Abstract
Cochlear endolymph, an extracellular solution containing 150 mM K+, exhibits a positive potential of +80 mV. This is called the endocochlear potential (EP) and is essential for audition. The mechanism responsible for formation of the EP has been an enigma for the half century since its first measurement. A key element is the stria vascularis, which displays a characteristic tissue structure and expresses multiple ion-transport apparatus. The stria comprises two epithelial layers: a layer of marginal cells and one composed of intermediate and basal cells. Between the two layers lies an extracellular space termed the intrastrial space (IS), which is thus surrounded by the apical membranes of intermediate cells and the basolateral membranes of marginal cells. The fluid in the IS exhibits a low concentration of K+ and a positive potential similar to the EP. We have demonstrated that the IS is electrically isolated from the neighboring extracellular fluids, perilymph, and endolymph, which allows the IS to sustain its positive potential. This IS potential is generated by K+ diffusion across the apical membranes of intermediate cells, where inwardly rectifying Kir4.1 channels are localized. The low K+ concentration in the IS, which is mandatory for the large K+-diffusion potential, is maintained by Na+,K+-ATPases and Na+,K+,2Cl−-cotransporters expressed at the basolateral membranes of marginal cells. An additional K+-diffusion potential formed by KCNQ1/KCNE1-K+ channels at the apical membranes of marginal cells also contributes to the EP. Therefore, the EP depends on an electrically isolated space and two K+-diffusion potentials in the stria vascularis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sounds from the outside world propagate through the external and middle ears and then stimulate the cochlea of the inner ear, a specialized peripheral organ of hearing. Hair cells, receptors on the basilar membrane that vibrates when acoustic stimuli reach the inner ear, transform the mechanical energy of sounds into electrical signals (Fig. 1a). The mammalian cochlea is a highly sensitive sensor of hearing; it can detect basilar-membrane motion near ±0.1 nm that is elicited by a threshold stimulus [31]. To achieve such exquisite sensitivity, the cochlea is equipped with multiple systems enhancing its mechanical inputs. The endocochlear potential, the topic of this article, is one of the key systems. Model mice lacking the endocochlear potential (EP) are deaf [11, 22, 42], indicating that this potential is mandatory for proper hearing.
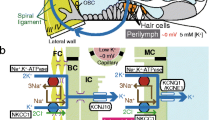
Structure of the cochlea and its lateral wall. a The ionic composition and potential of the endolymph are maintained by K+ circulation through the lateral cochlear wall (left). There are two possible routes of K+ transport from hair cells to the lateral wall: one through the gap-junctional network of epithelial cells on the basilar membrane and the other through perilymph. The locations of five types of fibrocyte are indicated by Roman numerals. b A schematic enlargement of the boxed region in (a) depicts the ion-transport apparatus involved in the formation of the EP (endocochlear potential). NKCC Na+,K+,2Cl−-cotransporter, ClC ClC-K/barttin-type Cl− channel, TJ tight junction. Reproduced and modified from [69]
The snail-shaped mammalian cochlea comprises three tubular structures—the scala media, scala tympani, and scala vestibuli—and various cell types such as epithelial cells and fibrocytes (Fig. 1a). The scala tympani and scala vestibuli are filled with perilymph whose ionic composition resembles that of ordinary extracellular solutions. On the other hand, the scala media holds a distinct solution termed endolymph. The endolymph contains 150 mM K+, 2 mM Na+, and 20 µM Ca2+, and exhibits an EP of about +80 mV relative to blood plasma and perilymph [107, 108]. Such a specialized electrochemical milieu is not observed in the extracellular fluids of any other organ in mammals. Each cochlear hair cell bathes its cell body in perilymph and exposes its apical membrane, which is surmounted by a mechanosensitive hair bundle comprising dozens of stereocilia, to endolymph. The basilar-membrane motion elicited by acoustic stimuli deflects the bundle and opens cation-permeable mechanoelectrical transduction channels at the top of the stereocilia. This process permits endolymphatic K+ to enter hair cells, resulting in their electrical excitation [29, 30, 32]. By forming a large driving force, the EP accelerates not only the influx of K+ but also the entry of Ca2+ that enhances the motility of the hair bundle [10]. The EP therefore serves as a biological battery to increase the sensitivity of hair cells.
Since Georg von Békésy, who was awarded with Nobel Prize for his research on traveling waves in the cochlea, first measured the EP in 1952 [107, 108], the mechanism underlying its formation has remained an enigma. Our recent electrophysiological assays have clarified key elements in the mechanism, which will be described in this article. We will also discuss the physiological merit of the EP as an amplifier for sound signaling.
K+ circulation in the cochlea
After entering hair cells, K+ exits across their basolateral membranes through K+ channels [3, 18, 39, 47, 48, 52, 71, 75, 87] and reaches the lateral cochlear wall either by a perilymphatic route or through the gap-junctional network comprising Deiters’ cells and epithelial cells on the basilar membrane [6, 7, 24, 40, 56] (Fig. 1a). K+ is subsequently transported across the lateral cochlear wall and finally returned back to endolymph (Fig. 1a).
This cochlear K+ circulation from endolymph to perilymph and then to endolymph, which is also referred to as cochlear K+ recycling, is thought to be involved in the establishment of the EP and possibly the high K+ concentration in endolymph [27, 40, 41, 113, 114]. The concept of the K+ circulation was originally proposed on the basis of the following observations. First, radioactive K+ applied in perilymph can be detected in endolymph much more efficiently than when perfused in blood plasma [45, 90]. Second, perilymphatic but not vascular perfusion of K+-free solution rapidly and prominently suppresses the EP [59, 109]. Zidanic and Brownell [118] electrophysiologically measured the current flow from endolymph to perilymph and estimated it as 0.8–1.6 µA per 1-mm wedge of cochlea. This may represent a fraction of the total K+ circulation.
Architecture of the lateral cochlear wall
The lateral cochlear wall is composed of two components, the spiral ligament containing five distinct types (I–V) of fibrocytes and connective tissue, and an epithelial tissue, the stria vascularis, comprising marginal, intermediate, and basal cells (Fig. 1a, b). In the ligament, types II and IV fibrocytes are characterized by numerous extensions of their plasma membranes [88]. All of the fibrocytes, which are bathed in perilymph, and the basal and intermediate cells in the stria vascularis are connected together through gap junctions and are therefore considered to share the same electrochemical properties [11, 93]. These three types of cells appear to form a functional syncytium; its basolateral and apical surfaces correspond to the membranes of fibrocytes and to the apical membranes of intermediate and basal cells, respectively (Fig. 1b).
Marginal cells, whose apical surfaces directly face endolymph, constitute a monolayer of epithelial cells (Fig. 1b). Thus, the lateral wall beside scala media can be considered to be made up of the epithelial syncytium and the marginal-cell layer. Between the two layers, there are numerous capillaries and an extracellular space called the intrastrial space (IS) [28, 89] (Fig. 1b). The basolateral membranes of marginal cells and the apical membranes of intermediate cells are highly infolded and twisted together, and the IS is very narrow, with a width of only 15 nm. The interior of the stria is separated from perilymph, endolymph, and blood by the tight-junctional networks of basal cells in the epithelial-syncytium layer, of marginal cells, and of vascular endothelial cells [35] (Fig. 1b).
The tissues and ion-transport apparatus critically involved in formation of the EP
Contribution of the spiral ligament to the EP
Types II and IV fibrocytes in the spiral ligament express on their membranes two K+-uptake transporters: Na+,K+-ATPase composed of α1 and β1 subunits [82, 83] and Na+,K+,2Cl−-cotransporter (NKCC) made up of NKCC1 subunit [12, 78] (Fig. 1b). Ouabain or furosemide, a specific blocker for Na+,K+-ATPase or NKCC, respectively, dramatically suppresses the EP when applied to perilymph [46, 49–51, 59]. It is therefore likely that both transporters contribute to the EP by carrying K+ from perilymph into the fibrocytes across their membranes that correspond to the basolateral surface of the epithelial-syncytium layer. K+ taken up by the fibrocytes then moves through the gap junctions of basal and intermediate cells to the apical surface in the epithelial-syncytium layer (Fig. 1b).
The stria vascularis is essential for the EP
Since the first measurement of the EP, audiologists have wondered which cochlear tissue generates the potential. Tasaki and Spyropoulos found by electrophysiological techniques that a deaf guinea pig called waltzing exhibited an EP of normal amplitude although it had no hair cells [100]. This observation ruled out an involvement of hair cells in the establishment of the EP. They additionally conducted an experiment with a wild-type guinea pig to precisely identify the tissue responsible for formation of the EP. After removing Reissner’s membrane separating endolymph from perilymph (Fig. 1a), they sucked most of the endolymph in the scala media into a glass pipette. In this condition, a thin film of endolymph might have remained over the stria vascularis and the organ of Corti. They then measured the potential along the surface of these tissues with the electrode, and recorded positive potential of +30 to +50 mV near the stria vascularis, whereas the potential was near 0 mV in the proximity of the organ of Corti [100]. These two observations strongly suggest that the stria vascularis but not the organ of Corti is essential for generation of the EP.
Strial ion-transport apparatus crucial for the EP
Anoxia or vascular perfusion of either ouabain or furosemide dramatically reduces the EP [44, 51, 110]. Of importance, these drugs affect the EP much more rapidly when perfused by a vascular route than by a perilymphatic one [51, 110]. The Na+,K+-ATPase and NKCC expressed in the stria vascularis are therefore more critically and directly involved in the generation of the EP than the corresponding transporters in the spiral ligament. NKCC and Na+,K+-ATPase, which consist respectively of NKCC1 subunits and of a complex of α1 plus β1 or β2 subunits, occur at the basolateral membrane of marginal cells [12, 34, 38, 82, 116]. Moreover, vascular perfusion of Ba2+, a blocker of K+ channels, sharply decreases the EP [26, 57, 60, 96], so the channels expressed in the stria vascularis are suggested to play a pivotal role in the formation of the EP. It is now known that a target of Ba2+ is the inward rectifier Kir4.1 [26], and that this K+ channel is localized at the apical membranes of intermediate cells [25, 94, 96]. Slc26a4-knockout mice, which lack the strial Kir4.1 for unknown reasons, and Kir4.1-null mice completely lose the EP [61, 115]. In addition, several human mutations of the gene encoding Kir4.1, which are expected to impair the channel’s function, cause sensorineural deafness accompanied by neuronal disorders and electrolyte imbalance [5, 81].
Previous model for the mechanism of formation of the EP
As for the mechanism for formation of the EP, the following two-cell model [112, 113], also called the five-compartment model [96], has been widely accepted. Salt et al. [79] measured the electrochemical properties of the stria vascularis by inserting a K+-selective electrode. They found that the fluid of the IS contained a low K+ concentration like that of perilymph and exhibited a highly positive potential of +90 mV [79] (Fig. 1b). This positive IS potential was presumed to represent the majority of the EP [33, 79]. Some groups reported that the membrane potential of basal cells in the epithelial syncytium relative to the perilymph was −5 mV and their intracellular K+ concentration was high [33, 63, 79] (but see [69] reporting 0 to +4 mV). In general, if the cell membranes were selectively K+ permeable and there was a concentration gradient across them, the K+-diffusion potential, which is the K+-equilibrium potential (E K) represented by Nernst’s equation, would occur across the membranes. The potential difference across the apical surface of the epithelial syncytium is close to the E K with respect to the IS [79]. Because vascular perfusion of Ba2+ prominently suppresses the EP [26, 60], it was hypothesized that the K+-diffusion potential formed by Kir4.1 produces the IS potential and is therefore critical for the EP [79, 113, 114, 116]. In this model, Na+,K+-ATPase and NKCC at the basolateral membranes of marginal cells sustain a low K+ concentration in the fluid of the IS to facilitate K+ diffusion through Kir4.1 channels. The IS potential of +90 mV decreases across the apical membrane of marginal cells by 10 mV [63, 70], resulting in a potential of +80 mV in endolymph.
The current model for generation of the EP based on our findings
Questions to be addressed in the previous hypothesis
Although the two-cell or five-compartment model seems reasonable, no in vivo experiments have been performed to verify it because of technical difficulties. In addition, Melichar et al. reported that strong inhibition of Na+,K+-ATPase reduces the EP to a value of −10 mV, which is called the negative EP, but leaves the stria vascularis at a positive potential of +14 mV [63]. This potential difference cannot be explained by the two-cell model, which proposes that the IS potential is nearly equivalent to the EP. Therefore, some elements that are essential for formation of the EP may be missing in the two-cell model. Furthermore, it is not clear how the stria vascularis maintains the positive IS potential. It is reported that, in two lines of knockout mice, the tight-junctional networks of the stria vascularis are disrupted and the EP is eliminated: mice lacking claudin-11, a major constituent of tight junctions of basal cells [22, 42, 43], and mice missing the gene encoding connexin30 (Cx30) [11, 102]. Although Cx30 is a gap-junctional protein of fibrocytes [41, 53, 54], all cell types in the stria vascularis of the mutant mice display an elevation of homocysteine, which damages endothelial cells and disrupts their tight junctions [11]. Because the IS potential is important for the EP, the results from the two knockout mice suggest the involvement of a unique electrical property of the stria vascularis in sustaining the IS potential. However, neither this property nor the process inducing the phenotype of the mutant animals has been clearly identified [11, 22, 42, 102].
Measurement of the electrochemical properties in the lateral cochlear wall
To clarify the precise mechanism underlying the generation of the EP, we measured the potential, K+ activity, and input resistance by inserting a double-barreled K+-selective electrode into the lateral cochlear wall under various conditions [69]. The experimental results are summarized in Fig. 5.
An ionic activity is an effective concentration of an ion, or in other words, a measure of the fraction of the ions that are free and active and actually elicit a variety of chemical and biological phenomena including the diffusion potential monitored with the ion-selective electrode. An ionic activity is the product of the ion’s concentration and its activity coefficient. Because the coefficient is variable and unmeasurable in intact cells and tissues, the K+ activity was measured in our assays. In some experiments, the EP was simultaneously monitored with a separate electrode.
First, in normal conditions, we found four compartments in the lateral wall, each of which shows distinct electrochemical property as follows (see Fig. 5): (1) epithelial syncytium—slightly positive potential (0 to +4 mV) and 60 mM K+ activity; (2) IS—high potential similar to the EP and low K+ activity like perilymph (5 mM); (3) marginal cells—high potential similar to the EP and 80 mM K+ activity; and (4) endolymph—high potential of +80 mV and high K+ activity exceeding 100 mM.
The stria vascularis may have a barrier function to maintain the positive potential in the IS. To test this idea, we measured the input resistance in the IS with the K+-selective electrode (Fig. 2). The electrode was driven through the lateral wall and, when it encountered the IS, the potential changes of this region in response to current pulses became much larger than those of perilymph and endolymph (Fig. 2). This finding indicates that the resistance inside the IS is much higher than those of the perilymph and endolymph. This space is therefore electrically isolated from neighboring extracellular fluids to sustain the positive potential.
Input-resistance measurement of lateral-wall compartments. A double-barreled, K+-selective electrode was driven through the lateral wall of the cochlea to record the potential (red) and K+ activity (blue). The input resistance was measured simultaneously as downward deflections in the potential record in response to 50-nA current pulses 200 ms in duration. The upward deflections in the recording of K+ activity are artifacts resulting from the current pulses applied to the neighboring electrode. The electrode encountered successively the perilymph (PL), the epithelial syncytium (Syn, filled arrowheads), the intrastrial space (IS, filled arrows), and finally the endolymph (EL, open arrows). Note the decline in the IS potential during a period of anoxia (see Fig. 3a). In this and subsequent recordings, the gray wedge above the trace indicates the period during which the electrode was advanced. Reproduced and modified from [69]
Next, we examined the electrochemical property of the IS that would be crucial for formation of the EP (Fig. 3). During inhibition of Na+,K+-ATPase by anoxia, the potential in the IS fluid falls and then rebounds slightly, whereas the K+ activity shows a reverse pattern (Fig. 3a). Under the same conditions, the electrochemical milieu inside the epithelial syncytium barely changes. Because the apical membranes of intermediate cells of the epithelial-syncytium layer express Kir4.1 channels [1, 25] (see Fig. 1b), these two observations suggest that the IS potential is mainly generated by K+ diffusion. Indeed, the E K calculated by Nernst’s equation with measured K+-activity values in the epithelial syncytium and in the IS corresponds well to the IS potential recorded during anoxia (Fig. 3b). Similar results are obtained when ouabain or bumetanide is arterially applied to the stria vascularis to specifically inhibit Na+,K+-ATPase and NKCC. Furthermore, perfusion of Ba2+ to the stria vascularis decreases the potential of the IS with little change of its K+ activity (Fig. 3a). Taken together, the IS potential is likely to be formed mainly by K+ diffusion through Ba2+-sensitive Kir4.1 channels in the apical membranes of intermediate cells. The low K+ concentration in the IS seems to be maintained by Na+,K+-ATPase and NKCC expressed basolaterally in marginal cells. During anoxia and perfusion of Ba2+, the EP changes roughly in parallel with the IS potential (Fig. 3a). Thus, the IS potential represents a significant fraction of the EP.
Effects of blocking Na+,K+-ATPase and K+ channels on the property of the IS. a After passing through the epithelial syncytium (filled arrows), a K+-selective electrode (upper panel) was held in the intrastrial space (IS, open arrows). The endocochlear potential (EP) in a different portion of the cochlear turn was monitored simultaneously with a single-barreled electrode (lower panel). Open and filled arrowheads show the peak changes in the potentials (red) and K+ activity (blue) during anoxia and vascular perfusion of 1 mM Ba2+. Anoxia increased K+ activity in the IS (aK +IS ) much more than did the application of Ba2+, but both treatments reduced the IS potential to a similar value (thin horizontal line). b The IS potential during anoxia was predicted (green) with the equation IS potential = V Syn + (RT/F) ln(aK +i(Syn) /aK +IS ). The potential and K+ activity in the syncytium (V Syn and aK +i(Syn) ) and in the IS (aK +IS ) (blue) were obtained from (a) (upper panel, filled arrows, and box). The IS potential recorded in (a) (boxed) is also shown (red). Reproduced and modified from [69]
During anoxia, the IS potential remains positive but the EP decreases and finally reaches −15 mV (Fig. 3a; see also [63]). This potential difference may occur in the marginal-cell layer, for that is the sole tissue component between the IS and endolymph (see Fig. 1b). We thereby examined the K+ activity and potential of marginal cells (Fig. 4). After passing the epithelial syncytium and the IS, the K+-selective electrode encounters the inside of marginal cells (Fig. 4a). This compartment is distinguishable from the endolymph, because the K+ activity of the former (80 mM) is lower than that of the latter (>100 mM; Fig. 4a). In marginal cells, the K+ activity is barely changed by vascular perfusion of Ba2+ but prominently decreased in a sigmoidal fashion by application of anoxia, although the potential is sharply reduced in both cases (Fig. 4a). Of note, neither anoxia nor perfusion of Ba2+ alters the K+ activity in endolymph (data not shown). In the anoxic condition, when the K+-selective electrode is advanced from marginal cells to endolymph, a large potential difference is detected (Fig. 4a). It indicates that the potential difference occurs across the apical membranes of marginal cells. For two reasons, most of the difference is attributable to the K+-diffusion potential. First, during a period of anoxia, E K calculated by Nernst’s equation with the recorded K+ activity of marginal cells and that of endolymph [62, 86, 112] matches well the measured potential difference between these two compartments (Fig. 4b). Second, in the apical membranes of marginal cells, the K+ permeability considerably exceeds those of Na+ and Cl− [98]. The K+-diffusion potential is likely to be formed by the KCNQ1/KCNE1 channels that are strongly expressed at the apical membranes of marginal cells [9, 68, 77, 104]. Because the basolateral membranes of marginal cells express little K+ conductance [95], the K+-concentration gradient across this membrane barely produces a potential difference.
Analysis of the properties of marginal cells. a A K+-selective electrode (upper panel) advanced from the perilymph towards the endolymph first encountered the epithelial syncytium (filled arrows) and the intrastrial space (IS, filled arrowheads). Anoxia was imposed briefly when the electrode was in the IS. Entry into the marginal cells was signaled by a highly positive potential and elevated K+ activity (open arrow). Anoxia and 1 mM Ba2+ were tested while the electrode was held at that location. The electrode was ultimately inserted into the endolymph during a period of anoxia (open arrowheads). Throughout the experiment, the endocochlear potential (EP) was recorded with a second electrode (lower panel). b The EP during anoxia was predicted (green) with the equation EP = V MC + (RT/F) ln(aK +i(MC) /aK +EL ). The potential (VMC, red) and intracellular K+ activity (aK +i(MC) , blue) of marginal cells and the endolymphatic K+ activity (aK +EL ) were obtained from (a) (upper panel, box, and upper open arrowhead). The vertical scale of the EP in (a) (lower panel, box) was adjusted (scaled EP, gray line) to fit the curve of the potential that was recorded by the K+-selective electrode when it was in endolymph (red line highlighted by gray arrowheads). IS intrastrial space, EL endolymph. Reproduced and modified from [69]
According to all of the experimental data and calculations, the EP can be represented by the simple relation:
where V Syn is the potential of the epithelial syncytium (constant); aK +i(Syn) , aK +IS , aK +i(MC) , and aK +EL are the K+ activities of the epithelial syncytium (constant), the IS fluid, marginal cells, and endolymph (constant), respectively; and R, T, and F have their usual meanings. The first two terms on the right represent the IS potential, and the final term represents the potential difference across the apical membranes of marginal cells. The EP thus depends on the electrical isolation of the IS (Fig. 2) and two K+-diffusion potentials (Figs. 3 and 4) in the stria vascularis. See Fig. 5 to review the electrochemical properties of the lateral wall in different conditions.
Summary profile of electrochemical properties of the lateral cochlear wall. The top panel depicts the structure of the lateral wall and the K+-transport apparatus involved in the generation of the EP. The predicted potential and K+ activity in each compartment under normal conditions and during inhibition of the strial K+-uptake transporters and Kir4.1 are respectively shown in other panels. NKCC Na+,K+,2Cl−-cotransporter. Reproduced and modified from [69]
The key elements in formation of the EP
Our findings not only have experimentally confirmed the two-cell (five-compartment) model but also have demonstrated three additional factors crucial for formation of the EP. First, the IS is electrically isolated from the perilymph, endolymph, and blood (Fig. 2). This feature is required for maintenance of the high potential and seems to depend on the separation of this space by tight-junctional networks of basal cells in the epithelial syncytium, marginal cells, and endothelial cells. The loss of the EP observed in claudin-11- and Cx30-null mice [11, 22, 42, 102] must result from disruption of the electrical barrier constituted by basal and endothelial cells, respectively. In spite of the electrical isolation of the stria, arterially perfused Ba2+ appears to penetrate the endothelial cells and reach the IS. The endothelial barrier may therefore be loose enough to permit the permeation of Ba2+ but tight enough to guarantee the high input resistance of the IS. The abundantly infolded membranes of intermediate and marginal cells tightly wrap the capillaries. The IS between the membranes is very narrow, only 15 nm [28]. These anatomical features could provide an additional mechanism to increase the resistance between the IS and blood while permitting the effective diffusion of substances across this border. However, the precise properties of the barrier in the stria and the mechanism underlying its establishment are still largely unknown, and thus further experimental studies are needed.
A second important point is that the epithelial syncytium is virtually clamped at 0 to +4 mV. Because of this property, the K+-diffusion potential at the apical membranes of intermediate cells represents a significant fraction of the positive IS potential. Gap junctions interconnect many fibrocytes and constitute an extremely large functional cell volume that provides a large membrane capacitance for the basolateral surface of the epithelial-syncytium layer. Therefore, even if the amplitude of K+ transport across the lateral wall should alter, the potential of the syncytium would barely change. The mechanism that sets the potential of the epithelial syncytium at 0 to +4 mV has not yet been identified. Maintenance of a high K+ concentration in the epithelial syncytium is also important to set the large K+-diffusion potential across the apical surface. Perilymphatic perfusion of K+-free solution [59], ouabain, or furosemide [46, 49–51, 59] reduces the EP because it may attenuate K+ influx across the membranes of the fibrocytes by blocking Na+,K+-ATPase and NKCC, decreasing K+ concentration in the epithelial syncytium and thus decreasing the IS potential. Brn-4-null mice, which show reduction of the EP by 45–50 mV, lose membrane infoldings in their type II fibrocytes [64, 72] and are therefore expected to display a reduced density of K+-uptake transporters. This abnormality would decrease the K+ concentration in the epithelial syncytium, which could result in considerable reduction of the IS potential.
Third, the K+-diffusion potential at the apical membranes of marginal cells also contributes to the generation of the EP (Fig. 3). In the normoxic condition, the K+ activity of endolymph, which exceeds 100 mM, is significantly higher than the intracellular K+ activity of marginal cells, about 80 mM. The potential difference of 10 mV measured at the apical membranes of marginal cells [63, 70] is therefore likely to represent mainly the K+-diffusion potential. The negative EP observed during strong block of the K+-uptake transporters of marginal cells reflects the combination of a decrease in the IS potential and an increase in the diffusion potential across the apical membranes of marginal cells (Figs. 3, 4, and 5).
Unidirectional K+ transport through the lateral cochlear wall and its involvement in generation of the EP
As mentioned earlier, K+ is transported from perilymph to endolymph across the lateral cochlear wall: in each of the two epithelial layers, K+ is taken up across the basolateral membranes by Na+,K+-ATPase and NKCC and then secreted across the apical membranes by K+ channels (Fig. 1b). This unidirectional K+ transport is achieved by Na+ recycling between the two K+-uptake transporters as discussed below and drives K+ circulation throughout the cochlea. Disruption of K+ transport by blocking K+-uptake transporters prominently affects K+ concentration in the IS and in marginal cells and consequently changes the amplitude of the two K+-diffusion potentials that form the EP (Figs. 3, 4, and 5). Therefore, unidirectional K+ transport is critically involved in generation of the EP.
In general, if the potential of the membranes expressing K+ channels is identical to E K, then K+ is not moved across them. In the stria vascularis, the measured potential differences across the apical membranes of intermediate cells and those of marginal cells always modestly exceed E K of these membranes (Figs. 3 and 4). Thus, K+ should be secreted from these cells into the IS and the endolymph through Kir4.1 and KCNQ1/KCNE1 channels, respectively. This machinery supports unidirectional K+ transport across the lateral cochlear wall and thus K+ circulation. In addition, local Na+ homeostasis may be involved in unidirectional K+ transport. Because Na+,K+-ATPase and NKCC occur together in the basolateral membranes of marginal cells and in the fibrocyte membranes of the epithelial syncytium, Na+ is expected to cycle between the two K+-uptake transporters (Fig. 1b). This local cycling may couple the Na+ efflux through Na+,K+-ATPase and the influx through NKCC, which would be required for K+ uptake across the basolateral membranes. Then K+ would exit from intermediate cells of the epithelial syncytium and marginal cells to the IS and the endolymph through the K+ channels localized in the apical membranes, respectively. This mechanism could be called Na+-recycling, unidirectional transport of K+.
K+ secretion from the stria vascularis to endolymph
K+ transported to strial marginal cells may finally be excreted into endolymph through KCNQ1/KCNE1 K+ channels in their apical membrane [9, 77, 92, 104, 112]. In support of this idea, both KCNE1- and KCNQ1-null mice show a collapse of scala media, probably due to abrogation of K+ secretion, and a severe hearing impairment [74, 104]. Human loss-of-function mutations of either subunit cause Jervell and Lange–Nielsen syndromes, which involve sensorineural deafness as well as prolongation of the QT interval in the electrocardiogram [36, 67, 84, 103, 111].
Other ion-transport mechanisms in the lateral cochlear wall
Type 4 Bartter’s syndrome, which is caused by loss-of-function mutations in the pore-forming ClC-K subunits or barttin accessory subunits of Cl− channels, is accompanied by hearing disturbance [4, 80]. Barttin-knockout mice show deafness with loss of the EP [73]. These observations imply that Cl− transport in the stria vascularis is involved in formation of the EP. The basolateral membranes of marginal cells are Cl− permeable and express the subunits listed above [2, 19, 76, 97] (Fig. 1b). The contribution of Cl− channels to the EP may be the following. NKCC, when actively working, would cause local depletion of the Cl− concentration in the IS fluid surrounding the basolateral membranes of marginal cells, which would result in dysfunction of the transporter. The Cl− channels may provide intracellular Cl− for the extracellular K+ site of NKCC to sustain its activity. In barttin-knockout mice [73], dysfunction of the NKCC induced by loss of the Cl− conductance would reduce the K+ concentration in marginal cells. This reduction could further increase the K+-diffusion potential at the apical membranes of marginal cells and suppress the EP (see Fig. 5).
Patch-clamp analyses of isolated marginal cells demonstrated that their basolateral and apical membranes express non-selective cation channels [91, 97, 98]. TRPML3, whose mRNA is abundantly detected in marginal cells [66], could be a constituent of these cation channels, although its distribution pattern inside the stria vascularis is controversial (see [13, 117]). A gain-of-function mutation of TRPML3 observed in deaf mice called varitint-waddler disturbs the structure of the stria vascularis and results in reduction of the EP [8, 16, 23, 66, 117]. In spite of this evidence, the physiological significance of TRPML3 in the formation of the EP is unknown. Other molecular candidates of the non-selective cation channels in marginal cells are TRPV4 [13, 55, 99] and TRPC-family channels [65].
Comparison of ion-transport mechanisms between the cochlea and vestibule
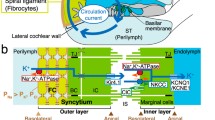
The vestibular labyrinth, which includes the sacculus, utriculus, and semicircular canals, is also filled with endolymph. Although vestibular endolymph contains 150 mM K+, its potential is 0 mV [58, 112]. A highly positive potential in endolymph may not be necessary in the vestibule, for the low-frequency response of the vestibular organs implies their transduction currents can be far smaller. Indeed, in the whole-cell configuration of in vitro condition, the transduction-current conductance of a mouse vestibular hair cell (2.6 nS) is about half of that of a cochlear hair cell (5.5 nS) [21]. Except in the sacculus, the endolymph of the vestibular system seems to be maintained by dark cells located in specialized epithelia of the ampullae [112] (Fig. 6a). Dark cells in the utriculus could be involved in the maintenance as well. The function of the dark-cell areas in ampullae may resemble that of the cochlear stria vascularis. Like marginal cells, dark cells form a monolayer by tight-junctional connection at the boundary between endolymph and perilymph (Fig. 6b).
Structure of the vestibule. a Vestibular endolymph is likely to be maintained by K+ transport from perilymph to endolymph in the dark cell area. b Ion-transport mechanisms in the dark cell area of ampullae (boxed region in (a)). NKCC Na+,K+,2Cl−-cotransporter, ClC ClC-K/barttin-type Cl− channel, TJ tight junction
Many similarities are observed between dark cells and marginal cells. Dark cells express Na+,K+-ATPase, NKCC1, and ClC-K/barttin in their infolded basolateral membranes [15, 19, 20, 58, 101, 116]. They also harbor KCNQ1/KCNE1 in the apical membranes [19, 68] (Fig. 6b). KCNE1-, KCNQ1-, and NKCC1-knockout mice exhibit a collapse of the vestibular endolymphatic space and balance disorders in addition to cochlear dysfunction [15, 17, 20, 74, 104]. Accordingly, like strial marginal cells, vestibular dark cells unidirectionally transport K+ from their basolateral to their apical sides.
On the other hand, several prominent differences are detected between the two organs (Figs. 1b and 6b). The dark-cell area has neither basal cells nor intermediate cells, so the vestibule possesses none of the characteristics corresponding to the IS, the K+ conductance generating the IS potential, and the electrical barrier. It is therefore reasonable that the potential of vestibular endolymph is 0 mV. Comparison of the epithelial architecture and function of the cochlea and vestibule clearly indicates that the two-layer system equipped with two K+-diffusion potentials plays an essential role in the formation of the positive potential in the endolymph while maintaining the K+ circulation and high endolymphatic K+ concentration. If the cochlea possessed only one epithelial layer that faced the endolymph and harbored only one K+-diffusion potential, then this organ, like the vestibule, would maintain a negligible EP because of the small K+-diffusion potential across the apical membrane. To achieve a highly positive potential in the endolymph with one epithelial layer and one K+-diffusion potential, the cochlea might be able to substitute high Na+ for high K+ in the endolymph, but this condition would not be suitable for the hair cell’s function.
The scala media of the cochlea is connected to the endolymphatic space of the sacculus by ductus reuniens, a narrow tunnel. In spite of this direct connection, cochlear endolymph exhibits a potential of +80 mV, whereas the endolymph in the vestibular system is at 0 mV. The mechanism underlying the maintenance of such a prominent difference is unknown. It is speculated that ductus reuniens provides an electrical barrier between the two endolymphatic spaces. Because the sacculus does not harbor any epithelial cells like the stria vascularis and the dark-cell area that maintains endolymph and its properties, Sellick and Johnstone proposed that the potential of saccular endolymph derives from the EP [85]. In support of this idea, the potential of saccular endolymph during a period of anoxia altered and became negative in parallel with the EP. They further assumed that current would flow through ductus reuniens and calculated that the resistance of the tunnel was 180 kΩ. However, a direct measurement of the current and the resistance has not yet been performed, and the nature of the electrical barrier remains uncertain. Further studies will be necessary to clarify these points.
Physiological merit of the EP and high endolymphatic K+ concentration
To amplify the mechanical inputs of sounds, the mammalian cochlea bears two mechanisms in hair cells: active hair-bundle motility and membrane-based electromotility. In the former system, the movement of hair bundles is enhanced by a myosin-based process in the stereocilia [31]. In the latter, depolarization shortens the outer hair cells, which amplify the motion of the basilar membrane [14]. Why have mammals acquired the EP in the course of evolution to further amplify sound signals in the cochlea? Even without the EP, if cochlear endolymph contained a high Na+ concentration and hair cells were excited by Na+ influx, the cochlea would be sufficiently sensitive to weak acoustic stimulation. Indeed, mechanoelectrical transduction channels are Na+ permeable [29, 32, 37]. The combination of the potential difference and the chemical gradient of Na+ between Na+-enriched endolymph and hair cells would establish the large driving force for Na+ influx. Nevertheless, why has the endolymph accumulated K+ instead of Na+ while maintaining the highly positive potential? Because mechanoelectrical transduction channels are open 15% of the time even at the resting position of stereocilia [29, 106], Na+ would continue to enter the hair cells from Na+-enriched endolymph. The hair cells would have to continuously spend large amounts of ATP to extrude Na+ into the perilymph against the concentration gradient, which would exhaust or even destroy the cells. To supply sufficient energy, an enormous number of capillaries should lie in the vicinity of the hair cells. In this situation, however, vibrations produced by blood flow would significantly interfere with the proper motion of the extremely sensitive basilar membrane and hair bundle. To avoid these disadvantages but simultaneously sensitize hearing, it is reasonable that the cochlea has acquired a high K+ concentration and a high endolymphatic potential. In this environment, K+, after exciting hair cells, can diffuse into the perilymph across their basolateral membrane without wasting their energy while the large driving force for K+ influx from endolymph to the cells is maintained.
The physiological significance of the large endolymphatic space remains uncertain. However, because the stria vascularis is positioned far from the hair cells and basilar membrane, any vibration elicited by blood flow in the strial capillaries can be dissipated before reaching them. The damping effect may be further strengthened by the viscosity of endolymph [105], which would act as a cushion, like the synovial fluid in joints, to absorb mechanical vibration.
Several properties of endolymph contribute to the proper operation of the basilar membrane and hair cells in response to subtle acoustic stimuli. It will be important for inner-ear physiologists to confirm the ideas mentioned above.
Conclusion
The key elements underlying formation of the EP have been now demonstrated: this potential depends on an electrical barrier function in the stria vascularis and two K+-diffusion potentials at the apical membranes of intermediate cells and of marginal cells [69]. These factors are established by a specific spatial arrangement of ion-transport apparatus and fine cellular and tissue architecture in the lateral cochlear wall including the stria vascularis. However, several enigmas remain. Moreover, although evidence has been accumulated to prove that dysfunction of the ion-transport mechanisms maintaining the cochlear K+ circulation and the EP causes hearing impairments, we do not know the genetic background and pathophysiological sources of many other disorders of the cochlear labyrinth including Méniere’s disease and sudden deafness. Further studies are necessary to completely understand the mechanisms underlying the formation of the EP and their physiological relevance to hearing function.
References
Ando M, Takeuchi S (1999) Immunological identification of an inward rectifier K+ channel (Kir4.1) in the intermediate cell (melanocyte) of the cochlear stria vascularis of gerbils and rats. Cell Tissue Res 298:179–183
Ando M, Takeuchi S (2000) mRNA encoding 'ClC-K1, a kidney Cl− channel' is expressed in marginal cells of the stria vascularis of rat cochlea: its possible contribution to Cl− currents. Neurosci Lett 284:171–174
Beisel KW, Rocha-Sanchez SM, Morris KA, Nie L, Feng F, Kachar B, Yamoah EN, Fritzsch B (2005) Differential expression of KCNQ4 in inner hair cells and sensory neurons is the basis of progressive high-frequency hearing loss. J Neurosci 25:9285–9293
Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F (2001) Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29:310–314
Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R (2009) Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360:1960–1970
Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ (2002) Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416:874–878
Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Volkl H, Hubner CA, Jentsch TJ (2003) Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. Embo J 22:5422–5434
Cable J, Steel KP (1998) Combined cochleo-saccular and neuroepithelial abnormalities in the Varitint-waddler-J (VaJ) mouse. Hear Res 123:125–136
Casimiro MC, Knollmann BC, Ebert SN, Vary JC Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K (2001) Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A 98:2526–2531
Chan DK, Hudspeth AJ (2005) Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci 8:149–155
Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C (2007) Connexin30 deficiency causes instrastrial fluid–blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A 104:6229–6234
Crouch JJ, Sakaguchi N, Lytle C, Schulte BA (1997) Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem 45:773–778
Cuajungco MP, Grimm C, Heller S (2007) TRP channels as candidates for hearing and balance abnormalities in vertebrates. Biochim Biophys Acta 1772:1022–1027
Dallos P, Fakler B (2002) Prestin, a new type of motor protein. Nat Rev Mol Cell Biol 3:104–111
Delpire E, Lu J, England R, Dull C, Thorne T (1999) Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22:192–195
Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K (2002) Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci U S A 99:14994–14999
Dixon MJ, Gazzard J, Chaudhry SS, Sampson N, Schulte BA, Steel KP (1999) Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet 8:1579–1584
Dulon D, Sugasawa M, Blanchet C, Erostegui C (1995) Direct measurements of Ca2+-activated K+ currents in inner hair cells of the guinea-pig cochlea using photolabile Ca2+ chelators. Pflugers Arch 430:365–373
Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl− channel beta-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 414:558–561
Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE (1999) Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274:26946–26955
Geleoc GS, Lennan GW, Richardson GP, Kros CJ (1997) A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc Biol Sci 264:611–621
Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DC, Kachar B (2004) Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci 24:7051–7062
Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S (2007) A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A 104:19583–19588
Hama K, Saito K (1977) Gap junctions between the supporting cells in some acoustico-vestibular receptors. J Neurocytol 6:1–12
Hibino H, Higashi-Shingai K, Fujita A, Iwai K, Ishii M, Kurachi Y (2004) Expression of an inwardly rectifying K+ channel, Kir5.1, in specific types of fibrocytes in the cochlear lateral wall suggests its functional importance in the establishment of endocochlear potential. Eur J Neurosci 19:76–84
Hibino H, Horio Y, Inanobe A, Doi K, Ito M, Yamada M, Gotow T, Uchiyama Y, Kawamura M, Kubo T, Kurachi Y (1997) An ATP-dependent inwardly rectifying potassium channel, KAB-2 (Kir4.1), in cochlear stria vascularis of inner ear: its specific subcellular localization and correlation with the formation of endocochlear potential. J Neurosci 17:4711–4721
Hibino H, Kurachi Y (2006) Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 21:336–345
Hinojosa R, Rodriguez-Echandia EL (1966) The fine structure of the stria vascularis of the cat inner ear. Am J Anat 118:631–663
Hudspeth AJ (1989) How the ear's works work. Nature 341:397–404
Hudspeth AJ (1997) How hearing happens. Neuron 19:947–950
Hudspeth AJ (2008) Making an effort to listen: mechanical amplification in the ear. Neuron 59:530–545
Hudspeth AJ, Corey DP (1977) Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A 74:2407–2411
Ikeda K, Morizono T (1989) Electrochemical profiles for monovalent ions in the stria vascularis: cellular model of ion transport mechanisms. Hear Res 39:279–286
Iwano T, Yamamoto A, Omori K, Akayama M, Kumazawa T, Tashiro Y (1989) Quantitative immunocytochemical localization of Na+, K+-ATPase alpha-subunit in the lateral wall of rat cochlear duct. J Histochem Cytochem 37:353–363
Jahnke K (1975) The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol Suppl 336:1–40
Keating MT, Sanguinetti MC (2001) Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104:569–580
Kennedy HJ, Evans MG, Crawford AC, Fettiplace R (2003) Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6:832–836
Kerr TP, Ross MD, Ernst SA (1982) Cellular localization of Na+, K+-ATPase in the mammalian cochlear duct: significance for cochlear fluid balance. Am J Otolaryngol 3:332–338
Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ (2006) Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. Embo J 25:642–652
Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T (2000) Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc 33:51–56
Kikuchi T, Kimura RS, Paul DL, Adams JC (1995) Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 191:101–118
Kitajiri S, Miyamoto T, Mineharu A, Sonoda N, Furuse K, Hata M, Sasaki H, Mori Y, Kubota T, Ito J, Furuse M, Tsukita S (2004) Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J Cell Sci 117:5087–5096
Kitajiri SI, Furuse M, Morita K, Saishin-Kiuchi Y, Kido H, Ito J, Tsukita S (2004) Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res 187:25–34
Konishi T, Butler RA, Fernandez C (1961) Effect of anoxia on cochlear potentials. J Acoust Soc Amer 33:349–390
Konishi T, Hamrick PE, Walsh PJ (1978) Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Otolaryngol 86:22–34
Konishi T, Mendelsohn M (1970) Effect of ouabain on cochlear potentials and endolymph composition in guinea pigs. Acta Otolaryngol 69:192–199
Kros CJ, Ruppersberg JP, Rusch A (1998) Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 394:281–284
Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ (1999) KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96:437–446
Kuijpers W, Bonting SL (1970) The cochlear potentials. I. The effect of ouabain on the cochlear potentials of the guinea pig. Pflugers Arch 320:348–358
Kuijpers W, Bonting SL (1970) The cochlear potentials. II. The nature of the cochlear endolymphatic resting potential. Pflugers Arch 320:359–372
Kusakari J, Ise I, Comegys TH, Thalmann I, Thalmann R (1978) Effect of ethacrynic acid, furosemide, and ouabain upon the endolymphatic potential and upon high energy phosphates of the stria vascularis. Laryngoscope 88:12–37
Langer P, Grunder S, Rusch A (2003) Expression of Ca2+-activated BK channel mRNA and its splice variants in the rat cochlea. J Comp Neurol 455:198–209
Lautermann J, Frank HG, Jahnke K, Traub O, Winterhager E (1999) Developmental expression patterns of connexin26 and -30 in the rat cochlea. Dev Genet 25:306–311
Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E (1998) Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 294:415–420
Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Mammano F, Bortolozzi M, Ortolano S, Anselmi F (2007) Ca2+ signaling in the inner ear. Physiology (Bethesda) 22:131–144
Marcus DC (1984) Characterization of potassium permeability of cochlear duct by perilymphatic perfusion of barium. Am J Physiol 247:C240–C246
Marcus DC, Liu J, Wangemann P (1994) Transepithelial voltage and resistance of vestibular dark cell epithelium from the gerbil ampulla. Hear Res 73:101–108
Marcus DC, Marcus NY, Thalmann R (1981) Changes in cation contents of stria vascularis with ouabain and potassium-free perfusion. Hear Res 4:149–160
Marcus DC, Rokugo M, Thalmann R (1985) Effects of barium and ion substitutions in artificial blood on endocochlear potential. Hear Res 17:79–86
Marcus DC, Wu T, Wangemann P, Kofuji P (2002) KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol 282:C403–C407
Melichar I, Syka J (1977) Time course of anoxia-induced K+ concentration changes in the cochlea measured with K+ specific microelectrodes. Pflugers Arch 372:207–213
Melichar I, Syka J (1987) Electrophysiological measurements of the stria vascularis potentials in vivo. Hear Res 25:35–43
Minowa O, Ikeda K, Sugitani Y, Oshima T, Nakai S, Katori Y, Suzuki M, Furukawa M, Kawase T, Zheng Y, Ogura M, Asada Y, Watanabe K, Yamanaka H, Gotoh S, Nishi-Takeshima M, Sugimoto T, Kikuchi T, Takasaka T, Noda T (1999) Altered cochlear fibrocytes in a mouse model of DFN3 nonsyndromic deafness. Science 285:1408–1411
Mori Y, Watanabe M, Inui T, Nimura Y, Araki M, Miyamoto M, Takenaka H, Kubota T (2009) Ca2+ regulation of endocochlear potential in marginal cells. J Physiol Sci 59:355–365
Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, Garcia-Anoveros J (2008) The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A 105:353–358
Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P (1997) A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange–Nielsen cardioauditory syndrome. Nat Genet 15:186–189
Nicolas M, Dememes D, Martin A, Kupershmidt S, Barhanin J (2001) KCNQ1/KCNE1 potassium channels in mammalian vestibular dark cells. Hear Res 153:132–145
Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y (2008) The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci U S A 105:1751–1756
Offner FF, Dallos P, Cheatham MA (1987) Positive endocochlear potential: mechanism of production by marginal cells of stria vascularis. Hear Res 29:117–124
Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B (2000) Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26:595–601
Phippard D, Lu L, Lee D, Saunders JC, Crenshaw EB 3rd (1999) Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J Neurosci 19:5980–5989
Rickheit G, Maier H, Strenzke N, Andreescu CE, De Zeeuw CI, Muenscher A, Zdebik AA, Jentsch TJ (2008) Endocochlear potential depends on Cl− channels: mechanism underlying deafness in Bartter syndrome IV. EMBO J 27:2907–2917
Rivas A, Francis HW (2005) Inner ear abnormalities in a Kcnq1 (Kvlqt1) knockout mouse: a model of Jervell and Lange–Nielsen syndrome. Otol Neurotol 26:415–424
Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Muller M, Kopschall I, Pfister M, Munkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M (2004) Deletion of the Ca2+-activated potassium (BK) α-subunit but not the BKβ1-subunit leads to progressive hearing loss. Proc Natl Acad Sci U S A 101:12922–12927
Sage CL, Marcus DC (2001) Immunolocalization of ClC-K chloride channel in strial marginal cells and vestibular dark cells. Hear Res 160:1–9
Sakagami M, Fukazawa K, Matsunaga T, Fujita H, Mori N, Takumi T, Ohkubo H, Nakanishi S (1991) Cellular localization of rat Isk protein in the stria vascularis by immunohistochemical observation. Hear Res 56:168–172
Sakaguchi N, Crouch JJ, Lytle C, Schulte BA (1998) Na-K-Cl cotransporter expression in the developing and senescent gerbil cochlea. Hear Res 118:114–122
Salt AN, Melichar I, Thalmann R (1987) Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 97:984–991
Schlingmann KP, Konrad M, Jeck N, Waldegger P, Reinalter SC, Holder M, Seyberth HW, Waldegger S (2004) Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med 350:1314–1319
Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP (2009) Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106:5842–5847
Schulte BA, Adams JC (1989) Distribution of immunoreactive Na+, K+-ATPase in gerbil cochlea. J Histochem Cytochem 37:127–134
Schulte BA, Steel KP (1994) Expression of alpha and beta subunit isoforms of Na, K-ATPase in the mouse inner ear and changes with mutations at the Wv or Sld loci. Hear Res 78:65–76
Schulze-Bahr E, Wang Q, Wedekind H, Haverkamp W, Chen Q, Sun Y, Rubie C, Hordt M, Towbin JA, Borggrefe M, Assmann G, Qu X, Somberg JC, Breithardt G, Oberti C, Funke H (1997) KCNE1 mutations cause Jervell and Lange–Nielsen syndrome. Nat Genet 17:267–268
Sellick PM, Johnstone BM (1972) The electrophysiology of the saccule. Pflugers Arch 336:28–34
Shen Z, Marcus DC (1998) Divalent cations inhibit IsK/KvLQT1 channels in excised membrane patches of strial marginal cells. Hear Res 123:157–167
Skinner LJ, Enee V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM, Dulon D (2003) Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the guinea pig cochlea. J Neurophysiol 90:320–332
Spicer SS, Schulte BA (1996) The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res 100:80–100
Spicer SS, Schulte BA (2005) Novel structures in marginal and intermediate cells presumably relate to functions of apical versus basal strial strata. Hear Res 200:87–101
Sterkers O, Saumon G, Tran Ba Huy P, Amiel C (1982) K, Cl, and H2O entry in endolymph, perilymph, and cerebrospinal fluid of the rat. Am J Physiol 243:F173–F180
Sunose H, Ikeda K, Saito Y, Nishiyama A, Takasaka T (1993) Nonselective cation and Cl channels in luminal membrane of the marginal cell. Am J Physiol 265:C72–C78
Sunose H, Ikeda K, Suzuki M, Takasaka T (1994) Voltage-activated K channel in luminal membrane of marginal cells of stria vascularis dissected from guinea pig. Hear Res 80:86–92
Takeuchi S, Ando M (1998) Dye-coupling of melanocytes with endothelial cells and pericytes in the cochlea of gerbils. Cell Tissue Res 293:271–275
Takeuchi S, Ando M (1998) Inwardly rectifying K+ currents in intermediate cells in the cochlea of gerbils: a possible contribution to the endocochlear potential. Neurosci Lett 247:175–178
Takeuchi S, Ando M (1999) Voltage-dependent outward K+ current in intermediate cell of stria vascularis of gerbil cochlea. Am J Physiol 277:C91–C99
Takeuchi S, Ando M, Kakigi A (2000) Mechanism generating endocochlear potential: role played by intermediate cells in stria vascularis. Biophys J 79:2572–2582
Takeuchi S, Ando M, Kozakura K, Saito H, Irimajiri A (1995) Ion channels in basolateral membrane of marginal cells dissociated from gerbil stria vascularis. Hear Res 83:89–100
Takeuchi S, Marcus DC, Wangemann P (1992) Ca2+-activated nonselective cation, maxi K+ and Cl− channels in apical membrane of marginal cells of stria vascularis. Hear Res 61:86–96
Takumida M, Kubo N, Ohtani M, Suzuka Y, Anniko M (2005) Transient receptor potential channels in the inner ear: presence of transient receptor potential channel subfamily 1 and 4 in the guinea pig inner ear. Acta Otolaryngol 125:929–934
Tasaki I, Spyropoulos CS (1959) Stria vascularis as source of endocochlear potential. J Neurophysiol 22:149–155
ten Cate WJ, Curtis LM, Rarey KE (1994) Na, K-ATPase α and β subunit isoform distribution in the rat cochlear and vestibular tissues. Hear Res 75:151–160
Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K (2003) Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet 12:13–21
Tranebjaerg L, Bathen J, Tyson J, Bitner-Glindzicz M (1999) Jervell and Lange–Nielsen syndrome: a Norwegian perspective. Am J Med Genet 89:137–146
Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J (1996) Inner ear defects induced by null mutation of the isk gene. Neuron 17:1251–1264
Vilstrup G (1955) The vitreous body and the endolymph as two related gelatinous substances; comparison of the two substances with reference to their hyaluronic acid and protein contents; preliminary report. Acta Ophthalmol (Copenh) 33:13–15
Vollrath MA, Kwan KY, Corey DP (2007) The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci 30:339–365
Von Bekesy G (1952) DC resting potentials inside the cochlear partition. J Acoust Soc Amer 24:72–76
Von Bekesy G (1952) Resting potentials inside the cochlear partition of the guinea pig. Nature 169:241–242
Wada J, Kambayashi J, Marcus DC, Thalmann R (1979) Vascular perfusion of the cochlea: effect of potassium-free and rubidium-substituted media. Arch Otorhinolaryngol 225:79–81
Wada J, Paloheimo S, Thalmann I, Bohne BA, Thalmann R (1979) Maintenance of cochlear function with artificial oxygen carriers. Laryngoscope 89:1457–1473
Wang Q, Bowles NE, Towbin JA (1998) The molecular basis of long QT syndrome and prospects for therapy. Mol Med Today 4:382–388
Wangemann P (1995) Comparison of ion transport mechanisms between vestibular dark cells and strial marginal cells. Hear Res 90:149–157
Wangemann P (2002) K+ cycling and the endocochlear potential. Hear Res 165:1–9
Wangemann P (2006) Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576:11–21
Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC (2004) Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med 2:30
Wangemann P, Liu J, Marcus DC (1995) Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear Res 84:19–29
Xu H, Delling M, Li L, Dong X, Clapham DE (2007) Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc Natl Acad Sci U S A 104:18321–18326
Zidanic M, Brownell WE (1990) Fine structure of the intracochlear potential field. I. The silent current. Biophys J 57:1253–1268
Acknowledgments
We thank Dr. Bernd Nilius (KU Leuven) for providing us with an opportunity to write this review article and Dr. A. J. Hudspeth (The Rockefeller University) for his critical reading of the text. HH and YK are supported by the following research grants and funds: Grant-in-Aid for Scientific Research on Priority Areas 17081012 (to HH), Grant-in-Aid for Young Scientists (B) 19790188 (to HH), the Global COE Program “in silico medicine” at Osaka University (to HH, FN, and YK), and a grant for “Research and Development of Next-Generation Integrated Life Simulation Software” (to YK), from the Ministry of Education, Culture, Sport, Science and Technology of Japan, Senri Life Science Foundation (to HH), Japan Foundation for Applied Enzymology (to HH), and the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical care (to HH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hibino, H., Nin, F., Tsuzuki, C. et al. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch - Eur J Physiol 459, 521–533 (2010). https://doi.org/10.1007/s00424-009-0754-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0754-z