Abstract
Heat shock proteins play a key regulatory role in cellular defense. To investigate the role of the inducible 70-kDa heat shock protein (HSP70) in skeletal muscle atrophy and subsequent recovery, soleus (SOL) and extensor digitorum longus (EDL) muscles from overexpressing HSP70 transgenic mice were immobilized for 7 days and subsequently released from immobilization and evaluated after 7 days. Histological analysis showed that there was a decrease in cross-sectional area of type II myofiber from EDL and types I and II myofiber from SOL muscles at 7-day immobilization in both wild-type and HSP70 mice. At 7-day recovery, EDL and SOL myofibers from HSP70 mice, but not from wild-type mice, recovered their size. Muscle tetanic contraction decreased only in SOL muscles from wild-type mice at both 7-day immobilization and 7-day recovery; however, it was unaltered in the respective groups from HSP70 mice. Although no effect in a fatigue protocol was observed among groups, we noticed a better contractile performance of EDL muscles from overexpressing HSP70 groups as compared to their matched wild-type groups. The number of NCAM positive-satellite cells reduced after immobilization and recovery in both EDL and SOL muscles from wild-type mice, but it was unchanged in the muscles from HSP70 mice. These results suggest that HSP70 improves structural and functional recovery of skeletal muscle after disuse atrophy, and this effect might be associated with preservation of satellite cell amount.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle atrophy is a morphological adaptation that normally occurs in response to lack of use, such as immobilization, denervation, response to aging, and various pathological conditions [11, 19, 38]. During immobilization, the absence of stimulation leads to a failure or inhibition of muscle growth, reduced protein synthesis, and increased protein degradation [11, 19, 38]. Thus, depending on the period of immobilization, the outcome can include decreased muscle mass, reduction of force production and fatigue resistance, and myofiber type I to type II conversion [11, 19, 38]. Skeletal muscle mass recovery from immobilization/disuse is a key issue in rehabilitation, and depending on the time involved, reestablishment of initial mass can be severely impaired [15, 37].
Although the process of skeletal muscle atrophy is well known at the structural level, the investigation of molecular mechanisms involved in this process is ongoing. It has been demonstrated that the number of satellite cells during muscle atrophy is reduced along with decreased expression of genes that are regulated during the activation and proliferation of satellite cells such as the receptor for hepatocyte growth factor c-Met, M-cadherin, and Myf5 [31]. In addition, expression of genes responsible for the modulation of muscle mass such as myostatin, atrogin-1, and muscle ring finger-1 (MURF-1) is intensely regulated in atrophied muscles [11, 19, 38].
The heat shock proteins (HSPs) have also been associated with the muscle atrophy process [8, 42]. HSPs are a family of proteins that restore protein homeostasis and contribute to cell survival; thus, they have diverse roles, which include chaperoning, assisting in the removal of damaged proteins, protein folding and transport, regulating cell signaling pathways, and protection against cellular stress [24, 45]. All these features regard HSPs to act as tissue protectors. The most prominent member of the heat shock family of proteins is the 70-kDa heat shock protein (HSP70) [2], and its expression levels are rapidly increased in response to cellular stress, conferring protection of myofibers [25, 32].
It has been reported that during immobilization of skeletal muscles, the HSP70 expression can increase in response to the oxidative damage that occurs in atrophied muscles [36, 44]. On the other hand, there is a depression of HSP70 expression in atrophied muscles previously submitted to long-term hindlimb unloading (28 days), which consequently impairs muscle mass recovery [22]. In order to address the role of HSPs in skeletal muscle atrophy, studies demonstrated that whole-body hyperthermia before [34] and during [39] skeletal muscle disuse promotes high levels of HSP70 and attenuates muscle wasting. Because the whole-body hyperthermia is an unspecific method to promote the expression of HSPs, more recent studies investigated the effect of overexpressing HSP70 in atrophied muscles provoked by electroporation method and found that increased levels of HSP70 attenuated muscle mass loss previously induced by immobilization in young and senescent rats suggesting that the HSP70 is involved in protection against muscle atrophy [7, 41, 42]. This effect is associated to the downregulation of atrogin-1 and MURF-1 [41, 42], ubiquitin ligases involved in the major proteolytic pathway in skeletal muscle, the ubiquitin–proteasome system [12, 23].
Although it has been shown that HSP70 is involved in protection against muscle wasting, the role of HSP70 in structural and functional key aspects of atrophy and subsequent recovery of phenotypically distinct muscles is still unknown. Moreover, the effect of HSP70 expression in atrophied muscles using a more physiological model, such as a transgenic mouse, has not been investigated. Therefore, this study aimed to investigate the role of HSP70 in histological and functional aspects of skeletal muscle atrophy and subsequent recovery of immobilized soleus (SOL) and extensor digitorum longus (EDL) in overexpressing HSP70 transgenic mice.
Materials and methods
This study was conducted according to the ethical principles in animal research followed by the National Institutes of Health (Bethesda, MD) and the Brazilian College of Animal Experimentation. All protocols were approved by the Institutional Animal Care and Use Committee of Loyola University Medical Center and the Institute of Biomedical Sciences/University of São Paulo Ethical Committee for Animal Research.
Animals
The transgenic mice overexpressing HSP70 were previously generated [28]. Briefly, the rat HSP70 was cloned into pCAGGS, a vector that produces high transgenic expression using a human cytomegalovirus enhancer upstream of the chicken β-actin promoter intron. Founders were generated by standard methods. Subsequently, they were screened using Southern blot analysis and then bred to homozygosity. Male HSP70-overexpressing transgenic mice (n = 24) and wild-type (WT) CB6F1 mice (n = 24; 2 months old) weighing 17.5 ± 1.5 and 23 ± 1.7 g, respectively, were kept in standard plastic cages in an animal room under controlled environmental conditions and maintained on standard food and water ad libitum.
Experimental design
All animals used in the present study, except those from the HSP70 and WT control groups, were anesthetized with pentobarbital sodium (50 mg/kg, respectively). The immobilization of the left hindlimb of each animal was held in the neutral position of the ankle using plaster. Great care was taken to make sure that the plaster did not cause ischemia. The EDL and SOL muscles were evaluated after 7 days of immobilization; other groups of animals had their left hindlimbs free after immobilization (7 days) and were assessed after 7 days. The muscles from intact animals served as control. In all three mentioned groups (control, immobilized for 7 days, and immobilized for 7 days and released from immobilization for 7 days), there were muscles from both HSP70 and WT mice, totalizing six groups. At the end of the experiments, the animals were sacrificed, and body and muscle weights were obtained. Then, the muscles were divided into two parts, one half for immunostaining experiments and another half to Western blot. Since entire muscles were required for in vitro contraction experiments, the other animals were exclusively used on those.
Antibodies used for immunostaining and Western blot analysis
The primary antibodies used for immunostaining were (1) mouse anti-myosin heavy chain (anti-MHC) type II monoclonal antibody, clone MY-32 (1:1,000 dilution; catalog no. M4276, Sigma); (2) mouse anti-skeletal MHC type I monoclonal antibody, clone NOQ7.5.4D (1:4,000 dilution, catalog no. M8421; Sigma); and (3) rabbit anti-neural cell adhesion molecule (NCAM) affinity-purified polyclonal antibody (2.5 μg/ml, catalog no. AB5032; Chemicon International, Temecula, CA). The corresponding secondary antibodies used for immunostaining were (1) goat anti-mouse IgG-FITC (1:50 dilution, catalog no. Sc-2010; Santa Cruz Biotechnology, Santa Cruz, CA); (2) goat anti-mouse IgG-FITC (1:50 dilution, catalog no. SC-2010; Santa Cruz Biotechnology); and (3) rhodamine red goat anti-rabbit IgG (1:50 dilution, catalog no. Rb394; Molecular Probes, Eugene, OR).
The primary antibody used for Western blotting was HSP70 (1:2,000 dilution) raised in the rabbit against a synthetic peptide as previously described [30]. The secondary antibody used for Western blotting was goat anti-rabbit IgG peroxidase-conjugated (1:5,000 dilution; catalog no. PI-1000; Vector Laboratories).
Immunostaining
Muscle cross sections were fixed with 4 % paraformaldehyde in 0.2 M phosphate buffer (PB) for 10 min at room temperature, blocked with 0.1 glycine in PBS for 5 min, and permeabilized in 0.2 % Triton X-100/PBS for 10 min. The slides were incubated overnight in a moisture chamber at 4°C with a solution containing the primary antibody, together with 3 % normal goat serum and 0.3 % Triton X-100/0.1 M PB. After the slides had been washed (three 10-min washes with 0.1 M PB), a solution containing the respective secondary antibody and 0.3 % Triton X-100/0.1 M PB was added, and the slides were maintained in this solution for 2 h in a dark room. The slides were again washed in 0.1 M PB (three 10-min washes), after which they were mounted with Vectashield mounting medium containing 4′, 6-diamidino-2-phenylindole (cat# H-1200; Vector Laboratories) and coverslipped. The stained sections were analyzed in a Nikon Eclipse light microscope (PCM2000 and E600, respectively; Nikon, Melville, NY).
Western blot analysis
Cellular protein extracts were prepared from the muscle tissue of both control and transgenic mice. The level of HSP70 was quantified using Western blot analysis as previously described [14] with a specific antibody to HSP70. Protein samples were fractionated for Western blot analysis on an 8 % SDS–PAGE gel and electrotransferred onto nitrocellulose membrane using a submersion electrotransfer apparatus (Bio-Rad Laboratories). The nitrocellulose blots were reacted with an antibody that binds specifically to HSP70. After, blots were reacted with an anti-rabbit IgG biotin–streptavidin horseradish peroxidase-conjugated antibody and developed using an ECL kit (catalog no. 34080; Pierce Biotechnology).
Quantitative and morphometric analysis
The quantitative and morphometric analyses were evaluated using a digitizing unit connected to a computer software (Image-Pro Plus; Media Cybernetics, Silver Spring, MD).
In order to assess the incidence of muscle fiber types I and II and their respective cross-sectional areas (CSAs), a total of ∼500 fibers per muscle in each group were counted, classified, and measured after immunostaining with antibodies against MHC types I and II in the SOL and EDL muscles.
As previously described [32], the frequency of NCAM per muscle was expressed as a percentage of the ratio of the number of NCAM-positive satellite cells (satellite cells) to the number of fibers associated or not associated with satellite cells. Three whole muscle cross sections from different animals in each group were used for NCAM analysis. The SOL and EDL muscle fiber CSAs were obtained from a total of ∼1,000 fibers per muscle. Approximately three or four cross sections of the SOL and EDL muscles from different animals were analyzed in all groups.
In vitro muscle contraction experiments
WT and HSP70 mice were euthanized by cervical dislocation, and SOL and EDL muscles were rapidly removed from both limbs in random order. As previously reported [13], the muscles were mounted at constant length in a tissue bath containing oxygenated, mammalian Ringer solution (in mM, 137 NaCl, 5 KCl, 2 CaCl2, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 0.025 tubocurarine chloride). The solution was gassed continually with 95 % O2–5 % CO2 throughout the experiment. Temperature was maintained at 37°C. Muscles from both WT and transgenic mice were from three distinct experimental groups: control (n = 5), immobilized for 7 days (n = 5), and immobilized for 7 days and recovered for 7 days (n = 5).
Muscle contractile properties
The preparation and experimental conditions used for in vitro contraction of SOL and EDL muscles have been described previously [13]. A muscle tendon was tied with 4-0 silk to a support and the other tendon was tied to the lever arm of a servomotor (Aurora Scientific, Richmond Hill, ON, Canada) that controls muscle length and measures the force developed by the muscle. The force transducer was connected to a computer that was used to collect and analyze force generated by the muscle contraction.
Optimal muscle length (L 0) for peak twitch force was established for the isolated muscles. All subsequent measurements were made at L 0. The muscles were electrically stimulated between two platinum electrodes with 0.1 ms square wave pulses of supramaximal voltage. The force generated by the muscle contraction was measured by the force transducer (Aurora Scientific, Richmond Hill, ON, Canada) coupled to an acquisition software package (Aurora Scientific, Richmond Hill, ON, Canada). Stimulation frequency was increased in EDL and soleus muscles until optimal force was obtained; muscles were rested for 2 min between each of these contractions [13]. For tetanic contractions, we used pulse train duration of 0.8 s, and the stimulation frequency was set at 100 Hz and then adjusted in increments of 50 Hz to obtain maximal isometric force. The contractions were obtained in grams and then converted to millinewtons.
Fatigue protocol
SOL and EDL muscles were electrically stimulated for 5 min with 0.1 ms square wave pulses at 40 Hz and 60 V for 0.1 s every 5 s [13]. The force generated was measured throughout the fatiguing protocol.
Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences version 11.01. Multiple comparisons of mean values were performed using ANOVA and a post hoc Tukey’s test when appropriate. A one-way repeated-measures ANOVA was used to analyze the effects of the fatigue protocol in the muscles. For comparisons between only two groups, an unpaired t test was used. For all comparisons, p < 0.05 was considered significant.
Results
HSP70 expression
Our results show, as expected, that both intact EDL and SOL muscles of WT mice had undetected levels of HSP70 (Fig. 1). Furthermore, HSP70 was substantially expressed in the intact transgenic muscles, in which SOL muscle showed reduced expression when compared to EDL muscle (Fig. 1a). Interestingly, the WT muscles immobilized for 7 days showed a great induction of HSP70 expression (Fig. 1b), overcoming HSP overexpressing muscles. After 7 days of recovery from immobilization, the HSP70 expression was undetected in EDL and SOL muscles from WT mice (Fig. 1c).
Western blot analysis of extensor digitorum longus (EDL) and soleus (SOL) muscles from HSP70 and WT mice from the groups control (A), immobilized for 7 days (B), and immobilized for 7 days and recovered from immobilization for 7 days (C). Blots were reacted with antibody specific for the inducible HSP70 and subsequently with antibody to GAPDH
Muscle weight, myofiber cross-sectional area, and NCAM-positive satellite cells
Control EDL and SOL muscles from HSP70 mice were significantly reduced when compared to those from WT mice (21 % and 32 %; respectively, p < 0.05, Table 1). The EDL muscle weight from both WT and transgenic animals was not altered in all groups. At this time of immobilization, we did not detect significant alterations in muscle weight in EDL muscle. However, immobilized SOL muscles from both WT and HSP70 mice showed a significant decreased weight (26 % and 40 %, respectively, p < 0.05, Table 1). After recovery from immobilization, SOL muscle from WT mice still showed reduced weight when compared to control (22 %, p < 0.05, Table 1); however, those from HSP70 mice had similar weight as compared with their controls (Table 1).
The EDL myofiber type I cross-sectional area did not change in the immobilized groups from both WT and HSP70 mice, and only the recovered HSP70 muscles had an increase of myofiber type I cross-sectional area when compared to their controls (33 %, p < 0.05, Fig. 2). The EDL myofiber type II showed a greater decrease of cross-sectional area in WT mice when compared to HSP70 mice (50 % and 16 %, respectively, p < 0.05, Fig. 2). After 7 days of recovery, the EDL myofiber type II cross-sectional area was still reduced in WT mice (54 % vs control, p < 0.05, Fig. 2) and returned to the control values in HSP70 mice.
Cross-sectional area (CSA; square micrometers) of immunohistochemically classified type I (MHC I) and II (MHC II) myofibers of extensor digitorum longus (EDL) and soleus (SOL) muscles from WT and transgenic HSP70-overexpressing mice. a, b CSAs of type I and II myofibers of EDL, respectively; c, d CSAs of type I and II myofibers of SOL, respectively. An ANOVA test followed by Tukey’s procedure for multiple comparisons was applied to test differences among the groups: black bars, control; white bars, immobilized for 7 days, and gray bars, immobilized for 7 days and recovered from immobilization for 7 days, respectively. Values are expressed as mean ± SD, n = 6–7.*p < 0.05 vs matched C group; # p < 0.05 vs matched immobilized group
In SOL muscles, the cross-sectional area of myofiber type I and II was diminished in immobilized groups from both WT and HSP70 mice (type I, 27 % and 17 %; type II, 31.5 % and 32 %; respectively, p < 0.05, Fig. 2). However, after 7 days of recovery, the SOL myofiber type I and II cross-sectional areas were still reduced in WT mice (type I, 16 %; type II, 31.5 %, p < 0.05, Fig. 2) and returned to the control values in HSP70 mice.
The percentage of NCAM-positive satellite cells was significantly decreased in both immobilized EDL and SOL muscles from WT mice (56 % and 36 % of control, respectively, p < 0.05, Table 1). After 7 days of recovery from immobilization, only WT mice showed reduced number of NCAM-positive satellite cells in both EDL and SOL muscles (69 % and 57 % of control, respectively, p < 0.05, Table 1). However, HSP70 mice did not have changes in the number of NCAM-positive satellite cells among groups (Table 1).
Muscle contraction measurements
The maximum tetanic contraction (MTC) of EDL muscle did not change in all groups evaluated (Table 2). On the other hand, the MTC of SOL muscle from immobilized and recovered WT mice was decreased (55 % and 47 %, respectively, p < 0.05, Table 2). HSP70 mice did not have alterations in the MTC, neither by immobilization nor by immobilization following recovery of both EDL and SOL muscles (Table 2).
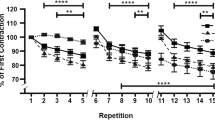
The loss of force generation by EDL and SOL muscles over time during repetitive tetanic contractions at 40 Hz was analyzed. Although there was an apparent reduction in force generation of immobilized EDL muscles and an improvement in force generation of recovered EDL muscles from both WT and HSP70 mice, they were not statistically significant (Fig. 3). There was a greater force generation in the groups control, immobilized, and recovered of EDL muscles from HSP70 mice when compared to the respective groups in the WT mice (p < 0.05, Fig. 3). The force generation in SOL muscles was unaltered in all groups evaluated over time during repetitive tetanic contractions (Fig. 3).
Force production of extensor digitorum longus (A) and soleus (B) muscles from transgenic HSP70-overexpressing mice (HSP 70) and wild-type mice (WT) from the groups control (C), immobilized for 7 days (I) and immobilized for 7 days and recovered from immobilization for 7 days (I + R) during protocol of 5-min fatigue. The data are expressed as percentage of initial force in the 10ª, 20ª, 30ª, 40ª, 50ª, and 60ª muscle contractions. Values are expressed as mean ± SE, n = 4–5. A one-way repeated-measures ANOVA was applied to test differences among the groups: *p < 0.05 vs matched WT
Discussion
Loss of skeletal muscle mass and function are important clinical consequences of certain diseases, immobilization/reduced activity, poor nutrition, use of specific medications, and aging [3, 17]. In addition, muscle wasting itself is known as an independent risk of increased morbidity and mortality [3]. The present study shows that the overexpression of inducible HSP70 in transgenic mice improved the recovery of muscle mass after immobilization of both EDL and SOL muscles, representative of fast and slow twitch muscles, respectively. These results provide further information in agreement with previous reports showing that the overexpression of HSP70 induced by either whole-body hyperthermia [34, 39] or plasmid electroporation [41, 42] was able to attenuate skeletal muscle disuse atrophy.
HSP70 is the most abundant inducible cytoprotective HSP and can be induced by a variety of factors (i.e., ischemia, hyperthermia, free calcium accumulation, stress on intermediate filaments, glycogen and ATP depletion, electro-mechanical coupling, oxidative stress, and acidosis) that can be present during prolonged exercise and also in immobilization atrophy [26]. In fact, we showed that 7-day immobilization induced a robust increase of HSP70 expression in both EDL and SOL muscles from WT mice; however, there was a slight induction of HSP70 expression in the respective muscles from transgenic mice. These results are in line with previous reports showing that immobilization induces oxidative stress [1, 40], which may consequently cause oxidative damage and increase of HSP70 levels during immobilization [36, 44]. In addition, we found that in WT 7-day recovery post-immobilization, HSP70 expression returns to control levels in both control and transgenic mice.
Differently from SOL muscles, EDL muscles were less affected by immobilization as demonstrated by unaltered muscle weight and size of myofiber type I after 7 days of immobilization. These results are in line with previous studies showing that slow twitch muscles, such as the SOL, are more susceptible to atrophic stimuli [9, 10] and also could be related to higher levels of HSP70 expression observed in EDL muscles under basal condition when compared to SOL muscles (Fig. 1).
As previously described [29, 32], the EDL and SOL muscle weights from HSP70 mice were smaller than those from WT mice. In contrast to unrecovered muscle mass in WT mice, the overexpression of HSP70 in transgenic mice significantly improved muscle mass recuperation post-atrophy. This could be related to the fact that the high expression of HSP70 in the muscles from transgenic mice was already present in the moment in which the mechanical stimulus was removed and consequently caused an attenuation of muscle mass loss shown in HSP70 mice under immobilization. Accordingly, HSPs could have retarded muscle atrophy during disuse by both maintaining protein synthesis and decreasing the rate of muscle protein breakdown. Considering that HSP70 binds to nascent polysomes and guides them through the ribosome channel [21, 35] and, has also been shown to affect initiation of translation [43], it is likely that the overexpression of HSP70 could be involved in maintenance of protein synthesis by sustaining polypeptide elongation rate.
Regarding protein degradation, it is known that muscle atrophy induced by immobilization is accompanied by oxidative injury in myofibers [1, 40]. This increase in the oxidative stress speeds muscle proteolysis because oxidatively modified proteins are very susceptible to proteolytic attack, [6] and thus, high levels of HSP70 at the moment that the mechanical stimulus was removed could reduce or prevent this proteolytic activity by binding to oxidatively modified proteins and assist in their refolding [27]. Another possibility is that the HSP70 could regulate specific atrophy signaling pathways and consequently attenuate skeletal muscle disuse atrophy, as plasmid-mediated overexpression of HSP70 in muscles from rats has been shown to prevent atrophy by regulating the key atrophy genes atrogin-1 and MURF-1 through, at least in part, the inhibition of transcriptional activity of Foxo3a and NF-kB [41, 42].
In addition to the complete recovery of muscle mass post-atrophy in overexpressing HSP70 muscles, there was a significant protection against loss of muscle force. Furthermore, although there was no effect of our fatigue protocol on gradual decrease of muscle force, we could see a better contractile performance of EDL muscles from overexpressing HSP70 groups as compared to their matched WT groups. The mechanism involved in protection of HSP against muscle force deficit is unclear. Some studies have also shown benefits in contractile function of muscles overexpressing HSPs, in which the overexpression of HSP70 in old mice prevented decrease of muscle force after lengthening contraction injury [29], and the overexpression of HSP10 preserved age-related loss of muscle contractile function [20] through a reduction in the accumulation of oxidative damaged mitochondrial proteins.
Considering that satellite cells have a crucial role in the maintenance of muscle mass [4, 16] and atrophy caused by immobilization leads to significant reduction in the number of satellite cells and impairs satellite cell proliferative potential [5, 33]; our results showing that overexpressing HSP70 muscles completely recovered their mass after disuse atrophy prompted us to assess the number of satellite cells. Our data demonstrated that high levels of HSP70 could prevent reduction in the number of NCAM-positive satellite cells in muscles exposed to immobilization, thus contributing to attenuation of muscle atrophy and consequent regain of muscle mass. Although the mechanisms involved in this effect is still unknown, it is possible that the overexpression of HSP70 allowed stabilization/survival of satellite cells, which consequently has a critical role in preservation of muscle mass [4, 16]. This finding is in line with the results from Kamanga-Sollo et al. [18], which demonstrated that increased HSP70 expression in muscle induced by heat stress increases satellite cell proliferation.
In summary, this work shows for the first time that the expression of HSP70 in skeletal muscle by transgenesis improves structural and functional recovery post-atrophy in mice. In addition, we also present evidence that this beneficial effect is associated with preservation of satellite cell amount. These findings have significant implications for clinical trials targeting the induction of HSP70 in an effort to improve recovery of muscle mass and function in diverse clinical conditions related to muscle wasting.
References
Appell HJ, Duarte JA, Soares JM (1997) Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med 18:157–160
Baba HA, Schmid KW, Schmid C, Blasius S, Heinecke A, Kerber S, Scheld HH, Bocker W, Deng MC (1998) Possible relationship between heat shock protein 70, cardiac hemodynamics, and survival in the early period after heart transplantation. Transplantation 65:799–804
Cassano M, Quattrocelli M, Crippa S, Perini I, Ronzoni F, Sampaolesi M (2009) Cellular mechanisms and local progenitor activation to regulate skeletal muscle mass. J Muscle Res Cell Motil 30:243–253
Ciciliot S, Schiaffino S (2010) Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des 16:906–914
Darr KC, Schultz E (1989) Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol 67:1827–1834
Dean RT, Fu S, Stocker R, Davies MJ (1997) Biochemistry and pathology of radical-mediated protein oxidation. Biochem J 324(Pt 1):1–18
Dodd S, Hain B, Judge A (2009) Hsp70 prevents disuse muscle atrophy in senescent rats. Biogerontology 10:605–611
Dodd SL, Hain B, Senf SM, Judge AR (2009) Hsp27 inhibits IKKbeta-induced NF-kappaB activity and skeletal muscle atrophy. FASEB J 23:3415–3423
Fitts RH, Riley DR, Widrick JJ (2000) Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89:823–839
Fitts RH, Riley DR, Widrick JJ (2001) Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204:3201–3208
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98:14440–14445
Gomez-Cabrera MC, Close GL, Kayani A, McArdle A, Vina J, Jackson MJ (2010) Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am J Physiol Regul Integr Comp Physiol 298:R2–8
Griffin TM, Valdez TV, Mestril R (2004) Radicicol activates heat shock protein expression and cardioprotection in neonatal rat cardiomyocytes. Am J Physiol Heart Circ Physiol 287:H1081–1088
Griffiths RD, Hall JB (2010) Intensive care unit-acquired weakness. Crit Care Med 38:779–787
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Jackman RW, Kandarian SC (2004) The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287:C834–843
Kamanga-Sollo E, Pampusch MS, White ME, Hathaway MR, Dayton WR (2011) Effects of heat stress on proliferation, protein turnover, and levels of heat shock protein mRNA in cultured porcine muscle satellite cells. J Anim Sci 89(11):3473–3480
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Kayani AC, Close GL, Dillmann WH, Mestril R, Jackson MJ, McArdle A (2010) Overexpression of HSP10 in skeletal muscle of transgenic mice prevents the age-related fall in maximum tetanic force generation and muscle cross-sectional area. Am J Physiol Regul Integr Comp Physiol 299:R268–276
Ku Z, Yang J, Menon V, Thomason DB (1995) Decreased polysomal HSP-70 may slow polypeptide elongation during skeletal muscle atrophy. Am J Physiol 268:C1369–1374
Lawler JM, Song W, Kwak HB (2006) Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve 33:200–207
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Liu Y, Gampert L, Nething K, Steinacker JM (2006) Response and function of skeletal muscle heat shock protein 70. Front Biosci 11:2802–2827
Liu Y, Steinacker JM (2001) Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6:D12–25
Locke M, Tanguay RM, Klabunde RE, Ianuzzo CD (1995) Enhanced postischemic myocardial recovery following exercise induction of HSP 72. Am J Physiol 269:H320–325
Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH (1995) Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest 95:1446–1456
McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ (2004) Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J 18:355–357
Mehta HB, Popovich BK, Dillmann WH (1988) Ischemia induces changes in the level of mRNAs coding for stress protein 71 and creatine kinase M. Circ Res 63:512–517
Mitchell PO, Pavlath GK (2004) Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol 287:C1753–1762
Miyabara EH, Martin JL, Griffin TM, Moriscot AS, Mestril R (2006) Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am J Physiol Cell Physiol 290:C1128–1138
Mozdziak PE, Pulvermacher PM, Schultz E (2000) Unloading of juvenile muscle results in a reduced muscle size 9 wk after reloading. J Appl Physiol 88:158–164
Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J (2000) Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol 88:359–363
Nelson RJ, Ziegelhoffer T, Nicolet C, Werner-Washburne M, Craig EA (1992) The translation machinery and 70 kd heat shock protein cooperate in protein synthesis. Cell 71:97–105
Powers SK, Kavazis AN, DeRuisseau KC (2005) Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288:R337–344
Rochester CL (2009) Rehabilitation in the intensive care unit. Semin Respir Crit Care Med 30:656–669
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23:160–170
Selsby JT, Dodd SL (2005) Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289:R134–139
Sen CK, Marin E, Kretzschmar M, Hanninen O (1992) Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J Appl Physiol 73:1265–1272
Senf SM, Dodd SL, Judge AR (2010) FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol 298:C38–45
Senf SM, Dodd SL, McClung JM, Judge AR (2008) Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22:3836–3845
Thomason DB (1998) Translational control of gene expression in muscle. Exerc Sport Sci Rev 26:165–190
Venojarvi M, Kvist M, Jozsa L, Kalimo H, Hanninen O, Atalay M (2007) Skeletal muscle HSP expression in response to immobilization and remobilization. Int J Sports Med 28:281–286
Welch WJ (1992) Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 72:1063–1081
Acknowledgments
The authors thank Dr. Timothy J. Koh for great initial advices. This study was financially supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of São Paulo) to E.H.M. as well as from an award by USAMRMC and TATRC contract # W81XWH-10-2-0119 to R.M. T.L.N. and D.C.R. received fellowships from FAPESP (grant# 10/04813-8) and the Programa Institucional de Bolsas de Iniciação Científica/Conselho Nacional de Desenvolvimento Científico e Tecnológico (PIBIC/CNPq, Institutional Program of Scientific Initiation Fellowships/The National Council for Scientific and Technological Development, grant# 136849/2009-1).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyabara, E.H., Nascimento, T.L., Rodrigues, D.C. et al. Overexpression of inducible 70-kDa heat shock protein in mouse improves structural and functional recovery of skeletal muscles from atrophy. Pflugers Arch - Eur J Physiol 463, 733–741 (2012). https://doi.org/10.1007/s00424-012-1087-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1087-x