicate to it [30]. Even if human Kir2.6 and zebrafish Kir2.2b were duplicates of Kir2.2, they are not orthologues to each other because they are outcomes from separate duplication events. Human Kir2.6 arose from duplication of a limited chromosomal region, whereas drKir2.2b is assumed to be an outcome of the whole genome duplication (2R) in the teleost fish lineage. drKir2.2b shares high homology (87.9 %) with crucian carp Kir2.5 (EU182584) which is presumably also a duplicate of Kir2.2 [17] and therefore renamed as ccKir2.2b.
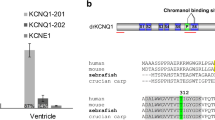
Next, we examined the transcript levels of the six drKir2 genes in the zebrafish heart. The main Kir2 subunits of zebrafish heart were drKir2.4 and drKir2.2a, which jointly represented about 99 and 94 % of ventricular and atrial drKir2 transcripts, respectively (Fig. 1). In the ventricle, drKir2.4 was clearly the main drKir2 isoform, comprising 92.9 ± 3.4 % of the total drKir2 transcripts. In the atrium, drKir2.2a was the dominant drKir2 channel subunit with a transcript expression level of 64.7 ± 3.1 %. drKir2.1 was a minor component in ventricle, where drKir2.1a and drKir2.1b comprised only 0.61 ± 0.08 % of the drKir2 transcripts. In the atrium, the relative proportion of drKir2.1 was slightly higher (4.39 ± 0.98 %) than in the ventricle (P < 0.05). drKir2b and drKir2.3 were expressed in atrium and ventricle only in trace amounts.
Normalized transcript expression of drKir2 genes in atrium and ventricle of the zebrafish heart. The bar chart shows mean (± SEM, n = 5) transcript levels for each drKir2 gene normalized to the geometric mean of DnaJA2 and ACTB reference genes. Note the logarithmic scale of the y-axis. The pie charts depict relative portions (%) of different drKir2 transcripts in the total drKir2 pool. An asterisk indicates statistically significant difference between atrial and ventricular gene expression (p < 0.05)
Sequence comparisons showed several consensus sites for inward rectification and Ba2+ block in all drKir2 channels (Fig. 2a, b). Eight amino acids critical for inward rectification have been previously identified for mammalian Kir2 channels [28]. Six of them (D172, E224, F255, D259, E299, and M301) were identical in all drKir2 channels (Fig. 2c). drKir2.2b differed from drKir2.2a and mammalian Kir2.2 in that F254 was replaced by Y254. Similar to mammalian Kir2.2 channels, drKir2.2a and drKir2.2b do not have C311 which is considered to affect inward rectification. Three amino acid residues, E125, T141, and T142, are considered to be crucial for Ba2+ sensitivity [1, 8]. drKir2.1a and b differ from the mammalian Kir2.1 in that glutamate E124 is replaced by asparagine N124.
Amino acid residues important in Ba2+ sensitivity and rectification in vertebrate Kir2 channels. a Amino acid alignment of genes represented in Table 3. Amino acids important for Ba2+ sensitivity and rectification are identified with light and dark gray, respectively. Amino acids are numbered according to human Kir2.1 (h human, cp guinea-pig (Cavia porcellus), rn rat (Rattus norvegicus), cc crucian carp (Carassius carassius)). b Schematic presentation of transmembrane topology of Kir2 channel. Cylinders indicate the transmembrane α-helices and the α-helix of the P-loop. Amino acids involved in Ba2+ sensitivity and rectification are indicated. c Conserved amino acid residues involved in inward rectification of the vertebrate Kir2 channels [28] and their presence in zebrafish Kir2 channels
Inward rectifier current (I K1) of zebrafish ventricular myocytes
Zebrafish cardiac myocytes showed a robust I K1 with typical electrophysiological characteristics of the vertebrate cardiac I K1 (Fig. 3). The zebrafish ventricular I K1 had a reversal potential (−81 ± 1.1 mV) close to the theoretical reversal potential (E rev) of K+ ions (−84.7 mV) (Fig. 3a), a large inward current at negative side of the E rev (−6.7 ± 1.2 pA pF−1 at −120 mV) and a peak outward current at the positive side of the E rev (0.68 ± 0.1 pA pF−1 at −59 mV) (Fig. 3b). The maximum outward current was 10.1 % of the inward current at -120 mV. There was clear negative slope conductance positive to -59 mV, but the current did not completely rectify at 0 mV. Half-maximal inward rectification occurred at the voltage of -79.3 ± 1.1 mV and with a slope of 6.9 ± 0.6 (Fig. 3c). The current was completely inhibited by external Ba2+ with the IC50 value of 3.8 μM (Fig. 3d).
The inward rectifier current (I K1) of the zebrafish ventricular myocytes. a A mean current voltage relationship of I K1 from eight ventricular myocytes. b Maximum inward current density at −120 mV and the maximum outward current density at −59 mV. The results are means ± SEM from eight myocytes. c Voltage-dependence of inward rectification of the Ba2+-sensitive I K1 (means ± SEM, n = 8). d A concentration-response curve of I K1 to external Ba2+ at −120 mV (n = 6). The inset (representative recordings) indicates the effect of different Ba2+-concentrations on the IK1 current
I K1 of the cloned drKir2 channels
The four most abundant Kir2 channels (drKir2.1a, drKir2.2a, drKir2.2b, drKir2.4) of the zebrafish heart were expressed in HEK cells for electrophysiological characterization (Fig. 4). All drKir2 channels generated strongly inwardly rectifying currents, which reversed direction at around −80 mV, the Nernst equilibrium potential of K+ ions (Fig. 4a). drKir2.1a channels passed more outward current (25 % of the current amplitude at −120 mV) than other drKir2 channels, i.e., it was the weakest inward rectifier. drKir2.2b was clearly the strongest inward rectifier as the maximum outward current was only 7 % of the current density at −120 mV. drKir2.2a and drKir2.4 were intermediate between those two channels (outward current 16 and 12 % of the current at −120 mV, respectively). In regard to the voltage-dependence of inward rectification drKir2.1a, drKir2.2a and drKir2.4 were similar with voltage for half-maximal rectification at around −77 mV, while inactivation of the drKir2.2b occurred at more negative voltages (−82 mV) (Fig. 4b).
Electrophysiological properties of the cloned drKir2 channels in HEK cells. a Mean current-voltage relationships of the I K1 generated by drKir2.1a, drKir2.2a, drKir2.2b and drKir2.4 channels (n = 10–14). The currents were normalized to the maximum inward current at −120 mV. b Voltage-dependence of inward rectification of the current produced by the cloned drKir2 channels. The inset shows the voltage for half-maximal inactivation of the current (V 1/2). The results are means ± SEM of 10–14 cells. c Ba-sensitivity of the I K1 generated by the cloned drKir2.4 channels. The inset shows concentration for half-maximal inhibition of the current (IC50) by Ba2+. The results are means ± SEM of 10–12 cells. Statistically significant differences (p < 0.05) between mean values are shown by dissimilar letters
External Ba2+ completely blocked all four drKir2 channels (Fig. 4c). There were, however, prominent differences in Ba2+-sensitivity between the drKir2 isoforms. drKir2.1a was the most insensitive channel to Ba2+ block with IC50 of 132 ± 14 μM, while drKir2.4 was the most Ba2+-sensitive channel with the IC50-value almost two orders magnitude higher (1.8 ± 1.1 μM) than that of the drKir2.1a. IC50-values for drKir2.2a and drKir2.2b channels were 14 ± 5.1 μM and 21 ± 8.5 μM, respectively.
Discussion
Kir2 composition of the zebrafish heart
The present results show that ventricular myocytes of the zebrafish heart have a robust inward rectifier K+ current, I K1, with typical characteristics for vertebrate cardiac I K1 including strong inward rectification and block by external Ba2+. Interestingly, the zebrafish cardiac I K1 is largely produced by drKir2.4 and drKir2.2a isoforms, thereby strongly deviating from the Kir2 subunit composition of the mammalian hearts [20]. Furthermore, some Kir2 orthologues of the zebrafish genome (drKir2.1a, drKir2.4) clearly differ from their mammalian counterparts in regard to the Ba2+-sensitivity of the generated I K1.
In mammalian hearts, three major Kir2 channels are expressed, Kir2.1, Kir2.2, and Kir2.3. In mammalian ventricular myocytes, Kir2.1 channels are the predominating isoform with a smaller contribution by Kir2.2 and Kir2.3 channels [46]. For example in the right ventricle of the human heart, Kir2.1, Kir2.2, and Kir2.3 transcripts form 47, 29, and 24 % of the total Kir2 transcripts, respectively [13]. In mammalian atria, Kir2.3 channels are abundantly expressed [26]. In the right atrium of the human heart, Kir2.3 forms 56 % of all Kir2 transcripts, while the relative portion of Kir2.2 and Kir2.1 is 31 and 13 %, respectively [13]. Contrary to the mammalian cardiac Kir2 composition, drKir2.1a, drKir2.1b, and drKir2.3 formed less than 6 and 1 % of the total drKir2 population in atrium and ventricle, respectively. Two homologues to the mammalian Kir2.2 channel, drKir2.2a and drKir2.2b, were present in the zebrafish heart. drKir2.2a was the main isoform of the zebrafish atrium (64.7 %) and expressed also in the ventricle (6.3 %). Synteny data strongly suggests that drKir2.2a and drKir2.2b are paralogues from a gene duplication event [24], but probably, the regulation of their gene expression have diverged from each other [17].
Surprisingly, an orthologue to the mammalian isoform of Kir2.4, drKir2.4, was the main Kir2 isoform in the zebrafish fish ventricle (93 %) and the second largest isoform in the zebrafish atrium (28.9 %). In mammals, Kir2.4 is strongly expressed in brain and retina [21, 39], but is not present or is weakly expressed in hearts [12, 36]. Even when it is expressed in the heart it may be confined to neuronal elements only [25]. Thus, our analysis of Kir2 channel composition in zebrafish heart reveals marked differences from mammals which may affect the extrapolation of zebrafish heart electrophysiology to human.
Inward rectification
Functionally, Kir2 channels are inward rectifiers, i.e., they pass little or no outward K+ current at the plateau voltage of cardiac AP while allowing some K+ efflux at more negative voltages. By this means, I K1 enables long plateau duration and accelerates the final phase 3 repolarization of the cardiac AP. However, Kir2 isoforms markedly differ in their inward rectifier properties [11, 28]. Similar to mammalian Kir2.1 channels, the zebrafish drKir2.1a subunit allows significant outward I K1 around −60 mV, shows a steep negative slope conductance between −60 and 0 mV, and completely rectifies at 0 mV. drKir2.2b channels are strong rectifiers, as are their mammalian counterparts [11, 28], passing relatively little outward current close to the E rev of K+ ions and completely rectifying at 0 mV. They will contribute to repolarization of the cardiac AP at the very late phase, when membrane potential approaches RMP. However, drKir2.1a and drKir.2.2b are weakly expressed in the zebrafish heart and therefore unlikely to have any significant effect on atrial or ventricular I K1. The prevailing Kir2 isoforms of the zebrafish heart drKir2.2a and drKir2.4 are intermediate between drKir2.1a and drKir2.2b channels in their rectification properties. It is notable, however, that unlike other drKir2 channels, drKir2.4 subunits and the native I K1 of zebrafish ventricular myocytes do not completely rectify at 0 mV, i.e., the negative slope conductance is shallower than that of the drKir2.1a. In this regard, drKir2.4 isoform seems to be more similar to the mammalian Kir2.3 channels, which are mainly expressed in mammalian atria [11].
Inward rectification of Kir2 channels is produced by voltage-dependent block of the channel by intracellular polyamines and Mg2+ ions. Several critical amino acid residues necessary for polyamine block of Kir2 channels have been found and examined including D172, E224, F254, D255, D259, E299, M301, and C311 (Fig. 2). All these critical residues also exist in drKir2.1a and drKir2.4 channels. drKir2.2a and drKir2.2b differ in regard to one of those residues: in drKir2.2b, the nonpolar phenylalanine in position 254 (F254) is replaced by a polar amino-acid tyrosine (Y254). Similar to the mammalian Kir2.2 and Kir2.3 channels, the zebrafish drKir2.2a and drKir2.2b do not have cysteine in the position 311. The polar cysteine is replaced by nonpolar amino-acids alanine and valine in drKir2.2a and drKir2.2b, respectively. Site-directed mutagenesis is needed to examine what kind of effects those two residues (254, 311) might have on inward rectification and other electrophysiological properties of the zebrafish channels.
Ba2+ sensitivity of drKir2 channels
There were two striking features in Ba2+-sensitivity of zebrafish drKir2 channels. Divergent from the mammalian Kir2.4 channels, which are characterized by low sensitivity to Ba2+ block [21, 38, 39], the zebrafish drKir2.4 was highly sensitive to Ba2+. The difference between mammalian and zebrafish Kir2.4 is almost two orders of magnitude (Table 3). Comparison of the amino acid residues E125, T141, and T142, known to be important for Ba2+ sensitivity [1, 7], shows that these amino acid residues are identical in zebrafish and rat Kir2.4 (Fig. 2a). Evidently, other amino-acid residues in addition to those three sites must be involved in regulation of Kir2 Ba2+ binding. Another marked deviation appeared in Ba2+ sensitivity of the drKir2.1a, because of its low affinity to Ba2+ in comparison to Kir2.1 channels of mammals and other fish species [17]. Both crucian carp Kir2.1 and drKir2.1a have asparagine instead of the E125 of the mammalian Kir2.1 channels. However, this residue is unlikely to be associated with lower Ba2+ sensitivity of the drKir2.1a, because the crucian carp (Carassius carassius) orthologue is five times more sensitive to Ba2+ than the drKir2.1a (Table 3).
Implications for a zebrafish model
I K1 is involved in some ion channel diseases of the human heart [10, 40]. A long QT7 (Andersen-Tawil) syndrome, a short QT syndrome, catecholaminergic polymorphic ventricular tachycardia and familial atrial fibrillation of the human heart, are all due to mutations of the main ventricular isoform, Kir2.1 and thus associated with the ventricular I K1 [3]. Because of short AP duration, high HR, and a divergent repertoire of the repolarizing K+ currents, the murine heart may not always be a useful arrhythmia model despite similarities in Kir2 channel composition between human and murine hearts [46]. The zebrafish is increasingly used as model for human cardiac electrophysiology and drug screening due to its amenability for genetic modification and similarities to human cardiac excitation. Recently, an orthologue to human KCNJ2 gene (drKir2.1a) was cloned from the zebrafish and the mutated gene with a delta95–98 deletion (producing an Andersen-Tawil syndrome in humans), was introduced into zebrafish embryos [24]. Although several dysmorphologies and malfunctions of skeleton and skeletal muscles, typical for the syndrome, appeared in the fish embryos, the cardiac phenotype was almost untouched. The current study shows that drKir2.1a forms less than 0.7 % of the total drKir2 transcripts, and therefore, it is likely that the trafficking-defect mutant of the drKir2.1a is either not produced in cardiac myocytes or it does not co-assemble with the dominant cardiac isoforms drKir2.4 and drKir2.2a. In order to manipulate the zebrafish cardiac I K1, the target for manipulation should be the main cardiac isoforms drKir2.4 and/or drKir2.2a. Although drKir2.4 and drKir2.2a channels are stronger rectifiers than the Kir2.1 isoforms, loss and gain of drKir2.4 and/or drKir2.2a function might produce cardiac phenotypes similar to long QT and short QT syndromes of the human heart, respectively.
Why is drKir2.4 the dominant isoform in zebrafish ventricle?
Kir2.1–3 subunits are expressed in mammalian hearts with some clear differences in Kir2 channel composition between species [20]. Kir2 composition of the zebrafish heart markedly deviates from the mammalian cardiac Kir2 composition in that drKir2.4 is the main subunit. This raises a question about possible physiological significance of this special Kir2 composition. Also, there exists clear interspecies differences in cardiac Kir2 composition among fish species. For example, in the heart of rainbow trout (Oncorhynchus mykiss), Kir2.1 channels are dominating while in crucian carp (Carassius carassius), Kir2.2a and Kir2.2b are the main cardiac isoforms [16, 17]. As noted above (Inward rectification), inward rectification properties of the drKir2.4 are not strikingly different from those of other drKir2 channels but rather an intermediate between the extremes. drKir2.4 channels have a clear negative slope conductance which provides repolarizing power during phase 3 of the cardiac AP and passes less outward current at the plateau level. Fish are ectotherms, and thermal tolerance range of the zebrafish extends from +6 to +36 °C [9]. Since Kir2 channel composition and I K1 density of fish hearts is strongly affected by environmental temperature [14, 17, 19], it remains to be shown what significance of drKir2.4 and drKir2.2a channels might have in thermal acclimation of the tropical zebrafish. Temperature changes are also associated with variation of blood pH. In this regard, the high pH sensitivity of Kir2.4 channels [21] might play some role in excitability of the fish heart.
Conclusions
The I K1 current of the zebrafish heart is produced by markedly different Kir2 channel composition in comparison to mammalian hearts. This difference emphasizes the importance of clarifying the molecular genetic background of zebrafish ion channels when using zebrafish as a model for human cardiac electrophysiology and cardiac diseases. Furthermore, significant differences are evident in Ba2+-sensitivity between orthologous mammalian and zebrafish Kir2 gene products, which suggests that the sensitivity of zebrafish cardiac ion channels to ion channel blockers can markedly differ from those of the human heart. This is consistent with previous studies which have shown marked differences in chromanol 239B sensitivity of the delayed rectifier K+ current (I Ks) and tetrodotoxin sensitivity of Na+ current (I Na) between fish and mammalian hearts [15, 18, 44].
References
Alagem N, Dvir M, Reuveny E (2001) Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol 534(2):381–393
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250. doi:10.1158/0008-5472.CAN-04-0496
Anumonwo JMB, Lopatin AN (2010) Cardiac strong inward rectifier potassium channels. J Mol Cell Cardiol 48:45–54. doi:10.1016/j.yjmcc.2009.08.013
Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC (2007) Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A 104:11316–11321
Brette F, Luxan G, Cros C, Dixey H, Wilson C, Shiels HA (2008) Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem Biophys Res Commun 374:143–146. doi:10.1016/j.bbrc.2008.06.109
Briggs JP (2002) The zebrafish: a new model organism for integrative physiology. Am J Physiol 282:R3–R9
Chatelain FC, Alagem N, Xu Q, Pancarglu R, Reuveny E, Jr LMD (2005) The pore helix dipole has a minor role in inward rectifier channel function. Neuron 47:833–843
Chatelain FC, Gazzarrini S, Fujiwara Y, Arrigoni C, Domigan C, Ferrara G, Pantoja C, Thiel G, Moroni A, Minor DL Jr (2009) Selection of inhibitor-resistant viral potassium channels identifies a selectivity filter site that affects barium and amantadine block. PLoS One 4:e7496. doi:10.1371/journal.pone.0007496
Cortemeglia C, Beitinger TL (2005) Temperature tolerance of wild-type and red transgenic zebra danios. Trans Am Fish Soc 134:1431–1437
Dhamoon AS, Jalife J (2005) The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm 2:316–324
Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JMB (2004) Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res 94:1332–1339
Eleawa SM, Sakr HF, Hussein AM, Assiri AS, Bayoumy NMK, Alkhateeb M (2013) Effect of testosterone replacement therapy on cardiac performance and oxidative stress in orchidectomized rats. Acta Physiol 209:136–147. doi:10.1111/apha.12158
Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S (2007) Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol 582:675–693
Galli GL, Lipnick MS, Block BA (2009) Effect of thermal acclimation on action potentials and sarcolemmal K+ channels from Pacific bluefin tuna cardiomyocytes. Am J Physiol 297:R502–R509. doi:10.1152/ajpregu.90810.2008
Hassinen M, Laulaja S, Paajanen V, Haverinen J, Vornanen M (2011) Thermal adaptation of the crucian carp (Carassius carassius) cardiac delayed rectifier current, IKs, by homomeric assembly of Kv7.1 subunits without MinK. Am J Physiol 301:R255–65. doi:10.1152/ajpregu.00067.2011
Hassinen M, Paajanen V, Haverinen J, Eronen H, Vornanen M (2007) Cloning and expression of cardiac Kir2.1 and Kir2.2 channels in thermally acclimated rainbow trout. Am J Physiol 292:R2328–R2339
Hassinen M, Paajanen V, Vornanen M (2008) A novel inwardly rectifying K+ channel, Kir2.5, is upregulated under chronic cold stress in fish cardiac myocytes. J Exp Biol 211:2162–2171. doi:10.1242/jeb.016121
Haverinen J, Hassinen M, Vornanen M (2007) Fish cardiac sodium channels are tetrodotoxin sensitive. Acta Physiol 191:197–204. doi:10.1111/j.1748-1716.2007.01734.x
Haverinen J, Vornanen M (2009) Responses of action potential and K+ currents to temperature acclimation in fish hearts: phylogeny or thermal preferences? Physiol Biochem Zool 82:468–482. doi:10.1086/590223
Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y (2010) Inwardly rectifying potassium channels: their structure, function and physiological role. Physiol Rev 90:291–366
Hughes BA, Kumar G, Yuan Y, Swaminathan A, Yan D, Sharma A, Plumley L, Yang-Feng TL, Swaroop A (2000) Cloning and functional expression of human retinal Kir2.4, a pH-sensitive inwardly rectifying K+ channel. Am J Physiol 279:C771–84
Jaillon O, Aury J, Brunet F, Petit J, Stange-Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf-Costaz C, Bernot A et al (2004) Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 431:946–957
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C et al (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi:10.1093/bioinformatics/bts199
Leong IUS, Skinner JR, Shelling AN, Love DR (2014) Expression of a mutant kcnj2 gene transcript in zebrafish. ISRN Mol Biol 324839:1–14
Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R (2001) Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol 532:115–126
Melnyk P, Zhang L, Shrier A, Nattel S (2002) Differential distribution of Kir2.1 and Kir2.3 subunits in canine atrium and ventricle. Am J Physiol 283:H1123–H1133
Nguyen CT, Lu Q, Wang Y, Chen JN (2008) Zebrafish as a model for cardiovascular development and disease. Drug Discov Today Dis Models 5:135–140. doi:10.1016/j.ddmod.2009.02.003
Panama BK, Lopatin AN (2006) Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol 571:287–302
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: bestkeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Ryan DP, da Silva MR, Soong TW, Fontaine B, Donaldson MR, Kung AW, Jongjaroenprasert W, Liang MC, Khoo DH, Cheah JS et al (2010) Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell 140:88–98. doi:10.1016/j.cell.2009.12.024
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York, p. 9.14–9.19
Schartl M (2014) Beyond the zebrafish: diverse fish species for modeling human disease. Dis Model Mech 7:181–192. doi:10.1242/dmm.012245
Schram G, Pourrier M, Wang Z, White M, Nattel S (2003) Barium block of Kir2 and human cardiac inward rectifier currents: evidence for subunit-heteromeric contribution to native currents. Cardiovasc Res 59:328–338
Shyng SL, Sha Q, Ferrigni T, Lopatin AN, Nichols CG (1996) Depletion of intracellular polyamines relieves inward rectification of potassium channels. Proc Natl Acad Sci U S A 93:12014–12019
Silver N, Best S, Jiang J, Thein SL (2006) Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7:33
Szuts V, Menesi D, Varga-Orvos Z, Zvara A, Houshmand N, Bitay M, Bogats G, Virag L, Baczko I, Szalontai B et al (2013) Altered expression of genes for Kir ion channels in dilated cardiomyopathy. Can J Physiol Pharmacol 91:648–656. doi:10.1139/cjpp-2012-0413
Taggart P, Sutton PMI, Boyett MR, Lab M, Swanton H (1996) Human ventricular action potential duration during short and long cycles. Am J Physiol 94:2526–2534
Tennant BP, Cui Y, Tinker A, Clapp LH (2006) Functional expression of inward rectifier potassium channels in cultured human pulmonary smooth muscle cells: evidence for a major role of Kir2.4 subunits. J Membr Biol 213:19–29. doi:10.1007/s00232-006-0037-y
Töpert C, Doring F, Wischmeyer E, Karschin C, Brockhaus J, Ballanyi K, Derst C, Karschin A (1998) Kir2.4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J Neurosci 18:4096–4105
Tristani-Firouzi M, Etheridge SP (2010) Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch 460:289–294. doi:10.1007/s00424-010-0820-6
Tu S, Chi NC (2012) Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 84:4–16. doi:10.1016/j.diff.2012.05.005
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Volff JN (2005) Genome evolution and biodiversity in teleost fish. Heredity 94:280–294
Vornanen M, Hassinen M, Haverinen J (2011) Tetrodotoxin sensitivity of the vertebrate cardiac Na+ current. Mar Drugs 9:2409–2422
Vornanen M, Haverinen J, Egginton S (2014) Acute heat tolerance of cardiac excitation in the brown trout (Salmo trutta fario). J Exp Biol 217:299–309. doi:10.1242/jeb.091272
Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL (2001) The consequences of disrupting cardiac inwardly rectifying K+ current (IK1) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol 533(3):697–710
Acknowledgments
The authors thank Nur Hidayah Jamar, Alex Leslie Thomas, and Robert Hallworth for their assistance with the zebrafish.
Funding
This study was supported by a grant from Jane and Aatos Erkko Foundation to MV (Project No. 64579) and the Leverhulme Trust to HAS (Project No. 240613).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassinen, M., Haverinen, J., Hardy, M.E. et al. Inward rectifier potassium current (I K1) and Kir2 composition of the zebrafish (Danio rerio) heart. Pflugers Arch - Eur J Physiol 467, 2437–2446 (2015). https://doi.org/10.1007/s00424-015-1710-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1710-8