Abstract
Polycystic kidney disease 2-like 1(PKD2L1), previously called transient receptor potential polycystin 3 (TRPP3), forms constitutively active voltage-dependent nonselective cation channels in the plasma membrane. The mechanism of regulation of PKD2L1 channels, however, has been poorly understood. In the present study, we found a bell-shaped alkaline pH dependence of PKD2L1 channel activity at the single-channel and whole-cell levels in patch-clamp recordings in HEK293T cells overexpressing mouse PKD2L1: alkalization to pH 8.0–9.0 increased the PKD2L1 currents, but alkalization to pH 10.0 decreased them. Single-channel analysis revealed that alkalization changed the open probability of PKD2L1 channels, but not their single-channel conductance. In addition, the voltage dependence of PKD2L1 channels was negatively and positively shifted by treatment with solutions of pH 8.0–9.0 and pH 10.0, respectively. These results indicate that the voltage-dependent gating of PKD2L1 channels was modulated by alkalization through two different mechanisms. Interestingly, we observed rebound activation of the PKD2L1 channel on washout of the alkaline solution after PKD2L1 channel inhibition at pH 10.0, suggesting that alkalization to pH 10.0 decreased PKD2L1 currents by inactivating the channels. Consistently, the PKD2L1 tail currents were accelerated by alkalization. These results suggest that alkalization is a bimodal modulator of mouse PKD2L1 channels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic kidney disease (PKD) 2-like 1 (PKD2L1) (previously called polycystin-L or transient receptor potential (TRP) polycystin 3 (TRPP3) but renamed TRPP2 in the newest nomenclature [28]) is a member of the TRP superfamily of nonselective cation channels that sense multiple stimuli such as temperature, taste compounds, and mechanical and osmotic stress [14, 16, 24]. It has recently been demonstrated that the mouse PKD2L1 channel that colocalizes with a PKD-like gene family member, PKD1L3, in a subset of taste receptor cells or human embryonic kidney HEK293T cells, functions as a sour receptor, which responds to washout of acidification [6–9, 11]. Although the assembly of PKD1L3 and PKD2L1 is reported to be essential for translocation of the functional sour receptor to the plasma membrane [9, 10], more recently, it has been reported that mutant mice lacking several transmembrane domains of PKD1L3 exhibit normal responses to acid in behavioral and electrophysiological assays when compared to wild-type mice [2, 12]. Therefore, it is controversial whether PKD1L3 expression is involved in sour sensing.
Besides taste receptor cells, PKD2L1 channels are also known to be expressed in excitable and non-excitable cells in the brain, heart, skeletal muscle, retina, testis, kidney, liver, pancreas, and spleen [1, 15, 23, 27]. It is unlikely that PKD2L1 channels are coexpressed with PKD1L3 in all tissues, suggesting that PKD2L1 channels have other functions. We have previously demonstrated that mouse PKD2L1 channels form a constitutively active nonselective cation channel with a large single-channel conductance, voltage dependence, and significant Ca2+ permeability in PKD2L1-overexpressing HEK293T cells not transfected with PKD1L3 [18]. In addition, the mouse PKD2L1 channel is reported to be inhibited by extracellular acidification in a way that is similar to that of human PKD2L1 channels [3], suggesting that PKD2L1 channels can directly respond to extracellular pH. However, how extracellular pH modulates the gating of PKD2L1 channels has not been analyzed.
In the present study, we therefore investigated the pH-dependent gating of PKD2L1 channels. We show that mouse PKD2L1 is an alkalization-activated channel with a bell-shaped pH dependence. Interestingly, although the PKD2L1 channel was inactivated by alkalization to pH 10.0, washout of the alkaline solution resulted in rebound activation of the PKD2L1 channel. These results indicate a bimodal modulation of mouse PKD2L1 channel gating by alkalization.
Materials and methods
Cell culture and transfection
Human embryonic kidney HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (Nissui, Tokyo, Japan) supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidity-controlled incubator with 5% CO2. HEK293T cells were transiently transfected with the mouse PKD2L1 gene using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). PKD2L1-overexpressing HEK293T cells were detached from the plastic substrate and cultured on cover slips (Matsunami Glass, Osaka, Japan) before electrophysiological experiments. All experiments were carried out more than 36 h after transfection.
Electrophysiology
Whole-cell recordings were performed with an EPC-9 patch-clamp amplifier (HEKA Elektronik, Lambrecht, Germany) or an Axopatch 200 patch-clamp amplifier (Molecular Devices, Sunnyvale, CA, USA) at room temperature. Patch master (HEKA Electronik) or pClamp 9.2 (Molecular Devices) was used for command pulse control and data acquisition, and pClamp 9.2 and WinASCD software (kindly provided by Dr. G. Droogmans, KU Leuven, Leuven, Belgium; ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/winascd.zip) was utilized for data analysis. Currents were filtered at 2.9 and 1 kHz and digitized at 10 and 5 kHz in the EPC-9 and Axopatch 200 amplifiers, respectively. Patch electrodes had a resistance of 2–4 MΩ when filled with pipette solutions. The access resistance (<10 MΩ) was electrically compensated by 70% to minimize voltage errors. To monitor voltage dependency of PKD2L1 channels, step pulses were applied from −100 to +160 mV in 20 mV increments with a post-pulse to −100 mV.
The amplitude of single-channel currents was measured as the peak-to-peak distance in Gaussian fits of the amplitude histogram. Channel activity (NP o, where N is the number of channels and P o is the open probability) at −60 mV was calculated by dividing the mean current amplitude of each recording lasting longer than 30 s by the single-channel amplitude in the same trace.
The pipette solution consisted of 130 mM Cs-aspartate, 2 mM Na2ATP, 10 mM MgCl2, 1 mM EGTA, and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), buffered at pH 7.3 with CsOH. The standard bathing solution contained 130 mM NaCl, 1 mM MgCl2, 10 mM HEPES, and 40 mM mannitol, buffered at pH 7.4 with NaOH. To prepare acidic or basic solutions, HEPES was replaced with MES (for pH 6.0), TAPS (for pH 8.0 and 9.0), or CAPS (for pH 10.0) at an equal concentration.
Statistics
Data are presented as means ± S.E.M. of n observations. Statistical differences of the data were evaluated by Student’s t test or one-way ANOVA with Tukey’s post-hoc test and were considered significant at P < 0.05.
Results
Effects of alkalization on single-channel activity of PKD2L1 channels
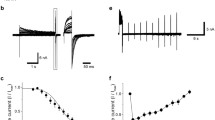
Spontaneous single-channel PKD2L1 currents can be recorded in whole-cell configurations because of the low open probability of PKD2L1 channels at hyperpolarized potentials [18]; we, therefore, first investigated alkaline effects on PKD2L1 single-channel currents. Figure 1a shows a typical holding current recorded at −60 mV under different alkaline pH conditions in PKD2L1-overexpressing HEK293T cells. Changing the pH of the bathing solutions from 7.4 to 8.0 and 9.0 increased single-channel activity of PKD2L1 channels in a pH-dependent manner. However, the channel activity was inhibited by application of a pH 10.0 solution. Interestingly, changing the bathing solution back to pH 9.0 caused rapid and robust activation of PKD2L1 channels followed by a slow recovery. In contrast, control GFP-expressing cells did not show any similar single-channel activity in response to alkaline pH (data not shown). These results suggest that PKD2L1 channels are activated by alkalization but would be inactivated under conditions more alkaline than pH 10.0.
Alkaline sensitivity of PKD2L1 channels at the single-channel level. a. Representative macroscopic currents recorded at −60 mV in the whole-cell configuration. Application of alkaline solutions is indicated by horizontal bars. b Time-expanded traces under different alkaline conditions. Arrowheads indicate the closed (C) and open (O) levels. The corresponding all-point amplitude histograms are shown on the right. PDF probability density function. c, d Effects of alkalization on the channel activity (NP o) (c) and the single-channel conductance (d) at −60 mV. The data are averaged from ten to 28 experiments. *P < 0.05; NS, P > 0.05
We analyzed how alkaline pH regulates PKD2L1 channels. Figure 1b shows time-expanded holding traces with the corresponding all-point amplitude histograms under different pH conditions. As shown in Fig. 1c, alkalization to pH 8.0 and pH 9.0 increased the channel activity (NP o) in a pH-dependent manner. In contrast, the single-channel conductance did not change in response to alkaline pH (Fig. 1d). These results suggest that alkalization modulates the gating of PKD2L1 channels.
Next, we investigated pH dependence of the rebound activation of PKD2L1 channels after removal of alkalization. A bathing solution of either pH 7.4 or pH 9.0 was applied after exposure to a pH 10.0 solution to quantify activity of the PKD2L1 channel. Figure 2a shows typical traces of PKD2L1 channel rebound activation under different pH conditions. Following application of a pH 10.0 bathing solution that decreased the single-channel activity of PKD2L1 channels, changing the bathing solution back to pH 7.4 or pH 9.0 induced rebound activation of PKD2L1 channels with equivalent peak current amplitudes, as summarized in Fig. 2b. In contrast, when we analyzed the time required to reach the peak current after washout, it was found that the rebound activation of PKD2L1 channels at pH 7.4 was much faster than that at pH 9.0 (Fig. 2c). These results suggest that alkalization inactivates the PKD2L1 channel.
pH dependence of the rebound activation of PKD2L1 channels after removal of a pH 10.0 bathing solution. a Representative macroscopic currents of rebound PKD2L1 channel activation under pH 7.4 (left) and pH 9.0 (right) conditions. The currents were recorded at −60 mV in whole-cell conditions. Application of alkaline solutions is indicated by horizontal bars. b, c The pH dependence of the peak current (b) and the time lag to the negative peak after washout of the pH 10.0 solution (c). The data are averaged from six to 13 experiments. *P < 0.05; NS, P > 0.05
Effects of alkalization on the voltage dependence of PKD2L1 channels
PKD2L1-expressing HEK293T cells are reported to exhibit outwardly-rectifying currents with large tail currents after repolarization to −100 mV [18]. To confirm the effects of alkalinity on the voltage dependence of PKD2L1 channels, we measured the tail currents of PKD2L1 channels under various alkaline conditions. As shown in Fig. 3a, application of pH 8.0 and pH 9.0 solutions increased PKD2L1 tail currents in a pH-dependent manner, but alkalization to pH 10.0 decreased the tail currents. PKD2L1 tail currents were enhanced to a greater degree 1 min after changing the solution from pH 10.0 back to pH 9.0 than after application of only the pH 9.0 solution. Finally, in cells exposed to a pH 7.4 solution for 4 min following exposure to a pH 10.0 solution, tail currents recovered to the initial amplitude seen with exposure to just pH 7.4. This result was consistent with the data for PKD2L1 holding currents at −60 mV (see Fig. 1a).
Modulation of the voltage dependence of PKD2L1 channels in response to alkalization. a Representative whole-cell PKD2L1 currents under different pH conditions. The currents were recorded 1 min after each perfusion, except for the current recorded 4 min after reapplication of the pH 7.4 solution. b The activation curves for PKD2L1 channels at the following pH values: pH 7.4 (open circles, n = 10), pH 8.0 (open squares, n = 6), pH 9.0 (open triangles, n = 10), pH 10.0 (open inverted triangles, n = 10), pH 9.0 after pH 10.0 (closed triangles, n = 10), and pH 7.4 after pH 9.0 (closed diamonds, n = 10). The apparent open probability (P o) as a function of voltage was determined as explained in the results section. Solid lines represent Boltzmann functions fitted to the data
The voltage dependence of PKD2L1 channels was estimated by calculating the apparent open probability (P o) from tail currents. Tail current amplitudes as a function of the prepulse potentials were fitted using the Boltzmann equation:
where V half is the potential of half-maximal activation, s is the slope factor, and I max is the saturating tail current. Global fits of apparent P o to the equation at different pH values are shown in Fig. 3b. Voltage dependence of PKD2L1 channels was shifted leftward by alkalization to pH 8.0 and pH 9.0. However, alkalization to pH 10.0 caused a rightward shift of the voltage dependence. Interestingly, changing the pH of the bathing solution from 10.0 back to 9.0 for 1 min shifted the curve of voltage dependence drastically to the left. The voltages for half-maximal activation (V half) were 231.9 ± 16.3 mV at pH 7.4 (n = 10), 195.5 ± 6.8 mV at pH 8.0 (n = 6), 177.3 ± 4.2 mV at pH 9.0 (n = 10), 208.1 ± 12.5 mV at pH 10.0 (n = 10), 138.9 ± 1.7 mV during reperfusion of a pH 9.0 solution (n = 10), and 235.6 ± 21.3 mV after washout at pH 7.4 (n = 10).
Next, we investigated effects of pH during washout on voltage dependence of PKD2L1 channels. As shown in Fig. 4, perfusion of a pH 7.4 bathing solution for 30 s after application of a pH 10.0 solution enhanced the tail currents of PKD2L1 channels and caused a leftward shift of the voltage dependence. The value for V half was 133.5 ± 1.1 mV after washout with a pH 7.4 bathing solution (n = 8), which is similar to that for rebound activation at pH 9.0. These results suggest that alkaline pH modulates the voltage-dependent gating of PKD2L1 channels, although the shift of V half is not dependent on the pH of the washing solution.
The rebound activation of PKD2L1 channels after reapplication of pH 7.4 solutions has a voltage shift similar to that after pH 9.0 reperfusion. a Representative whole-cell PKD2L1 currents under different pH conditions. The currents were recorded 1 min after each perfusion, except for the current recorded 30 s after reapplication of the pH 7.4 solution. b The activation curves for PKD2L1 channels at the following pH values: pH 7.4 (open circles), pH 9.0 (open triangles), pH 10.0 (open inverted triangles), and pH 7.4 after pH 10.0 (closed circles). Solid lines represent Boltzmann functions fitted to the data. The voltages for half-maximal activation (V half) were 244.8 ± 19.4 mV at pH 7.4, 178.4 ± 1.9 mV at pH 9.0, 191.5 ± 4.2 mV at pH 10.0, and 133.5 ± 1.1 mV 30 s after reperfusion of a pH 7.4 solution. The data are obtained from eight experiments
Dual action of PKD2L1 channels by alkalization
We further analyzed the pH dependence of PKD2L1 channels by calculating relative tail currents obtained using a prepulse of +160 mV at various pHs, normalizing to the initial tail currents at pH 7.4 in each series of experiments. Figure 5 summarizes the pH dependence of PKD2L1 tail current amplitudes. The tail currents of PKD2L1 channels were increased by alkalization, although application of a pH 10.0 solution decreased the tail currents. The rebound activation of PKD2L1 channels after washout of the pH 10.0 solution was observed at not only pH 7.4 but also pH 9.0. The amplitudes of tail currents were equivalent 30 s and 1 min after washout at pH 7.4 and pH 9.0, respectively. In addition, we observed that the tail currents recovered to their initial levels 4 min after exposure to a bathing solution of pH 7.4. We acquired PKD2L1 tail currents during rebound activation at different times because the rebound activation of PKD2L1 channels at pH 7.4 was faster than that at pH 9.0 (see Fig. 2c). In contrast, acidification to pH 6.0 completely inhibited the PKD2L1 currents at the single-channel and whole-cell levels (Fig. 5; Figs. S1 and S2), which is similar to previous results at pH 2.9 [18].
pH-dependent regulation of the tail currents of PKD2L1 channels. The tail currents from a prepulse of +160 mV at various pHs were normalized to the tail currents for the first pH 7.4 application in the same series of experiments. Closed circles indicate the relative tail currents of PKD2L1 channels during the first application of bathing solutions with different pH. Open circles show the relative tail currents during rebound activation of PKD2L1 channels 0.5 and 1 min after exchanging the pH 10.0 bathing solution with pH 7.4 and 9.0 solutions, respectively. The open square indicates the relative tail currents of PKD2L1 channels 4 min after the second application of the pH 7.4 bathing solution. The data are averaged from three to 18 experiments. *P < 0.05 compared to the first tail current at pH 7.4; # P < 0.05 compared to the first tail current at the corresponding pH
Another effect of alkalization on PKD2L1 tail currents was observed: the deactivation time course of the PKD2L1 tail currents was accelerated in response to alkalization (Fig. 6a; see Fig. 3a and Fig. 4a). When the tail currents were plotted on a semilog scale, it was found that the deactivation process of PKD2L1 channels was comprised of two components (Fig.6a inset). Therefore, to assess the effects of alkalinity on deactivation, time constants (τ) were calculated by fitting the tail currents from a prepulse of +160 mV to a two-exponential function. As shown in Fig. 6b, alkalization to pH 9.0 and pH 10.0 significantly decreased the fast time constant, but not the slow time constant. This result could be explained by the idea that alkalization caused a faster transition to inactivation.
Effects of alkalization on the time course of PKD2L1 tail currents. a Representative deactivation of tail currents from a prepulse of +160 mV under different pH conditions. The tail current was normalized to each peak amplitude. Solid and dotted curves represent actual data and their two-exponential fits. Inset: The difference between the inward current and the steady-state current at pH 10.0 was normalized to the peak current and plotted against time in the semilog plot. b Fast and slow time constants (τ f and τ s) of deactivation of tail currents in response to alkalization. The data are obtained from 20 experiments. *P < 0.05; NS, P > 0.05
Given the different sensitivities of tail current amplitudes and time constants to alkalization, alkalization has two disparate actions, an activating effect and an inhibitory effect, on PKD2L1 channel activity.
Discussion
We have previously reported that mouse PKD2L1 channels are spontaneously active nonselective cation channels with a large single-channel conductance and voltage-dependent activation. In addition, it has been demonstrated that PKD2L1 channels are regulated by cell volume and pH [18]. However, the regulation of PKD2L1 channels by alkalization has been poorly understood.
In the present study using patch-clamp experiments at the single-channel and whole-cell levels, we first showed dual effects of alkalization on the PKD2L1 channel. That is, alkalization to pH 8.0–9.0 increased the PKD2L1 currents, but alkalization to pH 10.0 decreased the currents, indicating a bell-shaped pH dependence of PKD2L1 channels. At the whole-cell level, the voltage dependence of PKD2L1 channels was shifted leftward in a pH-dependent manner, but the voltage shift was canceled by alkalization to pH 10.0. These shifts reflect changes in the open probability of PKD2L1 channels due to alkalization. Consistent with this, single-channel analysis demonstrated that alkalization modulated the open probability of PKD2L1 channels, but not the single-channel conductance. These results suggest that alkalization bimodally modified the gating properties of PKD2L1 channels.
The distinct effects of alkalization on PKD2L1 channels suggest the complexity of channel gating kinetics. In the present study, we observed a bell-shaped pH dependence of PKD2L1 currents, suggesting that alkalization induced not only the activation but also the inactivation of PKD2L1 channels. Indeed, the deactivation time course of PKD2L1 tail currents was accelerated in response to alkalization. Based on the result showing that PKD2L1 tail currents were comprised of two components, we hypothesized the following model which has three states: a closed state (C), an open state (O), and an inactivated state (I).
In this model, PKD2L1 channels are thought to exhibit slow deactivation and fast inactivation kinetics after activation; repolarization after depolarization causes a fast transition to an open state followed by slow channel closing and fast inactivation, which forms large tail currents. In the present study, we observed that alkalization increased PKD2L1 currents, indicating that alkalization caused the transition of the PKD2L1 channels from a closed state to an open state. In contrast, we demonstrated that alkalization decreased the fast time constant, but not the slow time constant, suggesting that alkalization accelerated the inactivation kinetics without changing the closing kinetics, resulting in faster tail currents being observed at pH 9.0 and pH 10.0. On the other hand, the shift from an inactivated state to an open state could be fast because reapplication of pH 9.0 and pH 7.4 solutions induced quick rebound activation of the PKD2L1 channel at single-channel and whole-cell levels following channel inactivation at pH 10.0. In addition, the time lag for reaching the peak current after exchanging the pH 10.0 bathing solution with the pH 9.0 one was longer than that after washout with pH 7.4. These results suggest that PKD2L1 channels easily undergo transition to an inactivated state from an open state in response to alkalization. Although the inactivation mechanism of PKD2L1 channels is not well understood, it might be the C-type inactivation reported in human ether-à-go-go (HERG) K+ channels because the gating mechanism of PKD2L1 channels is similar to that of HERG channels [21, 22]. Additional structural analyses are required to clarify the mechanism by which alkalization inactivates PKD2L1 channels.
It has been proposed that thermal and chemical stimuli act on voltage-dependent TRP channels by altering the voltage dependence of activation [13, 20, 25, 26]. The voltage-dependent TRP channels are generally activated upon strong membrane depolarization. Upon chemical or thermal stimulation, the activation curve of these channels is negatively shifted from depolarized potentials toward physiological potentials. In PKD2L1 channels, we have previously reported that changes of cell volume caused a shift of the voltage-dependent gating [18]. In the present study, we have also demonstrated that the voltage-dependent activation curve of PKD2L1 channels was modified by alkalization. Although the negative shift of half-maximum activation (V half) upon rebound activation of PKD2L1 channels was still small compared to that seen with stimulation of other voltage-dependent TRP channels [13, 20, 25], we observed the rebound activation of PKD2L1 channels at −60 mV in the single-channel analysis. This result indicates that the enhancement of PKD2L1 channel activity by alkalization would be effective within the physiological voltage range.
It has been recently demonstrated that other TRP channels such as TRPA1 and TRPV1 expressed in dorsal root ganglion neurons are activated by alkalization [4, 5], suggesting that the activation of these channels by alkalization is associated with noxious pain. In the present study, the effective pH range in alkalization-induced activation of PKD2L1 channels was pH 8.0–9.0, which is nearly equivalent to the alkaline pH range reported for TRPA1 and TRPV1 channel activation. This suggests that the alkaline regulation of PKD2L1 channels could contribute to their physiological or pathophysiological functions. In addition, since it is well-known that the interaction of a variety of stimuli modulates the gating of some voltage-dependent TRP channels [17, 19], it is possible that unknown stimuli shift the pH dependency of PKD2L1 channels toward the physiological range. More detailed analyses using mice lacking PKD2L1 channels are expected to shed light on the physiological or pathophysiological importance of alkalization-induced PKD2L1 activation.
References
Basora N, Nomura H, Berger UV et al (2002) Tissue and cellular localization of a novel polycystic kidney disease-like gene product, polycystin-L. J Am Soc Nephrol 13:293–301
Chang RB, Waters H, Liman ER (2010) A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci USA 107:22320–22325
Chen XZ, Vassilev PM, Basora N et al (1999) Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature 401:383–386
Dhaka A, Uzzell V, Dubin AE et al (2009) TRPV1 is activated by both acidic and basic pH. J Neurosci 29:153–158
Fujita F, Uchida K, Moriyama T et al (2008) Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest 118:4049–4057
Huang AL, Chen X, Hoon MA et al (2006) The cells and logic for mammalian sour taste detection. Nature 442:934–938
Inada H, Kawabata F, Ishimaru Y et al (2008) Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep 9:690–697
Ishii S, Misaka T, Kishi M et al (2009) Acetic acid activates PKD1L3-PKD2L1 channel—a candidate sour taste receptor. Biochem Biophys Res Commun 385:346–350
Ishimaru Y, Inada H, Kubota M et al (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci USA 103:12569–12574
Ishimaru Y, Katano Y, Yamamoto K et al (2010) Interaction between PKD1L3 and PKD2L1 through their transmembrane domains is required for localization of PKD2L1 at taste pores in taste cells of circumvallate and foliate papillae. FASEB J 24:4058–4067
Kawaguchi H, Yamanaka A, Uchida K et al (2010) Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells. J Biol Chem 285:17277–17281
Nelson TM, Lopezjimenez ND, Tessarollo L et al (2010) Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem Senses 35:565–577
Nilius B, Talavera K, Owsianik G et al (2005) Gating of TRP channels: a voltage connection? J Physiol 567:35–44
Nilius B, Owsianik G, Voets T et al (2007) Transient receptor potential cation channels in disease. Physiol Rev 87:165–217
Nomura H, Turco AE, Pei Y et al (1998) Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J Biol Chem 273:25967–25973
Ramsey IS, Delling M, Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68:619–647
Ryu S, Liu B, Qin F (2003) Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol 122:45–61
Shimizu T, Janssens A, Voets T et al (2009) Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflügers Arch 457:795–807
Sugiura T, Tominaga M, Katsuya H et al (2002) Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88:544–548
Talavera K, Yasumatsu K, Voets T et al (2005) Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438:1022–1025
Tristani-Firouzi M, Sanguinetti MC (2003) Structural determinants and biophysical properties of HERG and KCNQ1 channel gating. J Mol Cell Cardiol 35:27–35
Vandenberg JI, Torres AM, Campbell TJ et al (2004) The HERG K+ channel: progress in understanding the molecular basis of its unusual gating kinetics. Eur Biophys J 33:89–97
Veldhuisen B, Spruit L, Dauwerse HG et al (1999) Genes homologous to the autosomal dominant polycystic kidney disease genes (PKD1 and PKD2). Eur J Hum Genet 7:860–872
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Voets T, Droogmans G, Wissenbach U et al (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–754
Voets T, Talavera K, Owsianik G et al (2005) Sensing with TRP channels. Nat Chem Biol 1:85–92
Wu G, Hayashi T, Park JH et al (1998) Identification of PKD2L, a human PKD2-related gene: tissue-specific expression and mapping to chromosome 10q25. Genomics 54:564–568
Wu LJ, Sweet TB, Clapham DE (2010) International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62:381–404
Acknowledgments
We thank all members of the Toyama and Leuven laboratories for helpful discussions. We are grateful to Dr. Elbert L. Lee for careful reading of the manuscript. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 90.4 kb)
Supplementary Fig. S1
(GIF 36.7 kb)
Supplementary Fig. S2
(GIF 28.6 kb)
Rights and permissions
About this article
Cite this article
Shimizu, T., Higuchi, T., Fujii, T. et al. Bimodal effect of alkalization on the polycystin transient receptor potential channel, PKD2L1. Pflugers Arch - Eur J Physiol 461, 507–513 (2011). https://doi.org/10.1007/s00424-011-0934-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0934-5