Abstract
Pericytes have become a hot topic in renal biology. They play a critical physiological role in vessel development, maintenance and remodelling through active communication with their vascular partners—endothelial cells—and modulation of extracellular matrix proteins. Multiple functions for renal pericytes have been described; specialised perivascular populations participate in glomerular filtration, regulate medullary blood flow and contribute to kidney fibrosis by differentiation into collagen-generating myofibroblasts. Interestingly, the origin of renin-producing cells of the juxtaglomerular region is attributed to the perivascular cell lineage; we have observed the coincidence of renin and pericyte marker expression during human kidney development. Finally, pericytes have been shown to share features with mesenchymal stem cells, which places them as potential renal progenitor cell candidates. Since renal diseases are often associated with microvascular complications, renal pericytes may emerge as new targets for the treatment of kidney disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pericytes were originally described almost 140 years ago by Charles Rouget as a contractile component in capillaries [2]. Initially, light and electron microscopy were the only methods to visualise and study perivascular cells [3]. Pericytes appear as long, polymorphic cells that stabilise the microvascular network and communicate through direct contact with endothelial cells along the length of the vessel [12]. Some characteristics of pericytes, e.g., smooth muscle actin expression, resemble those of vascular smooth muscle cells (VSMC). Both cell types are commonly known as mural or simply perivascular cells. VSMC constitute a mid-layer in larger vessels called tunica media, have a higher content of contractile proteins and form their own basement membrane. In contrast, pericytes are ascribed to small vessels such as pre-capillary arterioles, capillaries and post-capillary venules and express lower levels of alpha-smooth muscle actin compared to VSMC, and share, together with endothelial cells, a common basement membrane [1].
In the kidney, pericytes within the glomerular region are known as mesangial cells, whereas in tubulointerstitium, they are referred to as peritubular pericytes. Both of these pericyte populations have been the subject of separate reviews (for example, see [36, 39]). Here, we will discuss the renal functions of pericytes, their distribution within the kidney, markers used for their identification and their involvement in renal pathology.
Pericyte identification and ontogeny
Pericytes are characterised by dynamic protein expression that varies among different tissues, development stage and physiological/pathological conditions. To date, a single suitable marker has not been found and pericyte isolation and characterisation remains a challenging task. The most effective approach has been to use a multiple criteria to determine pericyte identity and assess their location, morphology and gene/protein expression patterns. Commonly used pericyte markers are summarised in Table 1.
Antigens used to identify pericyte populations belong to different classes of proteins. Alpha-smooth muscle actin (αSMA) [31] and desmin [14] are contractile filaments. αSMA is expressed by a subset of pericytes and is up-regulated in pathological conditions such as inflammation or tumorigenesis [2]. Neuron-glial 2 (NG2, chondroitin sulphate proteoglycan) has been used to detect pericytes in developing tissues and tumours [2], but it is usually absent from venules [30]. Platelet-derived growth factor receptor beta (PDGFRβ), a tyrosine-kinase receptor, is one of the most studied pericyte antigens [16, 26] and has been used to identify renal pericytes in both human [10] and mouse [19]. The ‘regulator of G protein signalling 5’ (RGS5) is a GTPase-activating protein, the expression of which overlaps with that of both PDGFRβ and NG2 in developing vessels [7]. Another marker used for pericyte detection is CD146 (melanoma cell adhesion molecule) [8], which have been successfully used for the isolation of pericytes via fluorescent-activated cell sorting [11]. Expression of the mesenchymal stem cell antigen CD73 is correlated with pericyte identity [11] and CD248 is a marker of stromal pericytes and fibroblasts in the kidney; it also has been used as a marker of fibrosis [40]. CD146 is also expressed on a subset of endothelial cells hence counter-staining of other endothelial cell markers is indispensable.
Renal pericytes are thought to descend from the FoxD1 lineage (forkhead/winged helix transcription factor) [15]. During nephrogenesis, FoxD1 is expressed in metanephric mesenchyme by cells destined to be stromal cells: pericytes, perivascular fibroblasts, vascular smooth muscle cells and mesangial cells. Studies by Brunskil et al. recently demonstrated that podocytes may also belong to FoxD1 lineage with both microarray and in situ hybridization data showing that they have abundant expression of FoxD1 [4].
Localization of pericytes/pericyte-like cells in the kidney
The kidney is highly vascularised, receiving about 25 % of the cardiac output, and hence, a dense capillary network is essential to provide efficient blood circulation. Renal pericytes are heterogeneous in nature; they are found around and inside glomeruli in the cortical region, and in the vasa recta and/peritubular capillaries in the renal medulla (see Fig. 1). In each renal location, pericytes perform specific functions.
Mesangial cells
Mesangial cells have been described as specialised pericyte cells of kidney glomeruli [35]. They display pericyte characteristics by providing structural support for glomerular capillaries, controlling glomerular filtration due to their contractility, and modulating local injury response by cell proliferation and membrane remodelling. An interesting feature of mesangial cells is their ability to modify innate and adaptive immune responses [36]. PDGFR-β has a critical role in mesangial cell development; in mice genetically deficient for PDGFR-β or PDGFβ, endothelial cells fail to recruit pericytes, which leads to hyper-dilated glomerular capillaries that have one or several distended capillary loops [24]. Analysis of PDGFRβ, desmin and αSMA expression during development suggests that mesangial cells and VSMC of glomerular afferent and efferent arterioles are derived from common PDGFRβ+ progenitors [26].
Mural cells of kidney arterioles
Mural cells of kidney arterioles enfold vessels that enter and exit glomeruli. Afferent arterioles diverge into glomerular capillaries and efferent arterioles branch into peritubular capillaries; both types of vessels are involved in maintaining blood pressure as a part of the tubuloglomerular feedback mechanism. When blood pressure is reduced, renin-producing cells located within afferent arterioles secrete renin in order to maintain blood pressure. The nature of renin-expressing cells has been a matter of debate; however, some studies suggested their perivascular/mesenchymal origin [20, 38]. It was shown that murine mesenchymal stem cells (MSCs) can develop into renin-expressing cells upon activation of receptor liver X receptor-α and release renin following cAMP up-regulation [27]. In a recent microarray study, RGS5, one of the pericyte markers, has been associated with renin-secreting cells [5].
Descending vasa recta/peritubular pericytes
Descending vasa recta (DVR)/peritubular pericytes effect the distribution of blood coming from the juxtamedullary cortex to the medulla. Peritubular pericytes are difficult to study due to the inaccessibility of the medulla in vivo; therefore, a model of isolated DVR has been established. In the presence of vasoconstrictors and vasodilators, DVR has been shown to constrict or dilate respectively [33]. Recently, contractility of DVR pericytes has been confirmed in situ on the ‘live’ kidney slice model in which pericyte-mediated changes in vessel diameter were visualised [9]. Following kidney injury, peritubular pericytes become activated and migrate from the vessels to the interstitial space to become myofibroblasts, which leads to the destabilisation and regression of capillaries [25].
Physiological function of pericytes
Pericytes are important for blood vessel development, stabilisation, and dynamic remodelling, but the actual role of pericytes is much more than vessel scaffolding; pericytes are multi-tasking cells in which specific features facilitate different functions in the microvasculature.
Cross-talk with endothelial cells
Pericytes are embedded in the basement membrane of endothelial cells. Adhesion plaques and peg-socket contacts provide areas of direct contact for both cell types [1]. Due to the close physical relationship, pericytes and endothelial cells can interact with each other through secretion of bioactive molecules and via specific response, regulate their proliferation and differentiation. In the process of angiogenesis, endothelial cell sprouts release PDGFβ which attracts migrating pericytes and to the contrary, pericytes and VSMCs inhibit capillary growth [32].
Blood flow and vascular tone regulation
Historically, the concept of pericyte contractility has been proposed because of microscopic observations indicating the presence of microfilaments in these cells. However, experimental data demonstrating the ability of pericytes to synthesise and secrete a wide variety of vasoactive agents emerged much later. DVR pericytes have been found to express αSMA [34] and are described as muscle-like cells that can regulate vessel diameter and contribute to medullary blood flow regulation. Pallone and Silldorff provided a body of data to confirm the vasomotor function of pericytes by investigating a model of isolated DVR [33]. In glomeruli, mesangial cells can modulate single nephron glomerular filtration rate (SNGFR); however, the main regulators of SNGFR are the afferent and efferent arterioles [36]. A large number of vasoactive substances regulate pericyte contractility. Both in cortical and medullary regions, pericytes respond by contraction to multiple stimuli including angiotensin-II, endothelin-1, vasopressin and adenosine. Acetylcholine and norepinephrine induce relaxation similar to prostaglandins and nitric oxide [33, 36]. It has been proposed that pericytes provide a crosslink in the signalling pathways regulating medullary blood flow as they can respond to vasoactive stimuli released by neighbouring tubules, endothelium, red blood cells, sympathetic nerves and interstitial cells of the renal medulla (reviewed in: [23]).
Immune response modulation
Pericytes are a source of cytokines and chemokines, mesangial cells in particular are also known to exhibit phagocytic activity mediated by Fc and C3 receptors [35, 36]. In collagen 1(α1) green fluorescent protein (GFP) reporter mice (pericytes and myofibroblasts express GFP) microarray analysis of activated pericytes following kidney injury revealed up-regulation of immune response genes, suggesting that pericytes are major contributors to the inflammatory response [37]. The relevance of this finding was confirmed by a recent study, in which blocking platelet-derived growth factor (PDGF) signalling prevented kidney fibrosis and attenuated the inflammatory response [6].
Progenitor cell function
MSCs or multi-potent stromal cells are adult stem cells, however, their identity and distribution in vivo were unknown for many years. Crisan et al. demonstrated that pericytes and MSCs share markers, both in vivo and in vitro, and pericyte populations from multiple tissues exhibit mesodermal multi-lineage potential [11]. The ubiquitous presence of pericytes within blood vessels makes them excellent candidates for local tissue repair. Pericytes have not been tested yet in the context of kidney injury/regeneration, nevertheless, MSCs showed promising results in several studies [29, 42].
Plasticity of pericytes
Pericytes differentiate into many different cell types both in vitro and in vivo, including vascular smooth muscle cells, fibroblasts, adipocytes, chondrocytes and osteoblasts [1, 8, 12]. The emergence of a particular cell type depends on environmental cues and when homeostasis is challenged, pericytes respond by modifying their phenotype.
VSMCs
VSMCs and pericytes originate from the same PDGFβ + progenitor cells; PDGFRβ and PDGFβ knockout mice are deficient in both VSMCs and pericytes [16]. Histological studies describe ‘transitional pericytes’ around terminal arterioles and venules, which are intermediate forms between VSMC and pericytes and are characterised by an increase in myofilaments, insertional dense plaques and dense bodies [12]. In vitro, a VSMCs phenotype can be induced in mesenchymal cell lines by treatment with TGFβ [18] or Ang-II [22]. It has been speculated that during kidney vasculature development, αSMA is induced in afferent arterioles due to local up-regulation of AngII following renin expression (discussed in: [13]). The interrelationship and redundancy between pericytes and vascular smooth muscle cells is a topic in need of further study.
Renin-expressing cells
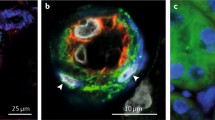
Renin-expressing cells located within afferent arterioles are the site of renin synthesis and release. When long-term homeostasis is threatened, VSMCs of preglomerular arterioles switch their phenotype from that of smooth muscle into a renin-producing secretory phenotype. Microarray studies of VSMCs of the renin lineage and kidney cortex in normal conditions and after renin induction treatment demonstrated that microRNAs, notably miR-330 and miR-125b-5p, control the contractile/endocrine switch of VSMCs [28]. In our experiments, we have observed co-expression of renin and pericyte markers between 8–20 weeks of gestation (CD146, NG2) in the human foetal kidney suggesting that renin-expressing cells are modified pericytes (Fig. 2). Moreover, primary cultures of pericytes isolated from the human foetal kidney can be induced to express and produce renin [Stefanska et al., unpublished].
Renin-positive cells co-express pericyte markers in the developing human kidney. Renin (red) was co-localised with NG2 (green) at 10 weeks of gestation (left) and CD146 (green) at 20 weeks of gestational age (right) in renal afferent arterioles. CD144 was used to distinguish endothelial cells as CD146 labels both pericytes and endothelial cells. Nuclei were counterstained with DAPI (blue) and the image was captured on a Leica confocal microscope with ×630 magnification
Myofibroblasts
Myofibroblasts are associated with reparative activity; they play a role in wound healing, injury repair and organ fibrosis. The hallmark of the myofibroblast phenotype is neo-expression of αSMA, thus they become contractile cells while retaining fibroblastic capabilities to synthetize extracellular matrix proteins (ECM) [17]. Various cell origins have been proposed for myofibroblasts, and among them are pericytes. Fate-tracking studies have been used to determine the origin of the cells contributing to kidney scarring. In an elegant study, Humphreys demonstrated that the major source of collagen deposition is a subset of CD73 + PDGFRβ + αSMA-perivascular interstitial cells derived from the FoxD1 cell lineage [19]. One possible scenario of events is that as a result of ischemic insult to the kidney or other stress stimuli, pericytes migrate from the vessels to the interstitial space, are exposed to local factors, and initiate a phenotypic change to become myofibroblasts.
Role for pericytes in pathophysiology and disease
Renal diseases are often associated with microvascular defects; pericytes, due to their prominent role in maintaining vascular homeostasis, play a central role in response to pathological stimuli. Pericyte loss or dysfunction can be an underlying cause of disease.
Kidney fibrosis
Pericytes and perivascular fibroblasts were recently identified as a main source of collagen I α1-producing cells in kidney disease. Pericyte activation, subsequent escape from the peritubular capillaries into the interstitium and differentiation into myofibroblasts result in scar tissue, progressive fibrosis and deterioration of renal function [19, 25, 39]. Therefore, targeting pericytes may constitute an efficient anti-fibrotic strategy. Studies by Chen et al., showed that alteration of PDGF-PBGFRβ signalling prevented interstitial kidney fibrosis and blocking this pathway via anti-PDGFR antibodies or treatment with imatinib (a PDGFRβ tyrosine-kinase inhibitor) reduced pericyte proliferation, macrophage infiltration and kidney fibrosis [6].
Diabetic glomerulopathy
A hallmark of diabetic nephropathy is increased mesangial matrix deposition which eventually leads to glomerulosclerosis and tubulo-intersitital fibrosis. In streptozotocin-induced diabetic rats renal NG2 expression was up-regulated; in a rat mesangial cell line, NG2 overexpression was associated with increased cell proliferation and ECM generation [41]. Furthermore, glomerular hypertension can accelerate diabetes-related complications. In the early phase of diabetes, mechanical stress applied to mesangial capillaries causes pericyte stretch, which activates various signalling pathways which are pro-mitogenic and promote matrix deposition [21].
Conclusions
Renal pericytes have justifiably been attracting a considerable amount of interest as the importance of these cells in both physiology and pathophysiology becomes more apparent. However, substantial gaps remain in our understanding of their latent potential and the mechanisms underlying their functions in vivo. Pericyte identification and isolation remain challenging and a better phenotype characterization would facilitate our understanding of pericyte roles in tissue development, homeostasis and diseases. Pericytes have the potential to become a target for the intervention and treatment of a range of renal diseases, and this will become more evident once we understand how to specifically alter different subsets of perivascular cells. The capacity of pericytes to act as resident renal stem cells in the context of kidney injury and repair is a topic of considerable interest and whilst still speculative, understanding the key events in pericyte activation and differentiation may enable the development of treatments for kidney fibrosis, and an increased understanding of the mechanisms regulating renal blood flow.
References
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523
Armulik A, Genové G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology 7:452–464
Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS (2011) Defining the molecular character of the developing and adult kidney podocyte. PLoS One 6:e24640
Brunskill EW, Sequeira-Lopez MLS, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA (2011) Genes that confer the identity of the renin cell. J Am Soc Nephrol 22:2213–2225
Chen Y-T, Chang F-C, Wu C-F et al (2011) Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80:1170–1181
Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH (2003) Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17:440–442
Covas DT, Panepucci RA, Fontes AM et al (2008) Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146 þ perivascular cells and fibroblasts. Exp Hematol 36:642–654
Crawford C, Kennedy-Lydon T, Sprott C, Desai T, Sawbridge L, Munday J, Unwin RJ, Wildman SSP, Peppiatt-Wildman CM (2012) An intact kidney slice model to investigate vasa recta properties and function in situ. Nephron Physiology 120:17–31
Crisan M, Chen C-W, Corselli M, Andriolo G, Lazzari L, Péault B (2009) Perivascular multipotent progenitor cells in human organs. Ann NY Acad Sci 1176:118–123
Crisan M, Yap S, Casteilla L et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Díaz-Flores L, Gutiérrez R, Varela H, Rancel N, Valladares F (1991) Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 6:269–286
Durik M, Sevá Pessôa B, Roks AJM (2012) The renin-angiotensin system, bone marrow and progenitor cells. Clin Sci 123:205–223
Fujimoto T, Singer SJ (1987) Immunocytochemical studies of desmin and vimentin in pericapillary cells of chicken. J Histochem Cytochem 35:1105–1115
Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E (1996) Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of winged helix transcription factor BF-2. Genes Dev 10:1467–1478
Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126:3047–3055
Hinz B (2010) The myofibroblast: paradigm for a mechanically active cell. J Biomech 43:146–155
Hirschi KK (1998) PDGF, TGF-beta, and heterotypic cell–cell interactions mediate endothelial cell-induced recruitment of 10 T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141:805–814
Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176:85–97
Jones C, Liang P, Pan L, Glenn S, Manly K, Gross K (2009) Renin-expressing cells from juvenile mouse kidneys are activated pericytes. Hypertension 54(4):E94
Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR (2008) Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med 233:4–11
Kim YM, Jeon ES, Kim MR, Jho SK, Ryu SW, Kim JH (2008) Angiotensin II-induced differentiation of adipose tissue-derived mesenchymal stem cells to smooth muscle-like cells. Int J Biochem Cell Biol 40:2482–2491
Kennedy-Lydon TM, Crawford C, Wildman SSP, Peppiatt-Wildman CM (2013) Renal pericytes: regulators of medullary blood flow. Acta Physiol 207:212–225
Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C (1994) Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 8:1875–1887
Lin S, Kisseleva T, Brenner DA, Duffield JS (2008) Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173:1617–1627
Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C (1998) Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125:3313–3322
Matsushita K, Morello F, Wu Y, Zhang L, Iwanaga S, Pratt RE, Dzau VJ (2010) Mesenchymal stem cells differentiate into renin-producing juxtaglomerular (JG)-like cells under the control of liver X receptor-alpha. J Biol Chem 285:11974–11982
Medrano S, Monteagudo MC, Sequeira-Lopez MLS, Pentz ES, Gomez RA (2012) Two microRNAs, miR-330 and miR-125b-5p, mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am J Physiol Renal Physiol 302:F29–37
Morigi M, Rota C, Montemurro T et al (2010) Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells 28:513–522
Murfee WL, Rehorn MR, Peirce SM, Skalak TC (2006) Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation 13:261–273
Nehls V, Drenckhahn D (1991) Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113:147–154
Orlidge A, D’Amore PA (1987) Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 105:1455–1462
Pallone TL, Silldorff EP (2001) Pericyte regulation of renal medullary blood flow. Exp Nephrol 9:165–170
Park F, Mattson DL, Roberts LA, Cowley AW (1997) Evidence for the presence of smooth muscle alpha-actin within pericytes of the renal medulla. Am J Physiol 273:R1742–R1748
Schlöndorff D (1987) The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J 1:272–281
Schlöndorff D, Banas B (2009) The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20:1179–1187
Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin S-L, Davis GE, Gharib SA, Humphreys BD, Duffield JS (2012) Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23:868–883
Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA (2001) Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281:F345–F356
Smith SW, Chand S, Savage COS (2012) Biology of the renal pericyte. Nephrol Dial Transplant 27:2149–2155
Smith SW, Eardley KS, Croft AP, Nwosu J, Howie AJ, Cockwell P, Isacke CM, Buckley CD, Savage COS (2011) CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney Int 80:199–207
Xiong J, Wang Y, Zhu Z, Liu J, Wang Y, Zhang C, Hammes H-P, Lang F, Feng Y (2007) NG2 proteoglycan increases mesangial cell proliferation and extracellular matrix production. Biochem Biophys Res Commun 361:960–967
Zoja C, Garcia PB, Rota C et al (2012) Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in adriamycin-induced nephropathy. Am J Physiol Renal Physiol 303:F1370–F1381
Acknowledgments
We acknowledge support from the British Heart Foundation Centre of Research Excellence Award. A.S. was the recipient of a BHF 4-year PhD studentship. We thank James Bailey for comments on the manuscript and Michael Mullins for the preparation of Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AM, S., B, P. & JJ, M. Renal pericytes: multifunctional cells of the kidneys. Pflugers Arch - Eur J Physiol 465, 767–773 (2013). https://doi.org/10.1007/s00424-013-1263-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1263-7