Abstract
Raloxifene is widely used for the treatment and prevention of postmenopausal osteoporosis. We examined the effects of raloxifene on the Kv4.3 currents expressed in Chinese hamster ovary (CHO) cells using the whole-cell patch-clamp technique and on the long-term modulation of Kv4.3 messenger RNA (mRNA) by real-time PCR analysis. Raloxifene decreased the Kv4.3 currents with an IC50 of 2.0 μM and accelerated the inactivation and activation kinetics in a concentration-dependent manner. The inhibitory effects of raloxifene on Kv4.3 were time-dependent: the association and dissociation rate constants for raloxifene were 9.5 μM−1 s−1 and 23.0 s−1, respectively. The inhibition by raloxifene was voltage-dependent (δ = 0.13). Raloxifene shifted the steady-state inactivation curves in a hyperpolarizing direction and accelerated the closed-state inactivation of Kv4.3. Raloxifene slowed the time course of recovery from inactivation, thus producing a use-dependent inhibition of Kv4.3. β-Estradiol and tamoxifen had little effect on Kv4.3. A preincubation of ICI 182,780, an estrogen receptor antagonist, for 1 h had no effect on the inhibitory effect of raloxifene on Kv4.3. The metabolites of raloxifene, raloxifene-4′-glucuronide and raloxifene-6′-glucuronide, had little or no effect on Kv4.3. Coexpression of KChIP2 subunits did not alter the drug potency and steady-state inactivation of Kv4.3 channels. Long-term exposure to raloxifene (24 h) significantly decreased the expression level of Kv4.3 mRNA. This effect was not abolished by the coincubation with ICI 182,780. Raloxifene inhibited Kv4.3 channels by interacting with their open state during depolarization and with the closed state at subthreshold potentials. This effect was not mediated via an estrogen receptor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Raloxifene, a selective estrogen receptor modulator, is widely used in the prevention and treatment of osteoporosis in postmenopausal women [18, 27, 40]. A number of clinical and experimental studies have shown that raloxifene exerts a multitude of favorable effects on the cardiovascular system [38, 52]. For example, this drug has direct cardioprotective effects against myocardial ischemia-reperfusion injury by improving coronary perfusion, cardiac contractility, and myocardiac metabolism in the canine ischemic heart [31]. Although the ionic mechanism for the antiarrhythmic effects of raloxifene was not investigated, in animal models, raloxifene has reduced the incidence of ventricular fibrillation and suppressed the duration of ventricular tachycardia elicited by myocardial ischemia [9, 30]. In this context, raloxifene has reportedly inhibited many different types of cardiac ion channels. Raloxifene directly inhibits the human atrial repolarizing voltage-gated K+ (Kv) currents, which are the transient outward and ultrarapid delayed rectifier Kv currents [25]. The inhibitory effects of raloxifene on these currents are independent of estrogen receptors. In addition, raloxifene inhibits the cloned human ether-a-go-go related gene (hERG) channel and the recombinant human cardiac KCNQ1/KCNE1 channel that are stably expressed in human embryonic kidney (HEK) cells [26]. Raloxifene also inhibits voltage-gated Ca2+ currents, including T- and L-type Ca2+ currents, which are involved in the depolarization of cardiac action potentials [24, 48, 50].
Kv4.3 channels give rise to transient, rapidly activating and inactivating Kv currents and have been identified as the primary α-subunits that contribute to the transient outward Kv currents in human myocytes [12, 32]. These currents play important roles in the initial repolarization phase of the action potential in the human heart [6, 36]. Consistent with this role, physiologic or pathologic conditions such as pregnancy, heart failure, and atrial fibrillation modulate the expression of this channel gene [17, 22, 44]. For instance, sex hormones, particularly estrogen, are associated with a functional downregulation of Kv4.3 channel gene expression [14]. Raloxifene exerts estrogenic agonistic or antagonistic effects on different tissues [28]. Because raloxifene is associated with estrogen-like effects on the cardiovascular system [38], it is possible that Kv4.3 channels are affected by raloxifene. Therefore, we have examined the direct effects of raloxifene on the Kv4.3 currents that are stably expressed in Chinese hamster ovary (CHO) cells using the patch-clamp techniques. Many women have received raloxifene over a long period of time [35], and, therefore, in addition to acute effects, the long-term modulation of Kv4.3 by raloxifene was investigated using real-time PCR.
Materials and methods
Cell preparation
CHO cells stably transfected with Kv4.3L complementary DNA (cDNA) were maintained in Iscove’s modified Dulbecco’s medium (Invitrogen, Grand Island, NY, USA) supplemented with 10 % fetal bovine serum, 2 mM glutamine, 0.1 mM hypoxanthine, 0.01 mM thymidine, and 0.3 mg/ml geneticin (Invitrogen), as described previously [1, 32]. For the coexpression of an ancillary β-subunit, CHO cells were transiently transfected with 1.4 μg KChIP2 plasmid DNA (GenBank No. NM 014591) and 4.2 μl FuGENE HD (Roche Diagnostics, Indianapolis, IN, USA) per 35-mm petri dish according to the manufacturer’s protocol. Patch-clamp experiments were performed 1–2 days after transfection. Only green fluorescing cells were used for recordings. Cells were detached from the culture flask using brief trypsin-EDTA (Invitrogen) treatments and were seeded onto glass coverslips (12-mm diameter; Fisher Scientific, Pittsburgh, PA, USA) in a 35-mm petri dish 24 h prior to use.
Electrophysiological recordings
Whole-cell currents were recorded at room temperature (22–24 °C) using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Patch pipettes were pulled from borosilicate glass capillaries (PG10165-4; World Precision Instruments, Sarasota, FL, USA) using a programmable horizontal microelectrode puller (P-97; Sutter Instrument Co., Novato, CA, USA) and had a tip resistance of 3–5 MΩ when filled with a pipette solution. When the Kv4.3 currents exceeded 1 nA, voltage errors were minimized using 80 % series resistance compensation. Cells were perfused with an extracellular solution containing (in mM) 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 10 glucose, which was adjusted to pH 7.3 using NaOH. The intracellular pipette solution contained (in mM) 140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 EGTA, adjusted to pH 7.3 with KOH. The measured osmolarity of the extracellular solution was 300–310 mOsm. Raloxifene, β-estradiol, tamoxifen (Sigma, St. Louis, MO, USA), ICI 182,780, raloxifene-4′-glucuronide, and raloxifene-6′-glucuronide (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) were dissolved in dimethyl sulfoxide (DMSO, Sigma) as stock solutions. The concentrations used in the present study were then diluted with the extracellular solution to obtain the desired concentration. The concentration of DMSO in the final dilution was less than 0.1 %, and this concentration had no effect on Kv4.3 currents.
Data analysis
Data were analyzed using Clampfit 10.0 (Molecular devices) and Origin 8.0 software (Origin Lab Corp., Northampton, MA, USA). The data of the concentration-response curve were fitted to the Hill equation:
where f is the fractional inhibition, [D] is the drug concentration, IC50 is the drug concentration necessary for a 50 % inhibition, and n is the Hill coefficient. A first-order blocking scheme was used to describe the drug-channel interaction. The apparent rate constants for association (k +1) and dissociation (k −1) were obtained from the following equation. [43]:
in which τ D is the time constant of the fast initial drug-induced current decay. The voltage dependence of steady-state inactivation was measured using a two-pulse protocol. A series of 1 s conditioning pulses with potentials ranging from −110 to +10 mV was delivered, followed by a test pulse of 500 ms to +40 mV. The curves were fitted with the following equation:
in which I max represents the maximum current from the absence of inactivation, and I c represents a noninactivating current at the most depolarized 1 s preconditioning pulse. V, V 1/2, and k are the test potential, the potential at which half of the channel population is inactivated, and the slope factor, respectively. The noninactivating residual current was removed by subtracting it from the actual value. The voltage dependence of the inhibition was determined as follows: the fractional inhibition was measured at individual test potentials and the voltage dependence of the fractional inhibition was fitted with a Woodhull equation:
where K d (0) is the apparent affinity at 0 mV (the reference voltage), z is the charge valence of the drug, δ is the fractional electrical distance (i.e., the fraction of the transmembrane electric field sensed by a single charge at the receptor site), F is Faraday’s constant, R is the gas constant, and T is the absolute temperature. In the present study, 25.4 mV was used as the value of RT / F at 22 °C.
RNA isolation and real-time PCR
Total RNA from the CHO cells was isolated using TRIzol® Reagent (Life Technologies, Carlsbad, CA, USA) and it was reverse-transcribed by PrimeScript™ RT kit (Takara, Otsu, Shiga, Japan), according to the manufacturer’s instructions. For the quantitative real-time PCR analysis, the cDNA was amplified with primers and a SYBR Green Q-PCR Master Mix (Takara) using an ABI 7300 thermal cycler (Applied Biosystems, Foster City, CA, USA). Real-time PCR was started at 95 °C for 10 s in the initial denaturation stage, followed by a PCR of 40 cycles at 95 °C for 5 s (denaturing step) and 60 °C for 30 s (annealing step). The PCR was validated via melting curve analysis performed in the dissociation stage. The primer sequences were as follows: Kv4.3 forward 5′-TAGCAAGAAGACCACGCACC-3′ and reverse 5′-GCTGGAGCGACTAGTGGTGA-3′; GAPDH forward 5′-CTGCCTTCACTTCTGGCAAA-3′ and reverse 5′-GCCTTGACTGTGCCTTTGAA-3′. The relative quantity of Kv4.3 expression was calculated using the ΔΔCt method with the GAPDH messenger RNA (mRNA) as an internal control, and four to six independent RNA samples were examined for statistical analysis.
Statistical analysis
All values are expressed as the mean ± SE. Statistical significance was tested using Student’s t test and one-way analysis of variance followed by Bonferroni test for comparisons of multiple groups. P values were considered significant at P < 0.05.
Results
Concentration- and time-dependent inhibition of Kv4.3
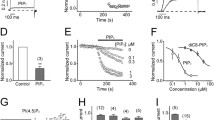
Figure 1 shows the whole-cell Kv4.3 currents evoked by 500-ms depolarizing pulses to +40 mV from a holding potential of −80 mV every 10 s, before and after the application of raloxifene. Under control conditions, the Kv4.3 currents were rapidly activated and inactivated during 500-ms depolarizing pulses as described previously [2, 32]. Figure 1a demonstrates the effect of cumulatively increased concentrations (0.3–10 μM) of raloxifene on Kv4.3 currents. When raloxifene was perfused, the inhibition of Kv4.3 reached a steady level within 3 min. The current amplitudes recovered to 95.8 ± 2.1 % (n = 12) of the control value after the drug washout. Raloxifene had two effects on the Kv4.3 current. It decreased the peak amplitude of Kv4.3 and accelerated the decay phase of inactivation in a concentration-dependent manner. Thus, the inhibition of Kv4.3 current was quantified as a reduction in the integral of the current during depolarization. A nonlinear least-square fit of the Hill equation yielded an IC50 value of 2.0 ± 0.1 μM with a Hill coefficient of 1.8 ± 0.1 (n = 8) (Fig. 1b). Under control conditions, the decay of the Kv4.3 current was well fitted by the double exponential function of the fast and slow time constants (Fig. 2a). The acceleration in the inactivation rate of Kv4.3 by raloxifene showed an apparent dependence on the raloxifene concentration. At 3 and 10 μM, raloxifene significantly accelerated both the fast and slow time constants as a function of its concentration. To investigate the effect on the activation kinetics, we normalized the Kv4.3 currents in the absence and presence of raloxifene. The activation kinetics (the dominant time constant of activation kinetics) was determined by fitting a single exponential function to the latter 50 % of activation [42, 53]. Under control conditions, the activation time constant was 0.75 ± 0.03 ms (n = 8) (Fig. 2b). The activation kinetics of the Kv4.3 current was accelerated by raloxifene in a concentration-dependent manner. The time constants of activation were decreased to 0.71 ± 0.02, 0.66 ± 0.03, 0.54 ± 0.01, and 0.46 ± 0.02 (F 4, 35 = 17.13, P < 0.01) for 0.3, 1, 3, and 10 μM of raloxifene, respectively. We also analyzed the activation kinetics by measuring the time from the onset of depolarization to peak Kv4.3 current (time-to-peak) [3], which reflects the transition from the closed to the open state of Kv4.3 channels, in the absence and presence of raloxifene. Raloxifene significantly decreased the time-to-peak in a concentration-dependent manner (Fig. 2c). The time-to-peak was also decreased for the potentials tested (Fig. 2d). Taken together, the time-to-peak was decreased secondary to the combination of acceleration in both activation and inactivation kinetics of Kv4.3. The time course for the development of a Kv4.3 inhibition by raloxifene is shown in Fig. 3. Drug-induced inhibition was plotted as a function of time after the onset of the pulse. The inhibition developed exponentially with time, and the rate of inhibition was increased with increase in concentration (Fig. 3a). The time constant of the fast component was concentration-dependent and was considered to represent the time constant for the development of the inhibition of Kv4.3. The linear regression fitted to the data at four concentrations yielded an apparent association rate constant of 9.5 ± 1.1 μM−1 s−1 and an apparent dissociation rate constant of 23.0 ± 3.2 s−1 (n = 8) (Fig. 3b). The theoretical K D value derived from these values by k −1 / k +1 was 2.6 ± 0.3 μM (n = 8), which was similar to the IC50 value obtained from the concentration-response of Fig. 1b.
Concentration-dependent inhibition of Kv4.3 by raloxifene. a Original Kv4.3 current traces were recorded following 500-ms depolarizing pulses from a holding potential of −80 to +40 mV at 10 s intervals under control conditions and after application of 0.3, 1, 3 and 10 μM of raloxifene. The inset shows the first 50 ms of the Kv4.3 currents on an expanded time scale. The dotted line marks zero current. b Concentration-response curve for Kv4.3 inhibition by raloxifene. The solid line represents the fit of the data to the Hill equation. Data are expressed as the mean ± SE
Inactivation and activation kinetics of Kv4.3. a The fast and slow inactivation time constants as a function of concentration of raloxifene. b The dominant activation time constants as a function of concentration of raloxifene. Time-to-peak as a function of concentrations of raloxifene (c) and test potentials (d) in the absence and presence of raloxifene. *P < 0.05, significantly different from the control value. Data are expressed as the mean ± SE
Time-dependent inhibition of Kv4.3. a Drug-sensitive currents were calculated by subtracting the ratio of the current amplitude before and after the addition of raloxifene from unity. The time courses of the inhibition were fitted to a double exponential function that yielded the concentration-dependent time constants. b The reciprocals of drug-induced fast time constants (τ D) were plotted versus raloxifene concentrations. The solid line represents the linear fit for τ D of the fast component in the presence of raloxifene. Data are expressed as the mean ± SE
Voltage-dependent inhibition of Kv4.3
The effects of voltage on the raloxifene inhibition of Kv4.3 were investigated by applying a series of 500-ms depolarizing pulses between −70 and +60 mV from a holding potential of −80 mV before and after the application of raloxifene (Fig. 4a, b). Figure 4c shows a representative I–V relationship for the peak current of Kv4.3 in the control and presence of raloxifene. For a voltage-dependent inhibition, the fractional integral inhibition of Kv4.3 (I Raloxifene / I Control) was plotted against the membrane potential (Fig. 4d). At test potentials from −30 to +10 mV, the raloxifene-induced inhibition increased from 56.4 ± 4.5 % at −30 mV to 72.4 ± 3.1 % at +10 mV (n = 7, P < 0.01), which corresponded to the voltage range of the channel activation. At a more positive potential where the conductance was saturated, raloxifene displayed a shallow voltage dependence. When the experimental data were fitted with a Woodhull equation, the calculated fractional electrical distance (δ) was 0.13 ± 0.04 (n = 7) in the presence of raloxifene.
Voltage-dependent inhibition of Kv4.3. Kv4.3 currents were elicited by 500-ms depolarizing pulses in the range −80 to +60 mV from a holding potential of −80 mV in the absence (a) and presence (b) of raloxifene. The inset shows the first 50 ms of the Kv4.3 currents on an expanded time scale. The dotted line marks zero current. c Current-voltage relationship for a Kv4.3 inhibition. d Relative integral currents from the data shown in a and b. The dashed line represents the normal activation curve of Kv4.3 [20]. The voltage dependence was fitted with Eq. 4 (see “Materials and methods”) and yielded δ = 0.13 ± 0.04 (n = 7). *P < 0.05, significantly different from the value at −30 mV. Data are expressed as the mean ± SE
Effects on the steady-state inactivation and closed-state inactivation of Kv4.3
Figure 5a illustrates the effects of raloxifene on the voltage dependence of the steady-state inactivation of Kv4.3. The steady-state inactivation was determined by measuring the Kv4.3 currents that were evoked by depolarizing pulses to +40 mV while the holding potential (1 s duration) was increased in 10 mV increments from −110 to + 10 mV. Under control conditions, the V 1/2 and k values were −50.5 ± 2.2 and 5.9 ± 0.4 mV (n = 7), respectively. After the administration of raloxifene, there was a hyperpolarizing shift in V 1/2 (−56.3 ± 2.5 mV, n = 7, P < 0.05). However, the k value did not change significantly in the presence of raloxifene (6.1 ± 0.5 mV, n = 7). Since Kv4.3 channels are inactivated predominantly from the closed state without opening at above subthreshold potentials [4], the closed-state inactivation was measured with prepulses of −60 and −50 mV [56]. The ratios of current measured at −60 and −50 mV to that measured at −110 mV represent the fraction of Kv4.3 currents directly entering into closed-state inactivation (Fig. 5b). Raloxifene produced a significant reduction in the ratios at −60 and −50 mV, suggesting that the kinetics of closed-state inactivation of Kv4.3 was accelerated in the presence of raloxifene. Detailed analysis of the closed-state inactivation of Kv4.3 was estimated under different durations of conditioning prepulse from 5 ms to 20 s (Fig. 6a). Under control conditions, the time course for the closed-state inactivation of Kv4.3 was fitted to a single exponential function with a time constant of 4.12 ± 0.17 s (n = 7) (Fig. 6b). In the presence of raloxifene, the closed-state inactivation was also fitted to a single exponential function and was accelerated with a time constant of 1.07 ± 0.27 s (n = 7, P < 0.05).
The effects of raloxifene on the steady-state inactivation of Kv4.3. a Original Kv4.3 current traces were recorded using a two-pulse protocol shown in the inset. The normalized currents are plotted against the membrane potential and fitted with a Boltzmann equation. b Kv4.3 currents elicited by preconditioning pulses to −50 and −60 mV followed by the test potentials to +40 mV in the absence and presence of raloxifene. The ratios of Kv4.3 currents measured at −60 and −50 mV to that measured at −110 mV. *P < 0.05, significantly different from the control value. Data are expressed as the mean ± SE
The effects of raloxifene on the kinetics of the closed-state inactivation of Kv4.3. a The control and conditioning pulses were applied from a holding potential of −100 mV. Kv4.3 currents were recorded using a two-pulse protocol with a control pulse followed by a conditioning pulse to −60 mV of variable duration (5 ms–20 s) in the absence and presence of raloxifene. b The current amplitudes elicited by the test pulses were normalized to the amplitude by the control pulse and plotted against the duration of the conditioning pulse. The data were fitted to a single exponential function. Data are expressed as the mean ± SE
Effects on the recovery from inactivation and use dependence of Kv4.3
Recovery from inactivation of Kv4.3 was investigated using a two-pulse protocol with prepulses and test pulses to +40 mV from a holding potential of −80 mV (Fig. 7a). The interval between the prepulse and test pulse was increased (10–10,000 ms), and the peak currents of Kv4.3 during test pulses were then normalized to that during prepulses. Under control conditions, the time course of recovery from inactivation of Kv4.3 was fitted to a single exponential function with a time constant of 198.4 ± 16.0 ms (n = 8) (Fig. 7b). The recovery from inactivation in the presence of raloxifene was fitted to a double exponential function with a fast time constant of 499.2 ± 46.1 ms and a slow time constant of 5,229.0 ± 464.1 ms (n = 8, P < 0.05), suggesting that raloxifene delayed the recovery from inactivation of Kv4.3. To study the use-dependent inhibition by raloxifene, Kv4.3 currents were evoked by 20 consecutive 200-ms pulses to +40 mV from a holding potential of −80 mV at frequencies of 1 and 2 Hz in the absence and presence of raloxifene (Fig. 8a). Under control conditions, the peak current of Kv4.3 decreased by 7.5 ± 1.8 % (n = 8) at a stimulation frequency of 1 Hz and 30.7 ± 2.9 % (n = 8) at a frequency of 2 Hz over the course of 20 repetitive depolarizing pulses (Fig. 8b). In the presence of raloxifene, there was a reduction in the peak amplitude of Kv4.3 current evoked by the first pulse applied after exposure of the cell to raloxifene for 3 min without pulsing (tonic block), which averaged 20.1 ± 0.4 % (n = 8). During the application of the train of pulses, the current amplitude progressively decreased to reach a final value of 57.8 ± 4.2 % at 1 Hz and 75.0 ± 2.1 % (n = 8) at 2 Hz.
The effects of raloxifene on the kinetics of recovery from inactivation of Kv4.3. a A two-pulse protocol was used to characterize the time course of recovery from inactivation of Kv4.3 in the absence and presence of raloxifene. b Data were plotted as the normalized amplitude of Kv4.3 against the interpulse interval. The recovery time course of Kv4.3 was best described by a single exponential function under control conditions and a double exponential function in the presence of raloxifene. Data are expressed as the mean ± SE
Use-dependent inhibition of Kv4.3 by raloxifene. a Original current traces of Kv4.3 obtained in the absence and in the presence of raloxifene when applying 20 depolarizing pulses from −80 to +40 mV at a frequency of 1 and 2 Hz. The dotted line marks zero current. b Peak current was normalized to the control value in the absence and presence of raloxifene and then plotted against the pulse number. Data are expressed as the mean ± SE
Effects of β-estradiol, tamoxifen, and ICI 182,780 on Kv4.3
To investigate whether the inhibitory effect of raloxifene is mediated through estrogenic receptors, we tested the effects of estrogen agonists, β-estradiol and tamoxifen on Kv4.3 (Fig. 9a, b). β-Estradiol and tamoxifen decreased Kv4.3 currents in a concentration-dependent manner. However, even 300 μM of β-estradiol and tamoxifen reduced the peak amplitude of the Kv4.3 current by only 28.3 ± 4.7 % (n = 9) and 22.7 ± 2.9 % (n = 9), respectively, and complete inhibition could not be achieved. A partial concentration-response curve yielded a tentative IC50 value of 1.2 ± 0.2 mM and a Hill coefficient of 0.8 ± 0.1 (n = 9) for β-estradiol and a tentative IC50 value of 1.3 ± 0.5 mM and a Hill coefficient of 1.1 ± 0.3 (n = 9) for tamoxifen. Thus, these agents are much less potent than raloxifene in inhibiting Kv4.3 currents by about 600-fold. After preincubation of 10 μM ICI 182,780 for 1 h, the application of 3 μM raloxifene to the bath solution containing ICI 182,780 decreased Kv4.3 currents by 57.1 ± 0.2 % (n = 5) (Fig. 9c). Since this concentration of ICI 182,780 is known to effectively block estrogen receptor activation, this result suggests that the inhibition of Kv4.3 currents by raloxifene is not mediated by estrogen receptors.
The effects of β-estradiol, tamoxifen, and ICI 182,780 on Kv4.3. Kv4.3 currents were elicited by 500-ms depolarizing pulses from a holding potential of −80 to +40 mV at 10 s intervals in the absence and presence of β-estradiol (a) and tamoxifen (b). c The control current recorded after preincubation with ICI 182,780 and the current measured after a subsequent 3 min treatment with raloxifene are shown. The dotted line marks zero current. Data are expressed as the mean ± SE
Effects of raloxifene-4′-glucuronide and raloxifene-6′-glucuronide on Kv4.3
Raloxifene is metabolized via systemic glucuronidation into the metabolites, raloxifene-4′-glucuronide and raloxifene-6′-glucuronide [21, 23]. Raloxifene-4′-glucuronide had little effect on Kv4.3: raloxifene-4′-glucuronide at 10 and 30 μM only inhibited the peak amplitude of Kv4.3 by 6.2 ± 0.3 and 20.1 ± 0.4 % (n = 7), respectively (Fig. 10a). Raloxifene-6′-glucuronide (30 μM) had no effect on Kv4.3 (96.2 ± 2.4 % of the control value, n = 4) (Fig. 10b). These results indicate that the metabolites of raloxifene were much less potent in inhibiting Kv4.3 than the parent drug.
Effects of KChIP2 on concentration dependence and steady-state inactivation of Kv4.3
Coexpression of KChIP2 (Kv4.3/KChIP2) modified the biophysical properties of Kv4.3 in two ways: (1) altered the inactivation kinetics and (2) shifted the steady-state inactivation curve in a hyperpolarizing direction, as reported previously [5, 33, 51]. Extracellular application of raloxifene (0.3–10 μM) decreased the peak amplitude of Kv4.3/KChIP2 currents together with an apparent acceleration of the inactivation rate in a concentration-dependent manner (Fig. 11a). The IC50 value required for the inhibitory effects of raloxifene on the integral of Kv4.3/KChIP2 currents was 2.8 ± 0.3 μM with a Hill coefficient of 0.8 ± 0.05 (n = 6). Therefore, raloxifene inhibited Kv4.3 alone and Kv4.3/KChIP2 channels with a similar potency. Under control conditions, the rate of inactivation of Kv4.3 at +40 mV is a biexponential process (Fig. 2a), but inactivation of Kv4.3/KChIP2 is well fit by a single exponential function with time constants of 34.3 ± 1.2 ms (n = 6). The steady-state inactivation of Kv4.3 was shifted by the coexpression of KChIP2 in a hyperpolarizing direction (V 1/2 = −62.4 ± 0.8 mV, k = 4.2 ± 0.1 mV, n = 6) (Fig. 11b). In the presence of raloxifene, Kv4.3/KChIP2 exhibited a hyperpolarizing shift in the voltage dependence of steady-state inactivation (V 1/2 = −70.9 ± 1.9 mV, n = 6, P < 0.05). However, the k value did not change in the presence of raloxifene (k = 4.4 ± 0.2 mV, n = 6).
Effects of KChIP2 on concentration dependence and steady-state inactivation of Kv4.3. a Original current traces of Kv4.3/KChIP2 were recorded following 500-ms depolarizing pulses from a holding potential of −80 to +40 mV at 10 s intervals under control conditions and after application of 0.3, 1, 3, and 10 μM of raloxifene. The inset shows the first 50 ms of the Kv4.3/KChIP2 currents on an expanded time scale. The dotted line marks zero current. Concentration-response curve for Kv4.3/KChIP2 inhibition by raloxifene. The solid line represents the fit of the data to the Hill equation. b The effects of raloxifene on the steady-state inactivation of Kv4.3/KChIP2. Original currents traces were recorded using the two-pulse protocol shown in the inset. The normalized currents are plotted against the membrane potential and fitted with a Boltzmann equation. Data are expressed as the mean ± SE
Effects of raloxifene on the expression of Kv4.3 mRNA
We studied the effect of raloxifene on the expression of the Kv4.3 channel mRNA using real-time PCR (Fig. 12a). Incubation of 3 μM of raloxifene for as long as 1 h had no effect on the expression of Kv4.3 mRNA. However, the expression of Kv4.3 mRNA was slightly decreased at 6 and 12 h, and a statistically significant reduction (66.1 ± 0.1 % of the control value, n = 4) was observed at 24 h, suggesting that long-term exposure of raloxifene decreased the expression level of Kv4.3 mRNA. To investigate whether the long-term effect of raloxifene on Kv4.3 mRNA expression is mediated through estrogenic receptors, we performed real-time PCR after 24 h coincubation with ICI 182,780 (Fig. 12b). The raloxifene-induced reduction in Kv4.3 mRNA was not abolished by the coincubation with ICI 182,780 (70.1 ± 0.4 % of the control value, n = 6). Incubation with ICI 182,780 alone was without effect. These results suggest that the long-term effect of raloxifene on Kv4.3 mRNA expression was independent of estrogenic receptors.
The effects of raloxifene on the expression of Kv4.3 mRNA. a The cells were incubated for 10 min to 24 h with 3 μM raloxifene. Kv4.3 mRNA was measured using a real-time PCR. b The cells were incubated with raloxifene, raloxifene and ICI 182,780, and ICI 182,780 alone for 24 h. Kv4.3 mRNA was measured using a real-time PCR. *P < 0.05, significantly different from the control value. Data are expressed as the mean ± SE
Discussion
Our study has demonstrated that raloxifene at concentrations of 0.3–10 μM significantly inhibits Kv4.3 currents in a concentration-dependent manner. The inhibition of Kv4.3 by raloxifene was characterized by an acceleration of the inactivation rate in a concentration-dependent manner. These characteristics of the Kv4.3 inhibition were similar to previous results showing an open channel block of transient outward Kv currents by raloxifene [25]. This finding could be explained by a preferential binding of raloxifene to the open state and/or by a drug-induced acceleration in the transition of the open state to the inactivated state of Kv4.3 channels [49, 57]. Although there are several similarities between an open channel block and channel inactivation [11], raloxifene produced a time-, voltage-, and use-dependent inhibition of Kv4.3 currents, which was interpreted as an open channel block with the following results. The characteristic feature of an open channel block is an induction of a fast time constant during depolarization. From the analysis of the concentration dependence of the channel block, the estimated K D (k −1 / k +1) value for the time-dependent block by raloxifene was similar to the IC50 value obtained from the concentration-response curve, suggesting that the time course of channel block during the depolarization was consistent with a bimolecular reaction scheme between drug molecules and channels in the open state [43]. These results imply that the effect of raloxifene on Kv4.3 was independent of the inactivation process during depolarization and was consistent with an open channel block mechanism. However, we cannot completely rule out the possibility that the fast time constant that was used as an approximation of the time course for drug-channel interaction kinetics may reflect the diffusion of the drug across the cell membrane. Therefore, it is possible that the drug acts through the membrane milieu to affect channel gating (mainly in the open state), which induced the apparent acceleration of the decay rate of Kv4.3 instead of the open channel block during depolarization. Raloxifene also accelerated the inactivation and activation kinetics and, thus, decreased the time-to-peak of Kv4.3, a result consistent with an open channel block mechanism [16, 47, 49]. In addition, both voltage- and use-dependent inhibition by raloxifene are also compatible with an open channel block of Kv4.3 [10, 49]. Since raloxifene has a pKa of 8.89 and is mainly charged at the intracellular pH of 7.4 (http://www.drugbank.ca/drugs/DB00481), the voltage dependence can be explained by an interaction between the charged form of the drug and the binding sites in the electrical field of the Kv4.3 channel. Raloxifene produced a hyperpolarizing shift of the steady-state inactivation curve of Kv4.3 with a slow rate of recovery from the inactivation. These results suggest that raloxifene interacts with the inactivated state of Kv4.3, particularly in the subthreshold range of membrane potentials. Since Kv4.3 is known to be inactivated from the closed state without opening at the subthreshold potentials [4], we further studied the closed-state inactivation in detail by using variable durations of condition prepulses. Raloxifene accelerated the rate of closed-state inactivation, suggesting a preferential interaction with the closed-inactivated states of Kv4.3 channels. Use-dependent inhibition by raloxifene was associated with a slow rate of recovery from inactivation [10], which reflected the interaction of the drug with the activated states (open and/or inactivated states) of the Kv4.3 channel [8, 49]. Thus, raloxifene is not a pure open channel blocker of Kv4.3 channels, and raloxifene inhibited Kv4.3 by interacting with the open state of the channel during depolarization and with the inactivated state at subthreshold potentials.
Kv4.3 encodes the pore-forming and voltage-sensing α-subunits of channels that conduct the transient outward Kv currents in the heart [12, 32]. The channel interacts with a number of ancillary β-subunits in native cells, which modulate the kinetic properties, the surface expression, and the drug potency of the Kv4.3 channels [5, 33, 51]. In the present study, raloxifene inhibited Kv4.3 and Kv4.3/KChIP2 with similar potency. Although raloxifene did not affect the steady-state inactivation of transient outward Kv currents in human atrial myocytes [25], the present study demonstrates that the steady-state inactivation curve of Kv4.3/KChIP2 was shifted to hyperpolarizing potentials by raloxifene. These results indicate that the coexpression of KChIP2 subunits in our study did not alter the drug potency and steady-state inactivation of Kv4.3 channels.
In the present study, the inhibitory effects of raloxifene on Kv4.3 appeared rapidly within 3 min after application, indicating either a direct or a nongenomic action. ICI 182,780, an estrogen receptor antagonist, is known to bind to the estrogen receptor with a similar binding affinity to raloxifene [54]. Incubation of ICI 182,780 for 1 h had no effect on the inhibitory properties of raloxifene on Kv4.3. In addition, β-estradiol and tamoxifen, another selective estrogen receptor modulator, had little effect on Kv4.3 currents. Because raloxifene has a high affinity for estrogen receptors that were identified in CHO cells [7, 46], these results suggest that raloxifene acts on Kv4.3 channel proteins independently of the effects on estrogen receptor-mediated pathways. However, it is known that Kv4.3 protein downregulation could result from nongenomic actions of estrogen on the channel itself or from genomic regulations (via the c-Src-Ras-MAPK pathway) of the Kv4.3 gene [15]. Therefore, further studies are required to investigate the possibility of direct actions on the channel itself or the involvement of signal transduction pathways in the mechanism of downregulation of Kv4.3 mRNA by raloxifene.
Raloxifene, a selective estrogen receptor modulator, is clinically used for treating postmenopausal osteoporosis [18, 27, 40]. Although this drug is known to exert cardiovascular protective actions via estrogen receptor-dependent and estrogen receptor-independent mechanisms in animal and human studies [38, 52], its effect on cardiac electrophysiology is not clearly understood. Raloxifene directly inhibited several types of Kv currents that participate in the repolarization of cardiac action potential: the human atrial transient outward and ultrarapid delayed rectifier Kv currents [25], the cloned hERG channel and recombinant human cardiac KCNQ1/KCE1 channel stably expressed in HEK cells [26], and the Kv4.3 currents in the present study. Accordingly, the inhibitory effects of raloxifene on these currents prolonged the repolarization phase of action potential duration in human atrial myocytes [25]. In cardiomyocytes, action potential duration is determined by the balance and interplay between the depolarizing and repolarizing currents [41]. On the other hand, raloxifene blocked L-type Ca2+ channels which are involved in the plateau phase of action potential and shortened action potential duration at 50 and 90 % repolarization in guinea pig ventricular myocytes [24], which can potentially cause proarrhythmic effects due to the concomitant shortening of the effective refractory period. In addition, raloxifene significantly decreased voltage-gated Na+ currents in guinea pig ventricular myocytes without QT interval prolongation [26]. Because Kv4.3 contributes to the initial phase of repolarization, pharmacological alteration of this channel, thereby, influences the amplitude of plateau potential and modulates the subsequent activation of the L-type Ca2+ channel and Ca2+ influx [12, 36]. Therefore, its impact on the duration of cardiac action potential is believed to be complex [45]. However, drug effects on cardiac action potential in guinea pig ventricular myocytes are difficult to extrapolate to humans because transient outward Kv currents, mainly encoded by Kv4.3, are entirely absent in guinea pig ventricular myocytes [29]. Since the overall effect of raloxifene on the action potential duration is determined by the relative potencies of the drugs on cardiac ionic currents, further study should focus on the effects of raloxifene on cardiac electrophysiology in human subjects.
In addition to acute pharmacological modulation, the alteration in the expression levels of the Kv4.3 gene has significant effects on cardiac electrophysiology. In humans, compared with males, females have a longer cardiac action potential duration and are more susceptible to arrhythmias in response to drugs [19]. Although the detailed mechanism of gender differences in the risk of arrhythmia is unclear, it is likely to be related to the influence of sex hormones on cardiac action potentials. For example, downregulation of Kv4.3 and Kv1.5 transcripts by estrogen may, in part, be responsible for gender-related differences in mouse ventricular repolarization [37]. The remodeling of the Kv4.3 gene expression by sex hormones was also demonstrated in the pregnant uterus [44]. In addition, estrogen treatment has been shown to downregulate cardiac Kv4.3 transcript that was antagonized by ICI 182,780 [14, 15]. Raloxifene directly inhibited T-type Ca2+ currents by affecting the channels and indirectly inhibited them by decreasing the level of the mRNA for channel protein (Cav3.2, Cav3.3) [50]. We found that long-term treatment with raloxifene (24 h) resulted in a significant decrease in the expression of Kv4.3 at the mRNA level independently of estrogen receptors, which may account for the chronic effects of raloxifene on cardiovascular events.
Raloxifene is clinically used in nanomolar plasma concentrations ranging between 0.9 and 2.7 nM [40], whereas the effective concentrations of raloxifene for the inhibition of Kv4.3 are at micromolar levels. Since raloxifene is rapidly absorbed from the gastrointestinal tract, its plasma concentrations can rapidly reach high levels under certain conditions. For example, the administration of a single dose of 185 mg of raloxifene resulted in a maximum plasma concentration of 12.5 mg/l (24.5 μM) within 30 min [40]. Furthermore, raloxifene has a long plasma elimination half-life of approximately 27 h [40], which may be attributed to a substantial accumulation of raloxifene in certain tissues. Accordingly, a buildup of raloxifene in different tissues has been known to reach micromolar concentrations [55]. Thus, the IC50 value of a Kv4.3 inhibition in the present study was within the range of therapeutic plasma concentrations of raloxifene. Raloxifene is rapidly absorbed from the gastrointestinal tract and undergoes extensive systemic glucuronidation, predominantly raloxifene-4′-glucuronide and raloxifene-6′-glucuronide [21, 23]. Both metabolites had little effect on Kv4.3 currents compared with the parent drug in the present study. However, the major metabolites of raloxifene are known to convert back to the parent compound in various tissues [13]. Thus, the chronic effects of raloxifene may also be modulated substantially by its reconversion in the tissues.
There are a number of potential limitations in the present study. In the present study, the electrophysiological recordings of Kv4.3 currents were performed at room temperature. The biophysical characteristics of Kv4.3 channels were temperature-dependent [34, 39]: the acceleration of the activation time course and the reduction in time-to-peak occurred at 37 °C. For these reasons, the clinical relevance of the observed electrophysiological effects of raloxifene on Kv4.3 channels requires further elucidation.
In summary, raloxifene produced a concentration-, time-, voltage-, and use-dependent inhibition of Kv4.3 currents. Raloxifene inhibited Kv4.3 channels by binding to the open state during depolarization and to the closed state at subthreshold potentials. These effects were not mediated via an estrogen receptor.
References
Ahn HS, Choi JS, Choi BH, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ (2005) Inhibition of the cloned delayed rectifier K+ channels, Kv1.5 and Kv3.1, by riluzole. Neuroscience 133:1007–19
Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ (2006) Interaction of riluzole with the closed inactivated state of Kv4.3 channels. J Pharmacol Exp Ther 319:323–31
Beck EJ, Bowlby M, An WF, Rhodes KJ, Covarrubias M (2002) Remodelling inactivation gating of Kv4 channels by KChIP1, a small-molecular-weight calcium-binding protein. J Physiol 538:691–706
Beck EJ, Covarrubias M (2001) Kv4 channels exhibit modulation of closed-state inactivation in inside-out patches. Biophys J 81:867–83
Bett GC, Morales MJ, Strauss HC, Rasmusson RL (2006) KChIP2b modulates the affinity and use-dependent block of Kv4.3 by nifedipine. Biochem Biophys Res Commun 340:1167–77
Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA (2004) Structure and function of Kv4-family transient potassium channels. Physiol Rev 84:803–33
Bryant HU, Glasebrook AL, Yang NN, Sato M (1999) An estrogen receptor basis for raloxifene action in bone. J Steroid Biochem Mol Biol 69:37–44
Butterworth JF, Strichartz GR (1990) Molecular mechanisms of local anesthesia: a review. Anesthesiology 72:711–34
Chung MT, Cheng PY, Lam KK, Chen SY, Ting YF, Yen MH, Lee YM (2010) Cardioprotective effects of long-term treatment with raloxifene, a selective estrogen receptor modulator, on myocardial ischemia/reperfusion injury in ovariectomized rats. Menopause 17:127–34
Delpon E, Valenzuela C, Gay P, Franqueza L, Snyders DJ, Tamargo J (1997) Block of human cardiac Kv1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels. Cardiovasc Res 35:341–50
Demo SD, Yellen G (1991) The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron 7:743–53
Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D (1996) Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res 79:659–68
Dodge JA, Lugar CW, Cho S, Short LL, Sato M, Yang NN, Spangle LA, Martin MJ, Phillips DL, Glasebrook AL, Osborne JJ, Frolik CA, Bryant HU (1997) Evaluation of the major metabolites of raloxifene as modulators of tissue selectivity. J Steroid Biochem Mol Biol 61:97–106
Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E (2005) Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96:1208–16
Eghbali M, Wang Y, Toro L, Stefani E (2006) Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med 16:285–91
Feng J, Wang Z, Li GR, Nattel S (1997) Effects of class III antiarrhythmic drugs on transient outward and ultra-rapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther 281:384–92
Grammer JB, Bosch RF, Kuhlkamp V, Seipel L (2000) Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation. J Cardiovasc Electrophysiol 11:626–33
Heringa M (2003) Review on raloxifene: profile of a selective estrogen receptor modulator. Int J Clin Pharmacol Ther 41:331–45
James AF, Choisy SC, Hancox JC (2007) Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol 94:265–319
Jeong I, Choi BH, Hahn SJ (2011) Rosiglitazone inhibits Kv4.3 potassium channels by open-channel block and acceleration of closed-state inactivation. Br J Pharmacol 163:510–20
Jeong EJ, Liu Y, Lin H, Hu M (2005) Species- and disposition model-dependent metabolism of raloxifene in gut and liver: role of UGT1A10. Drug Metab Dispos 33:785–94
Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF (1998) Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 98:1383–93
Kemp DC, Fan PW, Stevens JC (2002) Characterization of raloxifene glucuronidation in vitro: contribution of intestinal metabolism to presystemic clearance. Drug Metab Dispos 30:694–700
Liew R, Stagg MA, MacLeod KT, Collins P (2004) Raloxifene acutely suppresses ventricular myocyte contractility through inhibition of the L-type calcium current. Br J Pharmacol 142:89–96
Liu H, Jin MW, Xiang JZ, Huang Y, Sun HY, Chiu SW, Lau CP, Li GR (2007) Raloxifene inhibits transient outward and ultra-rapid delayed rectifier potassium currents in human atrial myocytes. Eur J Pharmacol 563:61–8
Liu H, Yang L, Jin MW, Sun HY, Huang Y, Li GR (2010) The selective estrogen receptor modulator raloxifene inhibits cardiac delayed rectifier potassium currents and voltage-gated sodium current without QTc interval prolongation. Pharmacol Res 62:384–90
Mitchner NA, Harris ST (2009) Current and emerging therapies for osteoporosis. J Fam Pract 58:S45–9
Muchmore DB (2000) Raloxifene: a selective estrogen receptor modulator (SERM) with multiple target system effects. Oncologist 5:388–92
Niwa N, Nerbonne JM (2010) Molecular determinants of cardiac transient outward potassium current (I to ) expression and regulation. J Mol Cell Cardiol 48:12–25
Ogita H, Node K, Asanuma H, Sanada S, Kim J, Takashima S, Minamino T, Hori M, Kitakaze M (2004) Raloxifene improves coronary perfusion, cardiac contractility, and myocardial metabolism in the ischemic heart: role of phosphatidylinositol 3-kinase/Akt pathway. J Cardiovasc Pharmacol 43:821–9
Ogita H, Node K, Asanuma H, Sanada S, Liao Y, Takashima S, Asakura M, Mori H, Shinozaki Y, Hori M, Kitakaze M (2002) Amelioration of ischemia- and reperfusion-induced myocardial injury by the selective estrogen receptor modulator, raloxifene, in the canine heart. J Am Coll Cardiol 40:998–1005
Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y (1997) Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel α-subunit, Kv4.3 in the rat. FEBS Lett 420:47–53
Patel SP, Parai R, Campbell DL (2004) Regulation of Kv4.3 voltage-dependent gating kinetics by KChIP2 isoforms. J Physiol 557:19–41
Radicke S, Riedel T, Cotella D, Turnow K, Ravens U, Schaefer M, Wettwer E (2013) Accessory subunits alter the temperature sensitivity of Kv4.3 channel complexes. J Mol Cell Cardiol 56:8–18
Recker RR, Mitlak BH, Ni X, Krege JH (2011) Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin 27:1755–61
Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH (2003) Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito). J Physiol 546:5–18
Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M (2009) Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res 105:343–52
Saitta A, Morabito N, Frisina N, Cucinotte D, Corrado F, D’Anna R, Altavilla D, Squadrito G, Minutoli L, Arcoraci V, Cancellieri F, Squadrito F (2001) Cardiovascular effects of raloxifene hydrochloride. Cardiovasc Drug Rev 19:57–74
Singleton CB, Valenzuela SM, Walker BD, Tie H, Wyse KR, Bursill JA, Qiu MR, Breit SN, Campbell TJ (1999) Blockade by N-3 polyunsaturated fatty acid of the Kv4.3 current stably expressed in Chinese hamster ovary cells. Br J Pharmacol 127:941–8
Snyder KR, Sparano N, Malinowski JM (2000) Raloxifene hydrochloride. Am J Health Syst Pharm 57:1669–75, quiz 1676–8
Snyders DJ (1999) Structure and function of cardiac potassium channels. Cardiovasc Res 42:377–90
Snyders DJ, Tamkun MM, Bennett PB (1993) A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression. J Gen Physiol 101:513–43
Snyders DJ, Yeola SW (1995) Determinants of antiarrhythmic drug action. Electrostatic and hydrophobic components of block of the human cardiac hKv1.5 channel. Circ Res 77:575–83
Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, Toro L, Stefani E (2001) Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem 276:31883–90
Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E (2004) Pharmacology of cardiac potassium channels. Cardiovasc Res 62:9–33
Thomas PB, Risinger KE, Klinge CM (2003) Identification of estrogen receptor beta expression in Chinese hamster ovary (CHO) cells and comparison of estrogen-responsive gene transcription in cells adapted to serum-free media. J Steroid Biochem Mol Biol 86:41–55
Thompson S (1982) Aminopyridine block of transient potassium current. J Gen Physiol 80:1–18
Tsang SY, Yao X, Essin K, Wong CM, Chan FL, Gollasch M, Huang Y (2004) Raloxifene relaxes rat cerebral arteries in vitro and inhibits L-type voltage-sensitive Ca2+ channels. Stroke 35:1709–14
Wang Z, Fermini B, Nattel S (1995) Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther 272:184–96
Wang Q, Lu L, Gao X, Wang C, Wang J, Cheng J, Gao R, Xiao H (2011) Effects of raloxifene on voltage-dependent T-type Ca2+ channels in mouse spermatogenic cells. Pharmacology 87:70–80
Wang S, Patel SP, Qu Y, Hua P, Strauss HC, Morales MJ (2002) Kinetic properties of Kv4.3 and their modulation by KChIP2b. Biochem Biophys Res Commun 295:223–9
Wenger NK (2002) Cardiovascular effects of raloxifene: the potential for cardiovascular protection in women. Diabetes Obes Metab 4:166–76
White MM, Bezanilla F (1985) Activation of squid axon K+ channels. Ionic and gating current studies. J Gen Physiol 85:539–54
Wijayaratne AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP (1999) Comparative analyses of mechanistic differences among antiestrogens. Endocrinology 140:5828–40
Yang Z, He X, Zhang Y (2007) The determination of raloxifene in rat tissue using HPLC. Biomed Chromatogr 21:229–33
Yuan LL, Chen X, Kunjilwar K, Pfaffinger P, Johnston D (2006) Acceleration of K+ channel inactivation by MEK inhibitor U0126. Am J Physiol Cell Physiol 290:C165–71
Zhang ZH, Steinberg MI (1995) LY 97241 accelerates the apparent rate of inactivation of transient outward K+ current: characterization of open channel block. J Pharmacol Exp Ther 274:249–57
Acknowledgments
We thank Dr. Imaizumi (Department of Molecular and Cellular Pharmacology, Nagoya City University, Japan) for the Kv4.3 cDNA. This work was supported by a grant from the Catholic Medical Center Research Foundation made in the program year of 2014.
Conflict of interest
None declared.
Authorship contributions
YJ Chae and SJ Hahn participated in the research design; YJ Chae, DH Kim, and Hong Joon Lee conducted the experiments; YJ Chae, DH Kim, Hong Joon Lee, Ki-Wug Sung, OJ Kwon, and SJ Hahn performed the data analysis; and OJ Kwon and SJ Hahn wrote or contributed to the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chae, Y.J., Kim, D.H., Lee, H.J. et al. Raloxifene inhibits cloned Kv4.3 channels in an estrogen receptor-independent manner. Pflugers Arch - Eur J Physiol 467, 1663–1676 (2015). https://doi.org/10.1007/s00424-014-1602-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1602-3