Abstract
Renal K+ retention is activated during pregnancy through a mechanism unknown to date. Here, we showed that the renal stimulation of H,K-ATPase type 2 (HKA2), whose expression was recently identified to be progesterone-dependent, is part of the mechanism favoring K+ accumulation during gestation. Moreover, investigation of the gestational phenotype of HKA2-null mice compared to their wild-type (WT) littermate revealed a decrease in fertility (gestation was successful in 33 % of HKA2-null mice vs. 83 % of WT mice) and in litter size (6.5 ± 0.6 and 7.8 ± 0.4 fetuses per litter, respectively). We also observed that urinary K+ excretion decreased by 20 % and plasma K+ concentration rose slightly (11 %) in WT mice during gestation (relative to basal conditions). In contrast, the renal excretion of K+ and plasma K+ levels in HKA2-null mice remained constant during gestation, whereas fecal K+ excretion increased. As a consequence, HKA2-null mice did not accumulate K+ in their extracellular compartment as efficiently as WT mice did. Finally, the link between inefficient K+ balance adaptations and gestational complications was established when we observed that these complications could be reversed with an increased K+ uptake. Altogether, these results define a novel physiological role for the HKA2 transporter and uncover a link between K+ metabolism and fertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregnancy is a physiological state that requires both physiological and anatomical modifications of the cardiovascular and renal systems to allow the gravid females to face the demands induced by fetal growth. Renal adaptation to pregnancy is of particular importance, and alterations in renal function or kidney diseases during pregnancy are particularly deleterious [34].
Electrolyte metabolism, which is strongly dependent on proper renal function, is modified during gestation in order to provide all required elements to the embryo. Fetal development requires in particular a large amount of K+, which is provided by the gravid female. At the end of gestation, women have accumulated around 300 mmol of K+ [30], which represents roughly 10–15 % of the total body K+ content or the total K+ intake over a 3-day period. Interestingly, a case–control study revealed that the risk of complications like preeclampsia is inversely proportional to the fiber and K+ dietary intake [19]. It is likely that the kidney is responsible for this accumulation of K+, but the mechanisms involved in this process are still unknown. It is also important to note that, during pregnancy, many factors favor K+ loss instead of K+ accumulation. For instance, the filtered load of K+ is higher during pregnancy because the glomerular filtration rate increases [5]. In addition, stimulation of Na+ retention through activation of the renin–angiotensin–aldosterone system [20, 28, 33], leading to increases in the activity of amiloride-sensitive epithelial sodium channels [48], should promote tubular K+ secretion through K+ channels in the distal part of the nephron.
Given these apparently divergent observations, on one hand, a pregnancy-induced potassium retention process and, on the other hand, a combination of factors that should contribute to renal K+ leak, we decided to investigate how the kidney adapts to gestation in the context of K+ homeostasis. Recently, we identified a new renal regulatory pathway involving progesterone, the H,K-ATPase type 2 (HKA2), and the renal retention of K+ [17]. We found that, during chronic dietary K+ restriction, adrenal steroidogenesis is modified so as to increase the production of progesterone and that this hormone promotes renal K+ retention in control mice but not in those deficient for the HKA2 gene. Moreover, the presence of the nuclear progesterone receptor in the distal part of the nephron [22] and the mifepristone sensitivity of HKA2 expression suggested that this receptor could be involved in the renal response to progesterone. HKA2 is an electroneutral transporter [7] that consists of two subunits, a catalytic α subunit that may combine, in heterologous systems, with different chaperon-like β subunits [12, 21]. The transporter exhibits pharmacological and transport features common to two closely related P-type ATPases, the Na,K-ATPase and the H,K-ATPase type 1 (HKA1). It is known that HKA2 may transport Na+ instead of H+ [9, 10, 12] and it is sensitive to ouabain [2, 11, 45] like Na,K-ATPase. It also transports H+ and is sensitive to Schering 28080 [36] as is HKA1. We recently demonstrated for the first time its physiological relevance in the kidney by showing its involvement in the circadian rhythm of urinary K+ excretion and in the preservation of stable plasma K+ concentrations throughout the day [42].
In this study, we tested the hypothesis that a regulatory pathway, similar to the one we described previously during dietary K+ depletion [17], is stimulated during gestation, whereby the increased production of progesterone switches the kidney to a “K+ reabsorption mode” through the activation of the HKA2 transporter.
Material and methods
Physiological analysis
Experiments were performed on C57BL/6 wild-type (WT) and knockout mice for the HKA2 α subunit gene [35]. In the experiments designed to investigate gestational parameters (percentage of successful fecundation and percentage of survival), WT and HKA2-null female mice were mated with WT males for three consecutive nights and then separated. In these experiments, the females were only weighted every day (without additional interference). To record physiological parameters, pregnant females were placed in metabolic cages at day 10 post-coitus; following 2 days of adaptation, food intake and 24-h-period urine samples were measured and collected from day 12 to 17 (late gestation period). Nonpregnant females of the same strains were placed in parallel in metabolic cages to record basal parameters. Urinary creatinine concentrations were determined using an automatic analyzer (Konelab 20i; Thermo, Cergy-Pontoise, France). Urinary K+ concentration was determined by flame photometry (IL943, Instrumentation Laboratory, Paris, France). Stools were collected over a 24-h period, dried, brushed (to eliminate food contaminants), and homogenized in distilled water (6 ml/g). Proteins were then precipitated using trichloroacetic acid (20 %) and removed by centrifugation (10,000×g; 10 min at 4 °C). In other experiments, females were kept in normal cages and, at day 17 post-coitus, plasma parameters were recorded by tail incision on the anesthetized animal with an ABL77 pH/blood gas analyzer (Radiometer, Lyon, France). After these measurements, nonpregnant and pregnant females were sacrificed for tissue and organ removal. All animal procedures were carried out in accordance with the French legislation for animal care and experimentation.
Quantitative polymerase chain reaction (PCR)
RNAs were extracted from whole kidneys using the TRI reagent (Invitrogen, Villebon sur Yvette, France) following the manufacturer’s instructions. One microgram of total RNA was then reverse-transcribed using the first-strand cDNA synthesis kit for reverse transcription (RT)-PCR (Roche Diagnostics, Meylan, France) according to the manufacturer’s instructions. Real-time PCRs were performed on a LightCycler (Roche Diagnostics, Meylan, France). No signal was detected in samples that did not undergo RT or in blank runs without cDNA. In each run, a standard curve was obtained using serial dilution of stock cDNA prepared from mouse kidney total RNA. Specific primers for targeted transcripts were chosen using the LC Probe Design 2.0 software.
Immunolabelling on microdissected tubules
Microdissected cortical collecting ducts (CCDs) from nonpregnant and pregnant female mice (day 17 post-coitus, late period) were treated as recently described [4, 18, 42] and incubated with an anti-HKA2 (1:400) antibody [38] that was previously characterized. After mounting, the slides were observed on a confocal microscope (Zeiss Observer.Z1) and images were analyzed by ImageJ software.
H,K-ATPase activity measurement
Kidney from nonpregnant and pregnant females (day 17 post-coitus) were perfused as described previously [29] and CCDs were distinguished and manually microdissected according to morphological and topographical criteria. ATPase activity in permeabilized nephron segments was determined by measuring the level of 32P formed after hydrolysis of [γ-32P]-ATP, as previously reported [51]. Briefly, to avoid contamination with Na,K-ATPase and to remove K+, pools of four to six CCDs were rinsed three times in a cold Na+-free and K+-free solution containing 0.8 mM MgSO4, 1 mM MgCl2, 0.5 mM CaCl2, 100 mM Tris(hydroxymethyl)aminomethane (Tris)–HCl, 1 mg/ml bovine serum albumin, and mannitol up to 400 mOsmol/kg, at pH 7.4. After being transferred into 96-well flat-bottom plates, CCDs were permeabilized with saponin (0.5 mg/ml) and a hypotonic solution (10 mM Tris–HCl) was then added. An assay medium containing 2.5 mM KCl, 10 mM MgCl2, 1 mM ethylene glycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid, 25 mM Tris–HCl, 5 mM Tris–ATP, and trace amounts of [γ-32P]-ATP at pH 7.4 was then added to the tubules, and the 96-well plates were incubated at 37 °C for 10 min. Three groups of tubules were treated in parallel: without inhibitors, with 50 μM Sch28080, or with 1 mM ouabain. Samples without nephron segments were treated in parallel in each experiment to determine the spontaneous breakdown of ATP. Preliminary experiments showed that no activity was detectable in the absence of added KCl, indicating that the entire measured activity could be ascribed to H,K-ATPase. This was also confirmed by the fact that the measured activity was fully inhibited by Sch28080 (see the “Results” section).
Tissue K+ content
Tissue K+ content was determined as described by Meneton et al. [35]. Briefly, fetuses or skeletal muscles (gastrocnemius) were weighed and freshly homogenized in distilled water and the proteins were precipitated with 10 % trichloroacetic acid. After centrifugation, at 10,000×g for 10 min at 4 °C, the K+ content of the supernatant was measured using a flame photometer (IL943, Instrumentation Laboratory, Paris, France).
Results
H,K-ATPase type 2 is stimulated during gestation
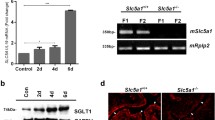
HKA2 mRNA expression was measured in the kidneys of nonpregnant (NP) and pregnant (P, day 17 of the gestation period) female mice. As shown in Fig. 1a, the level of expression of HKA2 increased twofold to threefold during the late phase of gestation compared to nonpregnant conditions. In parallel, we measured the expression of HKA1 (Fig. 1a) and found no difference between NP and P groups. At the protein level, we observed that the labeling of HKA2 on the luminal side of the CCD is stronger during the late gestation phase (Fig. 1b (a)) when compared to nonpregnant females, in which such labeling is hardly visible (Fig. 1b (b)). To confirm the stimulation of the HKA2 transporter, we measured its activity in the CCD of nonpregnant (Fig. 1c) and pregnant female mice (late phase; Fig. 1d). HKA2 function was assessed as the K+-dependent, ouabain-sensitive, and Schering 28080-sensitive ATPase activity measured in the absence of Na+. Similarly, HKA1 function was assessed as the K+-dependent, ouabain-insensitive, and Schering 28080-sensitive ATPase activity measured in the absence of Na+. As shown in Fig. 1c, HKA2 activity (i.e., ouabain-sensitive ATPase activity) was not detectable in the CCDs of nonpregnant female mice; the K+-dependent ATPase activity measured in these CCDs was fully inhibited by Schering 28080, indicating that it corresponds to HKA1. Conversely, in pregnant female mice, more than 95 % of the total K+-dependent ATPase activity was inhibited by both ouabain and Schering 28080, a specific characteristic of HKA2.
Expression of HKA2 during pregnancy. The mRNA levels of HKA2 and HKA1 α subunits (a), relative to the housekeeping gene cyclophilin A, were measured in the kidney of nonpregnant (NP, white bar) and pregnant female mice (day 17 post-coitus, P, black bar). Results are shown as the mean ± SEM (n = 6). **p < 0.01, nonpaired Student’s t test. b CCD from WT nonpregnant (a) and pregnant (late period of the gestation, b) were immunolabeled using a polyclonal anti-HKA2 antibody. K-ATPase activity in Na+-free medium was determined in CCD of nonpregnant (c) and pregnant (day 17 post-coitus, d) female mice in the absence of any inhibitor (total) or in the presence of 1 mM ouabain or of 50 μM Schering 28080. Results are shown as the mean ± SEM (n = 5 for the nonpregnant group and n = 8 for pregnant female mice). For each animal and each condition (basal, ouabain, and Sch28080), H,K-ATPase activity was determined in four replicate samples. **p < 0.01, nonpaired Student’s t test
Lack of HKA2 leads to gestational complications
Because the renal HKA2 is stimulated during the late gestation phase, we investigated the consequence of its genetic ablation on pregnancy. We first observed that HKA2-null mice had difficulties getting pregnant and we decided to quantify this effect. As shown in Fig. 2a, 83 % of WT mice became pregnant after two to three attempts (1 attempt = 3 nights in a row with a male), whereas only 33 % of HKA2-null mice did so. Another difference with HKA2-null mice is that around 30 % (4 out of 13) of the pregnant female went beyond their predicted delivery date (days 19–20), started to decline (i.e., exhibited weight; see Supplemental Fig. 1), and had to be sacrificed for ethical reasons (Fig. 2b). During this sacrifice, we observed that they were all bearing living fetuses. In contrast, only one WT mouse died during gestation for unknown reasons.
Gestational parameters of WT and HKA2-null mice. a The rate of fecundation leading to full-term pregnancy was evaluated under controlled conditions where a WT male was placed with a WT (white bar) and a HKA2-null (black bar) female for three consecutive nights in the same cage (this mating period corresponds to one attempt). Female mice were then isolated and their weight was measured every day for 9 days. In the absence of an increase in weight after 9 days, the female is replaced with a male for a second attempt. A maximum of five attempts was assayed for each female mouse. Results are shown as percentage of females getting pregnant over the total number (n) of females tested (n = 6 for WT and n = 12 for HKA2-null mice). For females that get pregnant, the mean number of attempts was similar between WT and HKA2-null mice (two to three attempts). b Percentage of WT (white bar) or HKA2-null (black bar) female mice that survive during the gestation period over the total number (n) of female tested (n = 6 for WT and n = 12 for HKA2-null mice)
K+ excretion is not adapted to gestation in HKA2-null mice
As shown in Fig. 3a, WT mice increased their food intake by 20 % during gestation. The increased K+ intake should have raised urinary K+ excretion in the same proportion; however, as shown in Fig. 3b and Table 1, gravid WT mice exhibited decreased urinary K+ excretion compared to the nonpregnant state. These results indicate that, as expected, during gestation, renal function is disconnected from the dietary intake and is switched to a “K+ reabsorption mode.” The fecal excretion of K+ is also modified during gestation with a decrease of about 15 μmol/day compared to the nonpregnant group (Fig. 3c). The gestation-induced renal and (to a lower extent) fecal K+ retention had an impact on plasma K+ concentrations, which increased from 3.70 ± 0.10 mM in nonpregnant WT mice to 4.10 ± 0.07 mM in the late gestation phase (Fig. 3d; unpaired Student’s t test p value = 0.006, n = 9–13). Similarly to WT mice, HKA2-deficient mice increased their food intake by 20 % during the late gestation phase (Fig. 3). In contrast with WT mice, however, their urinary K+ excretion was not reduced (Fig. 3f), indicating an inefficient renal adaptation. The fecal excretion of K+, in the nonpregnant state, was twice higher than in WT mice and significantly increased during gestation (Fig. 3g). In spite of these phenotypes, the plasma K+ concentration of HKA2-null mice (Fig. 3h) remained similar in the nonpregnant state (4.09 ± 0.1 mM, n = 12) and during the late gestation phase (3.85 ± 0.1 mM, n = 10).
Adaptation of the K+ balance during gestation in WT and HKA2-null mice. Nonpregnant (NP) and pregnant (P) WT (a–d, white bars) and HKA2-null mice (e–h, black bars) were placed in metabolic cages to measure their food intake (daily mean over a 6-day period, a and e), their urinary K+ excretion (daily mean over a 6-day period, b and f), their fecal K+ excretion (daily mean over a 6-day period, c and g), and their plasma K+ values (at day 17 post-coitus, d and h). Results are shown as the mean ± SEM (n = 7 for WT mice and n = 7 for HKA2-null mice). *p < 0.05, **p < 0.01; nonpaired Student’s t test
Tissue K+ content is modified during gestation
Because renal and intestinal K+ retention was not observed in HKA2-null mice, we examined how this may have affected intracellular K+ levels. As shown in Fig. 4a, the K+ content of muscles (gastrocnemius) from WT pregnant females decreased by 15 % at the end of gestation. This result indicates that WT mice not only retained K+ by activating their renal HKA2, but also mobilized their internal K+ pool. Unexpectedly, the muscle K+ content of HKA2-null mice did not decrease, even though these mice were not able to retain K+ (Fig. 4b). We then investigated whether fetus growth could be impacted by the lack of HKA2. As shown in Fig. 5a, WT mice had 7.8 ± 0.4 pups per litter, whereas the HKA2-null mice had 6.5 ± 0.6 pups per litter. However, weight (Fig. 5b) and K+ content (Fig. 5c) were similar in both strains. Therefore, on average, the need for K+ is less important in HKA2-null mice than in WT mice because their litter size is smaller.
Tissue K+ content during gestation in WT and HKA2-null mice. Muscle K+ contents were determined in WT (a) nonpregnant (NP) and pregnant (day 17 post-coitus, P) females or in HKA2-null nonpregnant and pregnant females (b). Results are shown as the mean ± SEM (n = 6–10 mice). *p < 0.05; nonpaired Student’s t test
Fetal growth parameters in WT and HKA2-null mice. a Number of fetuses per litter from WT (white dots) or HKA2-null (black dots) mice. Results are shown as individual values and as mean ± SEM. *p = 0.033; nonpaired Student’s t test. b Individual weight of fetuses at day 17 post-coitus in WT (white bar) or HKA2-null (black bar) mice. c Potassium content (in micromoles per gram of wet weight) of fetuses were determined in triplicate (three fetuses per litter) from three different pregnant WT (white bar) or HKA2-null (black bar) mice. Results are shown as the mean ± SEM
Acid–base status during pregnancy is altered in HKA2-null mice
The increase in food intake also leads to an increase in the acid load that needs to be excreted to avoid acidosis. Because HKA2 is also involved in this process [31, 47] along with H+-ATPase, we measured acid–base parameters in WT and HKA2-null mice during gestation. As shown in Fig. 6a, b and Table 1, in WT mice, gestation did not modify urine and plasma pH nor plasma bicarbonate concentration, indicating that the kidney adapted efficiently to excrete the acid load resulting from a higher food intake. Conversely, in HKA2-null mice during pregnancy, the urine pH became alkaline (Fig. 6c) and the plasma bicarbonate concentration decreased significantly (Fig. 6d), indicating the development of mild acidosis.
Acid–base status during gestation in WT and HKA2-null mice. Urine pH and plasma bicarbonate content were measured in WT mice (a, b) and in HKA2-deficient mice (c, d) before gestation (NP) and during the late period of gestation (P). Results are shown as the mean ± SEM (n = 6–10). *p < 0.05, **p < 0.01; nonpaired Student’s t test
Potassium supplementation restores normal gestational parameters in HKA2-null mice
To investigate whether the gestational problems of HKA2-null mice could be linked to their inefficient renal adaptation, which prevents K+ accumulation, we enriched their drinking water with K+ from the mating day until the delivery day. As shown in Fig. 7, fecundation rate (a), mortality (b) and the number of fetuses per litter (c) of HKA2-null mice (hatched bars) then became similar to those of WT mice (gray bars) and were not different from those measured previously in WT without K+ enrichment (Figs. 2 and 5). In other words, providing more K+ to HKA2-null mice allowed us to reverse their gestational phenotype.
Gestational parameters of WT and HKA2-null mice during K+ supplementation. Similar experiments as those reported in Fig. 2 were performed in the presence of 1 % KCl in the drinking water (from the mating day to the delivery) to measure fecundation rate (a), mortality rate (b), and number of fetuses per litter (c) of WT (gray bar) and HKA2-null (hatched bar) mice. For a and b, results are shown as the percentage of WT or HKA2-null female mice that became pregnant and survived during the gestation period over the total number (n) of females tested (n = 6 for WT and n = 8 for HKA2-null mice). For c, results are shown as the mean ± SEM
Discussion
The recent finding that progesterone stimulates the expression of HKA2 in the kidney during K+ depletion and promotes the renal reabsorption of K+ [17] suggests that this ion transporter is involved in pregnancy-induced K+ retention [30]. In the present study, we demonstrated that renal HKA2 is indeed stimulated in the late part of gestation: the mRNA and protein expression of the transporter and its activity all increase. The phenotype of female HKA2-null mice during pregnancy also highlighted the role of this transporter in the correct development of gestation and the link between the ability to retain K+ and fertility.
Renal solute transport during gestation
The strong physiological changes occurring during gestation impact the regulation of ion homeostasis. Indeed, two challenges need to be overcome: (1) maintaining the composition of the maternal internal milieu within strict limits and (2) providing the required resources for fetal growth. This is particularly important for K+, which is the main intracellular cation and thus an important “brick” in the building of fetuses. In mice for instance, after the 15th day post-coitus, each fetus almost doubles its weight every 24 h. Therefore, the need to provide sufficient amounts of K+ is particularly important during the late gestation phase, and this has to be achieved without modifying the physiological parameters of the pregnant female.
Many investigators had already observed that the accumulation of K+ was of renal origin and was due to an increase in tubular K+ retention [3, 25, 30]. However, the molecular mechanism of this retention was subject to debate. Indeed, it has been proposed that progestin compounds through their antimineralocorticoid action [41] could have natriuretic and antikaliuretic effects [8] in the distal part of the nephron. By itself, this explanation is not satisfying in the context of pregnancy because it would result in urinary Na+ loss instead of the reported Na+ retention [26]. Moreover, Quinkler et al. [40] showed, in vivo, that progesterone has only a weak antimineralocorticoid effect in humans.
Evidence for the presence of the nuclear progesterone receptor in the kidney and more particularly in the distal part of the nephron [22, 23], in addition to the finding of a novel regulatory pathway involving progesterone and K+ retention [17], opens the way for an alternate explanation in which the HKA2 plays a major role. As we showed in this study, this transporter is stimulated in the kidney of gravid females at the end of the gestation period, and its absence correlates with gestational defects. In parallel, we also observed that renal ATPase activity that is related to HKA1 (i.e., ouabain-insensitive) disappears during gestation but is not accompanied by changes in the mRNA level of the HKA1 α subunit. This shift in activity from HKA1 to HKA2 was previously observed when mice were switched from a normal to a low-K+ diet [15, 36]. However, whereas the expression of HKA2 increased during K+ depletion, the mRNA or protein expression of HKA1 was found either to be downregulated [36] or to remain constant [16, 27] or to increase [1]. The physiological relevance and the underlying mechanisms of this shift remain unknown but clearly indicate that, in a given physiological context, each H,K-ATPase has a specific role to play.
Potassium accumulation, HKA2, and fertility
We found that the absence of HKA2 affects both the K+ balance and gestational parameters. Are these two observations linked? It would be tempting to speculate that the expression of HKA2 in the uterus [39] and placenta [24, 32] could account for the lower fertility observed in HKA2-null mice. However, even though HKA2 has been detected at mRNA and protein levels, there is no evidence for its activity in these tissues. For instance, in attempting to characterize ATPase activity in the microvillus membranes of the human placental syncytiotrophoblast, Brunette et al. [6] did not observe any Schering 28080-sensitive activity. In the absence of clear evidence for HKA2 activity in these tissues, its putative physiological relevance remains obscure.
Since we showed that HKA2-null mice could recover normal gestational parameters by ingesting more K+, it is likely that the K+ homeostasis disturbances we measured in this study are the principal reason for the decrease in fertility in HKA2-null mice. The ability to accumulate K+, therefore, seems to be an advantage for reproduction. As mentioned earlier, K+ is required for fetal growth and also for maintaining stable plasma K+ concentrations under volume expansion conditions.
Obviously, this requirement is even more critical in species bearing multiple fetuses like rodents, with females that almost double their weight at the end of gestation. Conversely, in pregnant women at delivery, the weight gain normally does not exceed 20 %. Given these obvious differences, the need for accumulating K+ could be less important in humans than in mice. Another difference between rodent models and human beings concerns the sequence of the HKA2 α subunit. Mouse and human HKA2 α subunits are 86 % identical and most differences are localized in the first hundred amino acids. These discrepancies may be at the origin of some pharmacological and kinetic differences in the properties of the HKA2 α subunit between different species [44, 46]. However, reported values of the apparent affinity for K+ are similar between rodents and humans [46]. The significance of these human vs. mouse differences remains unclear in the context of gestation.
A successful pregnancy depends on many steps: ovulation, fertilization, preimplantation, implantation, and growth of the fetuses. We have not identified which of these steps are dependent on the accumulation of K+. However, we observed in some HKA2-null female mice, early post-coitus (between days 7 and 9), a slight and transient increase in body weight, followed by a return to the initial value. It is possible that these mice became pregnant but then lost their fetuses due to problems during fetal growth.
In addition to favoring fetus growth, relatively high K+ levels may have other positive consequences. For instance, in a recent study [49], K+ supplementation was shown to improve the acetylcholine-induced vasorelaxation of arterioles in a model of nonhypertensive DOCA/salt-fed mice. In terms of the Na+ balance, this model presents some similarities with gestation as in both cases the renal reabsorption of Na+ occurs in a hypervolemic state without an impact on blood pressure. We, therefore, propose that, as in this artificial model of forced Na+ reabsorption, a relatively high level of K+ is required to induce the vascular refractoriness to vasoconstrictors observed during gestation. The inability to maintain high extracellular K+ levels would then impede the pregnancy-specific, body-wide vasodilatation state.
Finally, the need for K+ accumulation needs to be confirmed in pregnant female patients suffering from diseases characterized by hypokalemia like Bartter’s or Gitelman’s syndrome. Interestingly, if these syndromes do not impede gestation, different reports indicate that 35 % (7 out of 20 [13]) to 55 % (6 out of 11 [14]) of women with Gitelman’s syndrome have complications during pregnancy (such as a miscarriage in the first trimester). This occurrence is much higher than in the general population. Even though the link between hypokalemia and gestational difficulties was not firmly established in these women, it is worth noting that some needed an intravenous administration of K+ to handle a full-term pregnancy.
How is the absence of HKA2 compensated for during gestation?
The HKA2-deficient female mice that have a full-term pregnancy do not display a significant reduction in plasma K+ concentrations, even though they do not retain K+ through their kidneys and colon. Even if we did not use a very sensitive method to measure muscle K+ content, it is clear that the HKA2-null mice do not compensate by the “altruistic” muscular process as defined by Youn and McDonough [50]. This occurs, in many cases, to the detriment of the litter size. However, we think that other possible compensation mechanisms help to maintain normal plasma K+ values and to provide K+ to fetuses. For instance, limiting the extracellular volume expansion that normally occurs during gestation could contribute to maintaining plasma K+ levels in the normal range. In WT mice, extracellular volume expansion is correlated to renal water retention, a vasopressin receptor-dependent mechanism involving stimulation of AQP2 [37, 43]. As shown in Table 1, the hematocrit decreased by 2 % during gestation in WT mice but remained unchanged in HKA2-null mice. This result suggests that the extracellular compartment volume does not increase as it should. This may allow the HKA2-null mice to maintain a relatively high plasma K+ concentration. However, this putative compensatory mechanism, if excessive, may induce a reduction in the perfusion of the placenta and, therefore, lead to partial abortion. This particular aspect would have to be investigated in more depth. Altogether, these results define a novel physiological role for the HKA2 transporter and reveal a link between K+ metabolism and fertility.
References
Ahn KY, Turner PB, Madsen KM, Kone BC (1996) Effects of chronic hypokalemia on renal expression of the “gastric” H(+)-K(+)-ATPase alpha-subunit gene. Am J Physiol 270:F557–F566
Asano S, Hoshina S, Nakaie Y, Watanabe T, Sato M, Suzuki Y, Takeguchi N (1998) Functional expression of putative H+-K+-ATPase from guinea pig distal colon. Am J Physiol 275:C669–C674
Atherton JC, Dark JM, Garland HO, Morgan MR, Pidgeon J, Soni S (1982) Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol 330:81–93
Azroyan A, Morla L, Crambert G, Laghmani K, Ramakrishnan S, Edwards A, Doucet A (2012) Regulation of pendrin by cAMP: possible involvement in beta adrenergic-dependent NaCl retention. Am J Physiol Renal Physiol. doi:10.1152/ajprenal.00403.2011
Baylis C (1987) The determinants of renal hemodynamics in pregnancy. Am J Kidney Dis 9:260–264
Brunette MG, Bastani B, Leclerc M, Narbaitz R (1995) Detection of different adenosine triphosphatases in human placental brush border membranes. J Membr Biol 145:285–293
Burnay M, Crambert G, Kharoubi-Hess S, Geering K, Horisberger JD (2001) Bufo marinus bladder H-K-ATPase carries out electroneutral ion transport. Am J Physiol Renal Physiol 281:F869–F874
Burton G, Galigniana M, De Lavallaz S, Brachet-Cota AL, Sproviero EM, Ghini AA, Lantos CP, Damasco MC (1995) Sodium-retaining activity of some natural and synthetic 21-deoxysteroids. Mol Pharmacol 47:535–543
Codina J, Pressley TA, DuBose TD Jr (1999) The colonic H+,K+-ATPase functions as a Na+-dependent K+(NH4 +)-ATPase in apical membranes from rat distal colon. J Biol Chem 274:19693–19698
Cougnon M, Bouyer P, Planelles G, Jaisser F (1998) Does the colonic H,K-ATPase also act as an Na,K-ATPase? Proc Natl Acad Sci USA 95:6516–6520
Cougnon M, Planelles G, Crowson MS, Shull GE, Rossier BC, Jaisser F (1996) The rat distal colon P-ATPase alpha subunit encodes a ouabain-sensitive H+,K+-ATPase. J Biol Chem 271:7277–7280
Crambert G, Horisberger JD, Modyanov NN, Geering K (2002) Human nongastric H+-K+-ATPase: transport properties of ATP1al1 assembled with different beta-subunits. Am J Physiol Cell Physiol 283:C305–C314
Cruz DN, Shaer AJ, Bia MJ, Lifton RP, Simon DB (2001) Gitelman’s syndrome revisited: an evaluation of symptoms and health-related quality of life. Kidney Int 59:710–717. doi:10.1046/j.1523-1755.2001.059002710.x
Daskalakis G, Marinopoulos S, Mousiolis A, Mesogitis S, Papantoniou N, Antsaklis A (2010) Gitelman syndrome-associated severe hypokalemia and hypomagnesemia: case report and review of the literature. J Matern Fetal Neonatal Med 23:1301–1304. doi:10.3109/14767051003678010
Dherbecourt O, Cheval L, Bloch-Faure M, Meneton P, Doucet A (2006) Molecular identification of Sch28080-sensitive K-ATPase activities in the mouse kidney. Pflugers Arch 451:769–775
DuBose TD Jr, Codina J, Burges A, Pressley TA (1995) Regulation of H(+)-K(+)-ATPase expression in kidney. Am J Physiol 269:F500–F507
Elabida B, Edwards A, Salhi A, Azroyan A, Fodstad H, Meneton P, Doucet A, Bloch-Faure M, Crambert G (2011) Chronic potassium depletion increases adrenal progesterone production that is necessary for efficient renal retention of potassium. Kidney Int 80:256–262. doi:10.1038/ki.2011.15
Fila M, Brideau G, Morla L, Cheval L, Deschenes G, Doucet A (2011) Inhibition of K+ secretion in the distal nephron in nephrotic syndrome: possible role of albuminuria. J Physiol 589:3611–3621. doi:10.1113/jphysiol.2011.209692
Frederick IO, Williams MA, Dashow E, Kestin M, Zhang C, Leisenring WM (2005) Dietary fiber, potassium, magnesium and calcium in relation to the risk of preeclampsia. J Reprod Med 50:332–344
Garland HO, Atherton JC, Baylis C, Morgan MR, Milne CM (1987) Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol 113:435–444
Geering K, Crambert G, Yu C, Korneenko TV, Pestov NB, Modyanov NN (2000) Intersubunit interactions in human X,K-ATPases: role of membrane domains M9 and M10 in the assembly process and association efficiency of human, nongastric H,K-ATPase alpha subunits (ATP1al1) with known beta subunits. Biochemistry 39:12688–12698
Grimont A, Bloch-Faure M, El Abida B, Crambert G (2009) Mapping of sex hormone receptors and their modulators along the nephron of male and female mice. FEBS Lett 583:1644–1648
Hofmeister MV, Damkier HH, Christensen BM, Olde B, Fredrik Leeb-Lundberg LM, Fenton RA, Praetorius HA, Praetorius J (2012) 17β-Estradiol induces nongenomic effects in renal intercalated cells through G protein-coupled estrogen receptor 1. Am J Physiol Renal Physiol 302:F358–F368. doi:10.1152/ajprenal.00343.2011
Johansson M, Jansson T, Pestov NB, Powell TL (2004) Non-gastric H+/K+ ATPase is present in the microvillous membrane of the human placental syncytiotrophoblast. Placenta 25:505–511. doi:10.1016/j.placenta.2003.11.008
Khraibi AA, Dobrian AD, Yu T, Solhaug MJ, Billiar RB (2005) Role of RIHP and renal tubular sodium transporters in volume retention of pregnant rats. Am J Hypertens 18:1375–1383. doi:10.1016/j.amjhyper.2005.04.022
Kim GH, Martin SW, Fernandez-Llama P, Masilamani S, Packer RK, Knepper MA (2000) Long-term regulation of renal Na-dependent cotransporters and ENaC: response to altered acid–base intake. Am J Physiol Renal Physiol 279:F459–F467
Kraut JA, Hiura J, Besancon M, Smolka A, Sachs G, Scott D (1997) Effect of hypokalemia on the abundance of HK alpha 1 and HK alpha 2 protein in the rat kidney. Am J Physiol 272:F744–F750
Ledoux F, Genest J, Nowaczynski W, Kuchel O, Lebel M (1975) Plasma progesterone and aldosterone in pregnancy. Can Med Assoc J 112:943–947
Lemale J, Bloch-Faure M, Grimont A, El Abida B, Imbert-Teboul M, Crambert G (2008) Membrane progestin receptors alpha and gamma in renal epithelium. Biochim Biophys Acta 1783:2234–2240
Lindheimer MD, Richardson DA, Ehrlich EN, Katz AI (1987) Potassium homeostasis in pregnancy. J Reprod Med 32:517–522
Lynch IJ, Rudin A, Xia SL, Stow LR, Shull GE, Weiner ID, Cain BD, Wingo CS (2008) Impaired acid secretion in cortical collecting duct intercalated cells from H-K-ATPase-deficient mice: role of HKalpha isoforms. Am J Physiol Renal Physiol 294:F621–F627
Marchand M, Horcajadas JA, Esteban FJ, McElroy SL, Fisher SJ, Giudice LC (2011) Transcriptomic signature of trophoblast differentiation in a human embryonic stem cell model. Biol Reprod 84:1258–1271. doi:10.1095/biolreprod.110.086413
Martin JD, Mills IH (1956) Aldosterone excretion in normal and toxaemic pregnancies. Br Med J 2:571–573
Maynard SE, Thadhani R (2009) Pregnancy and the kidney. J Am Soc Nephrol 20:14–22. doi:10.1681/ASN.2008050493
Meneton P, Schultheis PJ, Greeb J, Nieman ML, Liu LH, Clarke LL, Duffy JJ, Doetschman T, Lorenz JN, Shull GE (1998) Increased sensitivity to K+ deprivation in colonic H,K-ATPase-deficient mice. J Clin Invest 101:536–542
Nakamura S, Amlal H, Galla JH, Soleimani M (1998) Colonic H+-K+-ATPase is induced and mediates increased HCO3 − reabsorption in inner medullary collecting duct in potassium depletion. Kidney Int 54:1233–1239. doi:10.1046/j.1523-1755.1998.00105.x
Ohara M, Martin PY, Xu DL, St John J, Pattison TA, Kim JK, Schrier RW (1998) Upregulation of aquaporin 2 water channel expression in pregnant rats. J Clin Invest 101:1076–1083. doi:10.1172/JCI649
Pestov NB, Korneenko TV, Shakhparonov MI, Shull GE, Modyanov NN (2006) Loss of acidification of anterior prostate fluids in Atp12a-null mutant mice indicates that nongastric H-K-ATPase functions as proton pump in vivo. Am J Physiol Cell Physiol 291:C366–C374. doi:10.1152/ajpcell.00042.2006
Pestov NB, Romanova LG, Korneenko TV, Egorov MV, Kostina MB, Sverdlov VE, Askari A, Shakhparonov MI, Modyanov NN (1998) Ouabain-sensitive H,K-ATPase: tissue-specific expression of the mammalian genes encoding the catalytic alpha subunit. FEBS Lett 440:320–324
Quinkler M, Meyer B, Oelkers W, Diederich S (2003) Renal inactivation, mineralocorticoid generation, and 11beta-hydroxysteroid dehydrogenase inhibition ameliorate the antimineralocorticoid effect of progesterone in vivo. J Clin Endocrinol Metab 88:3767–3772
Rafestin-Oblin ME, Couette B, Barlet-Bas C, Cheval L, Viger A, Doucet A (1991) Renal action of progesterone and 18-substituted derivatives. Am J Physiol 260:F828–F832
Salhi A, Centeno G, Firsov D, Crambert G (2012) Circadian expression of H,K-ATPase type 2 contributes to the stability of plasma K+ levels. FASEB J 26:2859–2867. doi:10.1096/fj.11-199711
Schrier RW, Ohara M (2010) Dilemmas in human and rat pregnancy: proposed mechanisms relating to arterial vasodilation. J Neuroendocrinol 22:400–406. doi:10.1111/j.1365-2826.2010.01948.x
Shao J, Gumz ML, Cain BD, Xia SL, Shull GE, van Driel IR, Wingo CS (2010) Pharmacological profiles of the murine gastric and colonic H,K-ATPases. Biochim Biophys Acta Biochim Biophys Acta 1800:906–911. doi:10.1016/j.bbagen.2010.05.002
Swarts HG, Koenderink JB, Willems PH, De Pont JJ (2005) The non-gastric H,K-ATPase is oligomycin-sensitive and can function as an H+,NH4(+)-ATPase. J Biol Chem 280:33115–33122. doi:10.1074/jbc.M504535200
Swarts HG, Koenderink JB, Willems PH, De Pont JJ (2007) The human non-gastric H,K-ATPase has a different cation specificity than the rat enzyme. Biochim Biophys Acta 1768:580–589. doi:10.1016/j.bbamem.2006.10.010
Weiner ID, Frank AE, Wingo CS (1999) Apical proton secretion by the inner stripe of the outer medullary collecting duct. Am J Physiol 276:F606–F613
West C, Zhang Z, Ecker G, Masilamani SM (2010) Increased renal alpha-epithelial sodium channel (ENAC) protein and increased ENAC activity in normal pregnancy. Am J Physiol Regul Integr Comp Physiol 299:R1326–R1332. doi:10.1152/ajpregu.00082.2010
Wyss C, Wang Q, Golshayan D, Nussberger J, Burnier M, Lehr HA, Schaefer SC (2012) Potassium restores vasorelaxation of resistance arterioles in non-hypertensive DOCA/salt fed mice. Microvasc Res. doi:10.1016/j.mvr.2012.09.005
Youn JH, McDonough AA (2009) Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71:381–401
Younes-Ibrahim M, Barlet-Bas C, Buffin-Meyer B, Cheval L, Rajerison R, Doucet A (1995) Ouabain-sensitive and -insensitive K-ATPases in rat nephron: effect of K depletion. Am J Physiol 268:F1141–F1147
Acknowledgments
We thank Aurélie Edwards for her helpful reading of the manuscript and fruitful discussions and Lydie Cheval for the technical help. This study was supported by grants from the French Society of Nephrology (G.C. and A.S.) and by the Fondation du Rein (G.C.).
Disclosure
The authors have no conflicting interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Evolution of the maternal weight during gestation. Example of weight curves for three gravid HKA2-null mice. Two of them have developed their gestation to the delivery (black and white circles). Another one (black square), although having a similar progression of its weight, overpassed its scheduled delivery date and has had to be sacrificed. (TIFF 8694 kb)
Supplementary Fig. 2
Expression of H,K-ATPase type 2 in the colon of non-pregnant and pregnant female mice. The mRNA levels of HKA1 α subunits, relative to the housekeeping gene cyclophilin A, were measured in kidney of non-pregnant (NP) and pregnant female mice (day 17 post-coitus, black bar). Results are shown as the mean ± s.e.m. (n = 6). (TIFF 3565 kb)
Rights and permissions
About this article
Cite this article
Salhi, A., Lamouroux, C., Pestov, N.B. et al. A link between fertility and K+ homeostasis: role of the renal H,K-ATPase type 2. Pflugers Arch - Eur J Physiol 465, 1149–1158 (2013). https://doi.org/10.1007/s00424-013-1252-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1252-x