Abstract
We have previously reported a depolarization-activated 4-aminopyridine-resistant transient outward K+ current with inward rectification (I to.ir) in canine and guinea pig cardiac myocytes. However, molecular identity of this current is not clear. The present study was designed to investigate whether Kir2.1 channel carries this current in stably transfected human embryonic kidney (HEK) 293 cells using whole-cell patch-clamp technique. It was found that HEK 293 cells stably expressing human Kir2.1 gene had a transient outward current elicited by voltage steps positive to the membrane potential (around −70 mV). The current exhibited a current–voltage relationship with intermediate inward rectification and showed time-dependent inactivation and rapid recovery from inactivation. The half potential (V 0.5) of availability of the current was −49.4 ± 2.1 mV at 5 mM K+ in bath solution. Action potential waveform clamp revealed two components of outward currents; one was immediately elicited and then rapidly inactivated during depolarization, and another was slowly activated during repolarization of action potential. These properties were similar to those of I to.ir observed previously in native cardiac myocytes. Interestingly, inactivation of the I to.ir was strongly slowed by increasing intracellular free Mg2+ (Mg2+ i , from 0.03 to 1.0, 4.0, and 8.0 mM). The component elicited by action potential depolarization increased with the elevation of Mg2+ i . Inclusion of spermine (100 μM) in the pipette solution remarkably inhibited both the I to.ir and steady-state current. These results demonstrate that the Mg2+ i -dependent current carried by Kir2.1 likely is the molecular identity of I to.ir observed previously in cardiac myocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well recognized that depolarization-activated outward K+ currents play an important role in myocardial repolarization of different species, and they have distinctive biophysical and pharmacological properties [1]. These outward currents include transient outward currents (I to) [10, 13, 19, 44]; rapid and slow delayed rectifier K+ currents (I Kr and I Ks) in guinea pig [36], dog [43], pig [21], and human [24] cardiac myocytes; and inward rectifier K+ current (I K1) in cardiac myocytes of all species. Ultra-rapid delayed rectifier K+ current (I Kur) is also expressed in rat [3], dog [43], pig [21], and human [24] atrial myocytes.
I to has been identified using whole-cell voltage-clamp techniques in cardiac cells from a wide range of species since the 1960s, including rat [17], rabbit [13], elephant seal [31], ferret [4], dog [40, 44], pig [21], and humans [2, 23]. Kenyon and Gibbons [18] reported that 4-aminopyridine (4-AP) decreased I to in sheep cardiac Purkinje fibers. Subsequently, 4-AP has been used as a selective inhibitor of transient outward K+ current, and 4-AP-sensitive and 4-AP-resistant components of I to have been reported in sheep Purkinje fibers [6] and in rabbit [47] and dog [40, 44] cardiac myocytes. The 4-AP-sensitive and 4-AP-resistant components often are termed I to1 and I to2, respectively, by Tseng and Hoffman [40]; I to2 is a Ca2+-activated transient outward Cl− current (I Cl.Ca) [21, 44, 47]. The third element of transient outward current with inward rectification (I to.ir) was described in dog ventricular cells [25] and guinea pig cardiomyocytes [26]. I to.ir is another 4-AP-insensitive transient outward current carried by K+ ions and sensitive to inhibition by Ba2+ and/or the omission of extracellular K+ (K+ o). Zhabyeyev and colleagues also reported I to using whole-cell patch recording in guinea pig ventricular myocytes dialyzed with low-K+ (10 mM) solution [46]. It has been suggested the I to recorded using a low-K+ pipette solution [46] or the I to.ir determined using a physiological-K+ pipette solution [25, 26] is likely carried by the inward rectifier K+ channel; however, this is challenged by the classical notion that the cardiac I K1 channel acts as a diode [30] which activates upon hyperpolarization of the membrane [34], and limited current passes through the channel in the outward direction under physiological conditions [30, 34].
During the study of human Kir2.1 channels stably expressed in human embryonic kidney (HEK) 293 cells, we observed a 4-AP-insensitive I to, similar to the I to.ir observed in dog and guinea pig ventricular myocytes [25, 26]. The present study was designed to characterize this I to in HEK 293 cells stably expressing human Kir2.1 gene and to investigate whether Kir2.1 channel contributes to the molecular identity of I to.ir previously observed in cardiac myocytes.
Materials and methods
Gene transfection and cell culture
HEK293 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and grown in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum in 5% CO2 and 95% air at 37°C. Human Kir2.1 gene kindly provided by Dr. Carol A. Vandenberg (University of California at Santa Barbara, CA, USA) [35] was inserted into the pCDNA3 vector. The Kir2.1/pCDNA3 plasmid (4 μg) was then transfected into HEK 293 cells using Lipofectamine 2000TM and selected using 1,000 μg/ml G418 (GE Healthcare, Hong Kong). Colonies were picked with cloning cylinders (Sigma-Aldrich, St Louis, MO, USA) and examined for channel expression using whole-cell current recordings as described previously [9, 39]. The selected cell line stably expressing human Kir2.1 channel was maintained in DMEM medium containing 400 μg/ml G418 and 10% fetal bovine serum.
Solutions and chemicals
Tyrode solution contained (mM) NaCl 140, KCl 5.0, MgCl2 1.0, CaCl2 1.8, NaH2PO4 0.33, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10.0, and glucose 10 (pH adjusted to 7.3 with NaOH). For whole-cell recordings, the pipette solution contained (mM) KCl 20, K-aspartate 110, MgCl2 1.0, HEPES 10, ethyleneglycoltetraacetic acid (EGTA) 5, guanosine triphosphate (GTP) 0.1, Na-phosphocreatine 5, and Mg-adenosine triphosphate (ATP) 5 (pH adjusted to 7.2 with KOH, ∼10 mM). When accurate free Mg2+ concentrations in the pipette solution were administered, Mg-ATP was replaced by tris–ATP. The free Mg2+ in pipette solution was calculated using the Cabuf software created by Dr. G. Droogmans in the Department of Physiology, KU Leuven, Leuven, Belgium (http://www.kuleuven.be/fysiol/trp/cabuf). All reagents were obtained from Sigma-Aldrich. Stock solution of spermine was made with dimethylsulfoxide and was divided into aliquots and stored at −20°C.
Electrophysiology
Cells on a cover slip were transferred to an open cell chamber (0.5 ml) mounted on the stage of an inverted microscope and superfused with Tyrode solution at ∼2 ml/min. The whole-cell patch-clamp technique was used as described previously [9, 12, 39]. The whole-cell membrane currents were measured using an EPC-10 amplifier and Pulse software (Heka Elektronik, Lambrecht, Germany). Borosilicate glass electrodes (1.2-mm OD) were pulled with a Brown–Flaming puller (model P-97, Sutter Instrument, Nato, CA, USA) and had tip resistances of 1–2 MΩ when filled with the pipette solution. A 3-M KCl agar bridge was used as the reference electrode. The tip potential was zeroed before the patch pipette touched the cell. After the gigaohm seal was obtained, the cell membrane was ruptured by applying gentle pressure to establish a whole-cell configuration. Series resistance (Rs) was 2–3 MΩ and was compensated by 50–70% to minimize voltage errors. The liquid junction potential (13.7 mV) calculated with the software of Clampex (http://www.Axon.com) was not corrected in the experiment and data analysis. Cell membrane capacitive transient was electrically compensated with the Pulse software. Current and voltage signals were low-pass filtered at 5 kHz and stored in the hard disk of an IBM compatible computer. All experiments were conducted at room temperature (22–23°C).
Statistical analysis
Nonlinear curve-fitting was performed using Pulsefit (HEKA) and Sigmaplot (SPSS, Chicago, IL, USA). Paired and/or unpaired Student’s t test was used as appropriate to evaluate the statistical significance of differences between two group means, and analysis of variance was used for multiple groups. Group data are expressed as mean ± SEM. Values of P < 0.05 were considered to be statistically significant.
Results
Initial discovery of transient outward current carried by Kir2.1 channel
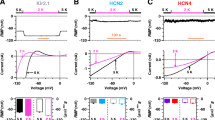
When we used a conventional voltage step protocol (inset of Fig. 1a) to study Kir2.1 channel stably expressed in HEK 293 cells, a current with fast inactivation was recorded at −40 mV as indicated in control of Fig. 1b,c. The current disappeared when the inwardly rectifying component of Kir2.1 channel elicited by the hyperpolarization voltage steps was inhibited by application of 200 μM Ba2+ or removal of external K+ ions (K+ o) (Fig. 1b,c). No transient outward current was observed in HEK 293 cells without Kir2.1 gene transfection. We therefore assumed that this current was a depolarization-elicited transient outward current of Kir2.1 channel when the hyperpolarization steps (−120 to −50 mV) were returned to the holding potential (−40 mV).
Transient outward current recorded in HEK 293 cells stably expressing human Kir2.1 channels. a Only a small background current was recorded with the voltage steps to between −120 and +40 mV from a holding potential of −40 mV in HEK 293 cells without Kir2.1 gene transfection (n = 5). Membrane capacitative transient was compensated electronically by the Pulse software. b Typical inward rectifier K+ current (I K1) was recorded in HEK 293 cells (n = 48) using the protocol as shown in a followed by a transient outward current as hyperpolarization potentials returned to the holding potential (−40 mV, broken arrow of left panel). Both the inward rectifier and transient outward currents were almost fully inhibited by the application of 200 μM Ba2+ (5 min; n = 6). c The inward rectifier and transient outward currents were abolished by omission of external K+ (K+ o, 5 min) in a representative cell expressing Kir2.1 gene (n = 5)
Depolarization-elicited transient outward current
To further confirm whether the transient outward current is elicited by depolarization voltage steps, a depolarization voltage protocol with 200 ms voltage steps to voltages between −70 and +40 mV from a holding potential of −80 mV (inset of Fig. 2a) was employed to characterize this current. Figure 2a shows the current tracings elicited by the depolarization voltage steps in a representative HEK 293 cell stably expressing Kir2.1 channel. A transient outward current that rapidly activated upon depolarization voltage steps positive to −70 mV (without correction of liquid junction potential) was insensitive to inhibition by the classical I to blocker 4-AP (5 mM), and no 4-AP-sensitive current was observed. Similar results were obtained in four other cells. This transient outward current was fully inhibited by the application of 200 μM Ba2+ or omission of K+ o from the bath medium, leaving a small background current. Ba2+- or K+ o-sensitive transient outward current was obtained by digital subtraction (Fig. 2b,c). Similar results were obtained in nine other cells in each experimental group. These results suggest that the I to is most likely a K+ current permeating through the Kir2.1 channel.
Transient outward current elicited by depolarization voltages. a Representative currents elicited by 200-ms depolarization steps from −80 mV to test potentials between −70 and +40 mV (inset) in a representative cell during control and after application of 5 mM 4-AP for 8 min. No significant 4-AP-sensitive current was obtained by digital subtraction of the currents before and after 4-AP treatment (n = 6). b Depolarization-elicited current was fully inhibited by the application of 200 μM Ba2+ in another cell. Ba2+-senstive current was obtained by digital subtraction (n = 10). c Depolarization-activated transient outward current was abolished by removing K+ o in different cells (n = 8). Arrows zero current level
Voltage dependence of the transient outward current
The voltage dependence of the depolarization-activated I to was determined using the same voltage-clamp protocol as in Fig. 2a. Ba2+-sensitive current was used to determine the I–V relation of the current. Figure 3a shows the Ba2+-sensitive current recorded in a representative cell at depolarization voltages between −70 and +40 mV from a holding potential of −80 mV. I to was observed at −70 mV, just positive to the membrane potential of −72 mV, as indicated by the voltage step from the holding current, and augmented as the depolarization potential was made more positive from −70 to −40 mV (left panel). The current showed voltage-dependent inward rectification in the voltage range from −30 to 0 mV (middle panel) and reached a steady-state level at potentials between +10 and +40 mV (right panel).
Current–voltage (I–V) relations of depolarization-elicited currents. a Voltage dependence of depolarization-activated Ba2+-sensitive current as shown in Fig. 2b. Current tracings are illustrated at potentials between −70 and −40 mV (left), between −30 and 0 mV (middle), and between +10 and +40 mV (right). b schematic showing current measurement in a Ba2+-sensitive current tracing recorded with 300-ms step to −20 mV from a holding potential of −80 mV. I peak time-dependent peak current, I SS steady-state current. c I–V relationships of Ba2+-sensitive currents (n = 10) for I peak and I SS. The liquid junction potential was not corrected for the current recording and data analysis
Figure 3b schematically illustrates the method of current measurement in a recording with 200-ms step to −30 mV from a holding potential of −80 mV and indicates the measurements of the I to (I peak) and the steady-state current (I SS). Figure 3c shows the mean values of I–V relationships of the I to and I SS in 11 cells. The amplitude of the I to increased on depolarization, reached a steady-state level at potentials positive to −20 mV, and showed an intermediate inward rectification, whereas the I–V relationship for I SS displayed a stronger inward rectification. These characteristics of I to are similar to those of the I to.ir described previously in cardiac ventricular myocytes from dog [25] and guinea pig [26] hearts. These results suggest that Kir2.1 channel likely carries two components of outward currents upon depolarization. One is the steady-state current with strong inward rectification (i.e., classical I K1) as widely observed in ventricular myocytes of mammalian hearts including those of humans [20]. Another is a transient outward current carried by Kir2.1 channel with intermediate inward rectification as I to.ir observed in ventricular myocytes of dog and guinea pig hearts [25, 26]. We therefore refer to this transient outward component as I to.ir.
Time- and voltage-dependent kinetics of I to.ir
Figure 4a shows the current trace recorded in a representative cell with a 200-ms voltage step from −80 to −20 mV. The transient outward component was best fitted to a bi-exponential function with time constants of 2.3 and 45.4 ms. The voltage dependence of the time constants τ fast and τ slow is shown in Fig. 4b. The fast inactivation τ fast was voltage-dependent (P < 0.01, n = 9), and rate of rapid inactivation increased on depolarization to −20 mV. The voltage-dependence of slow inactivation τ slow was not consistent, and rate of slow inactivation reduced only at −20, −10, and 0 mV (P < 0.05 or P < 0.01, n = 9). The relative contributions of fast and slow components likely reflect the influence of Mg2+ and/or gating polyamines.
Time-dependent inactivation of transient outward current. a Inactivation of the current on 200-ms depolarization from −80 to −20 mV was fitted to a bi-exponential function (curve shown as solid line, points are raw data) with time constants shown (τ fast and τ slow). b Voltage-dependence of time constants (τ fast and τ slow). (n = 9, *P < 0.05, **P < 0.01 vs −50 mV)
To study the time-dependent recovery from inactivation and voltage-dependent availability (inactivation) of I to.ir, we adopted the protocols used previously in dog and guinea pig ventricular myocytes [25, 26]. The recovery of I to.ir from inactivation was studied with a paired-pulse protocol (inset of Fig. 5a). The current during P2 (I 2) relative to the current during P1 (I 1) was determined as a function of the P1–P2 recovery interval. The recovery of I to.ir from inactivation was fitted to a monoexponential function with a time constant of 7.9 ± 0.4 ms (n = 10, Fig. 5b).
Recovery from inactivation and voltage dependence of availability of I to.ir. a Currents and protocol used to assess recovery of I to.ir from inactivation. Identical double pulses (200-ms, P1, and P2) from −80 to −30 mV (inset) were applied with a varying P1–P2 interval (Δt) delivered every 10 s. b Mean value of recovery curve (I 2/I 1) of I to.ir was fitted to a monoexponential function with a time constant of 7.9 ± 0.4 ms (n = 10). c Protocol and current traces used to assess the voltage dependence of steady-state inactivation (availability). Prepulses of 500-ms duration were applied to conditioning potentials between −120 and +10 mV, and currents were recorded during 200-ms test pulses to +20 mV. d Currents (I) recorded at +20 mV after 500-ms steps to the condition potential were normalized to the maximum current obtained (I max). Data were fit to the Boltzmann relationship: I/I max = {1 + exp[(V − V 0.5)/S]}−1, where V is the conditioning potential, V 0.5 is the potential for half-maximal inactivation (availability), and S is the slope factor. The V 0.5 of I to.ir availability was −49.2 ± 2.1 mV (n = 9)
Figure 5c illustrates the voltage protocol used for determining the availability of I to.ir and current traces recorded. The variable of I to.ir availability was determined as the current at a given prepulse potential divided by the maximum I to.ir in the absence of a prepulse. Figure 5d shows the mean values of the channel availability fitted to Boltzmann distribution. The half potential (V 0.5) of I to.ir availability was −49.2 ± 2.1 mV (n = 11), and the slope factor was 15.2 ± 0.6 mV. These results indicate that time- and voltage-dependent kinetics of I to.ir in HEK cells expressing Kir2.1 channel are similar to those of I to.ir in cardiac myocytes [25, 26].
Activation of I to.ir during cardiac action potential
The I to.ir in cardiac myocytes was activated by action potential waveform [25, 26]. To study whether it is the case for I to.ir carried by Kir2.1 channel stably expressed in HEK 293 cells, the action potential recorded from human right ventricular myocytes [22] was used as the protocol to record membrane current (Fig. 6a). Two components of outward current currents were activated by the action potential waveform. One was a transient outward component evident immediately after depolarization, which is likely contributed by I to.ir. Another component was activated during the phase 3 repolarization of the action potential. The two components of outward currents were abolished by the application 200 μM Ba2+ (Fig. 6b) or omission of K+ o (data not shown). Very little Ba2+-sensitive current was present during the plateau of the action potential (Fig. 6c). Similar results were obtained in a total of 11 cells. These results were similar to those observed in ventricular myocytes from dog and/or guinea pig hearts [25, 26], suggesting that the I to.ir observed in cardiac ventricular myocytes is most likely carried by Kir2.1 channel.
Contribution of Kir2.1 channel currents to action potential. a Action potential recorded from a human ventricular myocyte was used as a voltage clamp protocol. b Currents activated by the action potential waveform before (Control) and after exposure to 200 μM Ba2+. c Ba2+-sensitive current during the action potential was obtained by digitally subtracting membrane currents before and after application of 200 μM Ba2+. The early transient current is consistent with the properties of I to.ir, as determined from square voltage steps. The transient current during phase 3 likely reflects the inwardly rectifying I K1
Effects of intracellular free Mg2+ and spermine on I to.ir
To examine whether I to.ir was affected by intracellular Mg2+ i , we included different concentrations (0.03, 1, 4, or 8 mM) of free Mg2+ in the pipette solution. Figure 7a illustrates the currents recorded using the voltage steps as shown in the inset after 15 min dialysis for each concentration of free Mg2+ pipette solution. It is interesting to note that inactivation of I to.ir slowed with the increase of free Mg2+ i . At −20 mV, an increase in inactivation τ fast was observed at 8 mM free Mg2+ i (Fig. 7b, n = 8, P < 0.05 vs 0.03 mM), while increases in inactivation τ slow were observed at 1, 4, and 8 mM free Mg2+ i (n = 7–9, P < 0.01 vs 0.03 mM free Mg2+ i ). Figure 7c displays the Ba2+-sensitive current recorded in cells with action potential waveform using 0.03, 1, 4, or 8 mM free Mg2+ i pipette solutions. The action potential waveform protocol revealed that the duration of the component elicited by depolarization increased with the elevation of free Mg2+ i in the pipette solution; this is consistent with the slowing of I to.ir inactivation observed using the voltage step protocol (Fig. 7a,b). No significant change was observed for the second component activated by the repolarization during phase 3 of the action potential. Similar results were obtained in seven to nine cells for each free Mg2+ pipette solution concentration. These results indicate that I to.ir is greatly dependent on intracellular free Mg2+ concentration.
Effect of intracellular free Mg2+ on I to.ir. a Inactivation phase of I to.ir slowed with increasing free Mg2+ i from 0.03, to 1.0, 4.0, and 8.0 mM. b Inactivation time constant (τ slow and τ slow , at −20 mV, n = 7–10 for each group) increased (*P < 0.05; **P < 0.01 vs 0.03 mM free Mg2+ i ). c Ba2+-sensitive current elicited by action potential waveform, obtained by subtracting currents before and after application 200 μM Ba2+ with inclusion of different concentrations of free Mg2+ i . Duration of the early transient current elicited by phase 1 of action potential increased with free Mg2+ i elevation, consistent with the change of I to.ir inactivation determined from square voltage steps in a
It is well established that spermine blocks the outward component of I K1 or Kir channel. To study whether spermine decreases I to.ir, 100 μM spermine was included in a pipette solution containing 1 mM free Mg2+ i . Spermine inclusion almost completely abolished I to.ir and reduced the steady-state component of Kir2.1 channel (Fig. 8a). Action-potential clamp voltage protocol displays that the initial component elicited by action potential depolarization was almost completely inhibited, and the second component activated by repolarization of the action potential was remarkably reduced by spermine inclusion; similar results were obtained in a total of eight HEK cells stably expressing human Kir2.1 channel. These results indicate that spermine blocks K+ efflux through Kir2.1 channels, similar to those as described previously [11, 29, 42].
Effect of spermine inclusion on I to.ir. a Membrane current was recorded with the protocol shown in the inset with a pipette solution containing 1 mM free Mg2+ or 1 mM free Mg2+ plus 100 μM spermine. Spermine inhibited both I to.ir and I SS. b Ba2+-sensitive current elicited by the action potential waveform, obtained by subtracting currents before and after application 200 μM Ba2+ with a pipette solution containing 1 mM free Mg2+ i or 1 mM free Mg2+ i plus 100 μM spermine. No significant early transient current was observed during phase 1 of action potential, and only a small later transient current was recorded during phase 3
Discussion
The present study demonstrates direct evidence that human Kir2.1 channel carries a transient outward potassium current with inward rectification. I to.ir is only present in HEK 293 cells stably expressing human Kir2.1 gene. Although a small 4-AP-sensitive I to carried by A-type K+ channels was reported in HEK 293 cells using a protocol with very negative holding potentials (−110 mV) [16], we did not observe the endogenous I to with the protocol employed in the present study in HEK 293 cells that do not express human Kir2.1 channels (Fig. 1a). I to.ir was observed upon depolarization voltage steps only in cells stably expressing Kir2.1 gene (Fig. 1b,c) and was insensitive to the classical I to blocker 4-AP (Fig. 2a); therefore, possible contamination of I to.ir by endogenous A-type K+ channels should be very limited.
I to.ir was activated over a wide range of voltages positive to the membrane potential in HEK 293 cells stably expressing human Kir2.1 gene. The current was suppressed by the removal of Ko + or by application of Ba2+ (Figs. 1 and 2), indicating that the current is carried by human Kir2.1 channel. I to.ir inactivated rapidly after activation at more positive potentials (Figs. 3 and 4), recovered quickly from inactivation, and had an inactivation V 0.5 of −49 mV at normal K+ o of 5 mM (Fig. 5). Action potential waveform protocol experiments revealed two components of outward currents. One component is immediately activated during the depolarization phase and quickly inactivated at the plateau of the action potential. The other component is gradually activated during the repolarization (Fig. 6). These properties of I to.ir are similar to those of the I to.ir observed in dog [25] and guinea pig [26] ventricular myocytes. I to.ir was actually also observed in rabbit and human ventricular myocytes (the authors’ unpublished observations).
Several properties of I to.ir recorded in the present study are similar to the I to.ir observed in native cardiac ventricular myocytes [25, 26]: (1) The current was sensitive to Ba2+ block or K+ o removal; (2) the current displayed an intermediate inward rectification and a bi-exponential inactivation; (3) the current had similar values of availability potential (V 0.5) to I to.ir observed in native cardiac ventricular myocytes (−49.2 mV for I to.ir in this cell line, −44 mV and −51.6 mV for I to.ir, respectively, in dog and guinea pig ventricular myocytes); (4) the current recovered quickly from inactivation (recovery τ: 7.9 ms for I to.ir in HEK cell line and 10.7 ms for I to.ir in cardiac myocytes); and (5) the current activated immediately upon depolarization of the cardiac action potential and inactivated quickly at plateau of the action potential (Fig. 6).
It is well recognized that I K1 in cardiac ventricular myocytes is dominantly contributed by Kir2.1 channels [8, 45], though possible Kir2.2 contribution is suggested [45]. I to.ir observed in native ventricular myocytes [25, 26] is most likely carried mainly by Kir2.1 channel. In addition, the transient outward current recorded using a low K+ pipette solution in guinea pig ventricular myocytes [46] and the patch-duration-dependent 4-AP-insensitive I to in cat ventricular myocytes [27] may also be carried by Kir2.1 channels. Therefore, molecular identity of the non-classical I to observed under different conditions [25–27] is likely contributed mainly by Kir2.1 channel.
The previous arguments that I to.ir in native cardiac myocytes might be a novel current [25, 26] were based on the observation that the current is neither the classical I to1 or I to2, and the classical concept that (1) the cardiac ventricular I K1 channel is considered to act as a diode [30] that activates only on hyperpolarization of the membrane [34]; (2) there is very little current passing through the channel in the outward direction under physiological conditions [30, 34]; and (3) I K1 was inactivated during the upstroke and plateau phases of the action potential and is consequently available for repolarization only during phase 3 [37]. However, studies by others [27, 46] and ours [25, 26] on native ventricular myocytes of different species, in addition to the present study of HEK 293 cells stably expressing human Kir2.1 channels (Figs. 2 and 5), indicate that cardiac I K1 can be rapidly activated during depolarization, rapidly inactivated at plateau of the action potential, and re-activated gradually at phase 3 of the action potential (Figs. 6 and 7). Therefore, the contribution of cardiac I K1 during the depolarization of cardiac action potential should be revisited.
We found that the amplitudes of both I to.ir and I SS were significantly inhibited by application of spermine in the pipette solution (Fig. 8); this supports the notion that spermine blocks cardiac I K1 or Kir2.1 [11, 29, 42]. However, inactivation of I to.ir was remarkably regulated by different concentrations of free Mg2+ in the pipette solution (Fig. 7), although our previous studies did not find any effect of 5 μM spermine inclusion or Mg2+ omission in the pipette on I to.ir in native cardiac myocytes with 10 min dialysis [25, 26].
The reports for the effect of free Mg2+ i on outward current of classical cardiac I K1 or Kir2.1 channel were controversial and dependent on various experimental conditions. It seems that the Mg2+ i block of outward component in I K1 or Kir2.1 channels is usually observed under conditions where the channel conductance is increased when high K+ o or symmetrical K+ condition is applied to record the current [15, 30] but not under conditions where physiological K+ o is employed in cardiac myocytes [28, 38]. The conductance increase of I K1 or Kir2.1 channels by a high K+ o or symmetrical K+ is likely related to the alteration of channel conformation, which may be highly sensitive to internal block by Mg2+ i .
In the present study, a physiological concentration of K+ o was used to record Kir2.1 channel current; no evidence of Mg2+ blocking effect was observed for this current. Instead, inactivation of I to.ir carried by Kir2.1 channel greatly slowed with the increase of free Mg2+ i (Fig. 7a,b); the component elicited by phase 0 depolarization of the action potential significantly increased as the elevation of free Mg2+ i from 0.03 to 1.0, 4.0, and 8.0 mM without affecting the component activated by phase 3 repolarization of the action potential; and the increase of free Mg2+ i makes the current last long enough to contribute to phase 1 repolarization (Fig. 7c). Presumably, this is likely a complex interaction between Mg2+ and polyamines, with Mg2+ moving in and out of the channel with sufficient rapidity that current can flow but occupying enough to prevent polyamines like spermine from blocking.
It has been reported that cardiac physiological free Mg2+ i is in the range of 0.6–1.1 mM [5, 14, 32]. Thus, duration of I to.ir is significant at physiological free Mg2+ i (Figs. 6 and 7c). Therefore, I to.ir should contribute to action potential during the depolarization under physiological conditions. Due to its rapid activation and inactivation during the depolarization of the channel demonstrated in the previous reports [25, 26] and in the present study, the transient outward component of cardiac I K1 should make a significant contribution to K+ efflux during phase 1 of action potential. The rapid recovery of the transient outward of I K1 from inactivation suggests that its contribution to the action potential should be independent of the heart rate.
However, the potential significance of the Mg2+ i -dependent depolarization-activated component (I to.ir) of I K1 had been ignored for a long time because non-physiological K+ o and/or non-physiological Mg2+ i were used for most previous studies. For instance, if 1 mM MgCl2 is included in a pipette solution containing 5 mM K-ATP, 5 mM EGTA, and 0.1 mM GTP, the calculated free Mg2+ is only 0.014 mM using the Cabuf software (http://www.kuleuven.be/fysiol/trp/cabuf). At <0.03 mM free Mg2+ in pipette solution, I to.ir is quickly inactivated and is not easily differentiated from capacitative transient (Fig. 7a). Based on the Cabuf software, most Mg2+ molecules bind to ATP. In the present and our previous studies, Mg-ATP and 1 mM MgCl2 were applied in pipette solution, and the Mg-ATP may not require Mg2+ molecules to bind, in which significant I to.ir was demonstrated [25, 26].
Cell excitability has generally been associated with the ability of inward currents to generate an action potential upstroke. The classical component of I K1 plays a role in the excitability of cardiac cells by stabilizing the resting potential [7, 33]. Because significant outward current carried by I to.ir can be elicited by depolarization at very negative potentials over a time course comparable to I Na, I to.ir may play a role in maintaining cardiac excitability, especially under conditions where I Na is reduced, such as myocardial ischemia. Earlier work by Murphy et al. demonstrated that cytosolic free Mg2+ i level increased to >2.0 mM during cardiac ischemia [32]. Another study suggested that voltage-dependent changes in V max (maximal velocity) of action potential with increased [K+]o are poorly explained by changes in I Na [41]. This discrepancy may be due to a participation of I to.ir in determining the V max, which becomes particularly important when I Na is reduced.
In summary, human Kir2.1 channel carries a transient outward current (I to.ir) in HEK 293 cells stably expressing the Kir2.1 gene. Properties of the current are similar to those of I to.ir observed in dog and guinea pig ventricular myocytes. Therefore, the present study provides the direct confirmatory information that I to.ir observed in the present study and I to.ir previously observed in native cardiac myocytes shares the same molecular identity. Inactivation of I to.ir is strongly dependent on free Mg2+ i . In native cardiac myocytes, the depolarization-activated I to.ir contributes significantly to phase 1 repolarization of action potential [25, 26] and may play an important role in maintaining cardiac excitability.
References
Barry DM, Nerbonne JM (1996) Myocardial potassium channels: electrophysiological and molecular diversity. Annu Rev Physiol 58:363–394
Beuckelmann DJ, Nabauer M, Erdmann E (1993) Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 73:379–385
Boyle WA, Nerbonne JM (1991) A novel type of depolarization-activated K+ current in isolated adult rat atrial myocytes. Am J Physiol Heart Circ Physiol 260:H1236–H1247
Campbell DL, Qu Y, Rasmusson RL, Strauss HC (1993) The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. II. Closed state reverse use- dependent block by 4-aminopyridine. J Gen Physiol 101:603–626
Chen W, Steenbergen C, Levy LA, Vance J, London RE, Murphy E (1996) Measurement of free Ca2+ in sarcoplasmic reticulum in perfused rabbit heart loaded with 1,2-bis(2-amino-5,6-difluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid by 19F NMR. J Biol Chem 271:7398–7403
Coraboeuf E, Carmeliet E (1982) Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch 392:352–359
Dhamoon AS, Jalife J (2005) The inward rectifier current (I K1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm 2:316–324
Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JMB (2004) Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res 94:1332–1339
Dong MQ, Lau CP, Gao Z, Tseng GN, Li GR (2006) Characterization of recombinant human cardiac KCNQ1/KCNE1 channels (I (Ks)) stably expressed in HEK 293 cells. J Membr Biol 210:183–192
Dukes ID, Morad M (1991) The transient K+ current in rat ventricular myocytes: evaluation of its Ca2+ and Na+ dependence. J Physiol (Lond) 435:395–420
Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM (1994) Spermine and spermidine as gating molecules for inward rectifier K channels. Science 266:1068–1072
Gao Z, Sun HY, Lau CP, Chin-Wan FP, Li GR (2007) Evidence for cystic fibrosis transmembrane conductance regulator chloride current in swine ventricular myocytes. J Mol Cell Cardiol 42:98–105
Giles WR, van Ginneken AC (1985) A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol (Lond) 368:243–264
Headrick JP, Willis RJ (1991) Cytosolic free magnesium in stimulated, hypoxic, and underperfused rat heart. J Mol Cell Cardiol 23:991–999
Ishihara K, Mitsuiye T, Noma A, Takano M (1989) The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J Physiol (Lond) 419:297–320
Jiang B, Sun X, Cao K, Wang R (2002) Endogenous Kv channels in human embryonic kidney (HEK-293) cells. Mol Cell Biochem 238:69–79
Josephson IR, Sanchez-Chapula J, Brown AM (1984) Early outward current in rat single ventricular cells. Circ Res 54:157–162
Kenyon JL, Gibbons WR (1979) 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol 73:139–157
Li GR, Feng J, Wang Z, Fermini B, Nattel S (1995) Comparative mechanisms of 4-aminopyridine-resistant Ito in human and rabbit atrial myocytes. Am J Physiol 269:H463–H472
Li GR, Lau CP, Leung TK, Nattel S (2004) Ionic current abnormalities associated with prolonged action potentials in cardiomyocytes from diseased human right ventricles. Heart Rhythm 1:460–468
Li GR, Sun H, To J, Tse HF, Lau CP (2004) Demonstration of calcium-activated transient outward chloride current and delayed rectifier potassium currents in Swine atrial myocytes. J Mol Cell Cardiol 36:495–504
Li GR, Yang B, Feng J, Bosch RF, Carrier M, Nattel S (1999) Transmembrane I Ca contributes to rate-dependent changes of action potentials in human ventricular myocytes. Am J Physiol Circ Physiol 276:H98–H106
Li GR, Feng J, Yue L, Carrier M (1998) Transmural heterogeneity of action potentials and I to1 in myocytes isolated from the human right ventricle. Am J Physiol Heart Circ Physiol 275:H369–H377
Li GR, Feng J, Yue L, Carrier M, Nattel S (1996) Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res 78:689–696
Li GR, Sun H, Nattel S (1998) Characterization of a transient outward K+ current with inward rectification in canine ventricular myocytes. Am J Physiol Cell Physiol 274:C577–C585
Li GR, Yang B, Sun H, Baumgarten CM (2000) Existence of a transient outward K+ current in guinea pig cardiac myocytes. Am J Physiol Heart Circ Physiol 279:H130–H138
Martin RL, Barrington PL, Ten Eick RE (1994) A 3,4-diaminopyridine-insensitive, Ca(2+)-independent transient outward K+ current in cardiac ventricular myocytes. Am J Physiol Heart Circ Physiol 266:H1286–H1299
Martin RL, Koumi S, Ten Eick RE (1995) Comparison of the effects of internal [Mg2+] on I K1 in cat and guinea-pig cardiac ventricular myocytes. J Mol Cell Cardiol 27:673–691
Matsuda H, Oishi K, Omori K (2003) Voltage-dependent gating and block by internal spermine of the murine inwardly rectifying K+ channel, Kir2.1. J Physiol (Lond) 548:361–371
Matsuda H, Saigusa A, Irisawa H (1987) Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature 325:156–159
Maylie J, Morad M (1984) A transient outward current related to calcium release and development of tension in elephant seal atrial fibres. J Physiol (Lond) 357:267–292
Murphy E, Steenbergen C, Levy LA, Raju B, London RE (1989) Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem 264:5622–5627
Nichols CG, Makhina EN, Pearson WL, Sha Q, Lopatin AN (1996) Inward Rectification and Implications for Cardiac Excitability. Circ Res 78:1–7
Noble D (1984) The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol 353:1–50
Raab-Graham KF, Radeke CM, Vandenberg CA (1994) Molecular cloning and expression of a human heart inward rectifier potassium channel. Neuroreport 5:2501–2505
Sanguinetti MC, Jurkiewicz NK (1990) Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 96:195–215
Shimoni Y, Clark RB, Giles WR (1992) Role of an inwardly rectifying potassium current in rabbit ventricular action potential. J Physiol 448:709–727
Silver MR, DeCoursey TE (1990) Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence or absence of internal Mg2+. J Gen Physiol 96:109–133
Tang Q, Jin MW, Xiang JZ, Dong MQ, Sun HY, Lau CP, Li GR (2007) The membrane permeable calcium chelator BAPTA-AM directly blocks human ether a-go-go-related gene potassium channels stably expressed in HEK 293 cells. Biochem Pharmacol 74:1596–1607
Tseng GN, Hoffman BF (1989) Two components of transient outward current in canine ventricular myocytes. Circ Res 64:633–647
Whalley DW, Wendt DJ, Starmer CF, Rudy Y, Grant AO (1994) Voltage-independent effects of extracellular K+ on the Na+ current and phase 0 of the action potential in isolated cardiac myocytes. Circ Res 75:491–502
Xie LH, John SA, Weiss JN (2002) Spermine block of the strong inward rectifier potassium channel Kir2.1: dual roles of surface charge screening and pore block. J Gen Physiol 120:53–66
Yue L, Feng J, Li GR, Nattel S (1996) Characterization of an ultrarapid delayed rectifier potassium channel involved in canine atrial repolarization. J Physiol (Lond) 496:647–662
Yue L, Feng J, Li GR, Nattel S (1996) Transient outward and delayed rectifier currents in canine atrium: properties and role of isolation methods. Am J Physiol Heart Circ Physiol 270:H2157–H2168
Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL (2001) The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol 533:697–710
Zhabyeyev P, Asai T, Missan S, McDonald TF (2004) Transient outward current carried by inwardly rectifying K+ channels in guinea pig ventricular myocytes dialyzed with low-K+ solution. Am J Physiol Cell Physiol 287:C1396–C1403
Zygmunt AC, Gibbons WR (1992) Properties of the calcium-activated chloride current in heart. J Gen Physiol 99:391–414
Acknowledgment
The study was supported in part by a grant from Sun Chieh Yeh Heart Foundation. We appreciate Dr. Carol A. Vandenberg in University of California at Santa Barbara, CA, USA, for providing us human Kir2.1 channel gene. The authors thank Ms Hai-Ying Sun for the excellent technical support and Dr. G. Droogmans in the Department of Physiology, KU Leuven, Leuven, Belgium for the excellent Cabuf software which makes it possible to accurately calculate free Mg2+ concentrations in pipette solutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, DY., Lau, CP. & Li, GR. Human Kir2.1 channel carries a transient outward potassium current with inward rectification. Pflugers Arch - Eur J Physiol 457, 1275–1285 (2009). https://doi.org/10.1007/s00424-008-0608-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-008-0608-0