Abstract
Insulin secretion inhibitors (ISI) such as adrenaline and somatostatin act on the pancreatic β-cell by a number of mechanisms, one of which is plasma membrane hyperpolarization. Despite the ample evidence for this effect, the principal underlying channels have not been identified thus far. The G protein-gated inwardly rectifying potassium (Kir3.x/GIRK) channels, which are responsible for hyperpolarization in other excitable tissues, are likely candidates. In this paper, we show that GIRK channels are expressed and functional in mouse pancreatic islet cells. Reverse transcription polymerase chain reaction analysis revealed all four GIRK gene products in islet tissue. Immunofluorescent labeling of pancreatic sections demonstrated exclusive islet localization of all GIRK subunits, in part within insulin-expressing cells. Using the whole-cell configuration of the patch clamp technique, we found that the application of tertiapin-Q, a selective inhibitor of the GIRK channels, abolishes adrenaline-mediated inward currents and strongly attenuates adrenaline-induced hyperpolarization in a reversible manner. These results imply that GIRK channels are responsible for a major part of the electrical response to adrenaline in islet cells and suggest a role for these channels in pancreatic physiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose-stimulated insulin secretion (GSIS) is mediated by plasma membrane depolarization resulting from the closure of ATP-sensitive K+ channels (KATP) by ATP/ADP ratio increase following glucose metabolism. This depolarization leads to the opening of L-type calcium channels, a rise in cytoplasmic calcium levels, and a consequent fusion of insulin granules to the plasma membrane and insulin release (reviewed in [46]). Several Gi/o protein-coupled receptor (Gi/oPCR)-activating ligands, most notably catecholamines, somatostatin, and galanin, are known as GSIS inhibitors (ISIs). These agents act on the pancreatic β-cell through a number of mechanisms [50], one of which is the hyperpolarization of membrane potential through an increase in K+ conductance [1, 45, 51, 53]
The G protein-gated inwardly rectifying K+ potassium channel (GIRK) is activated in response to many PTX-sensitive G protein-coupled receptor ligands [62], including noradrenaline [5], somatostatin [31], and galanin [52]. The GIRK channel is expressed mainly in excitable cells, where it serves to reduce electrical activity in response to neurotransmitters and hormones. The channel is a tetramer constructed of various combinations of the four GIRK family subunits (GIRK1–4). While GIRK1 is expressed virtually ubiquitously in GIRK-expressing tissues, GIRK2 and GIRK3 are expressed primarily in neuronal tissues, and GIRK4 is expressed mainly in cardiac cells [62]. Although GIRK subunit gene expression has been detected in a variety of tissues [13, 62], little work has been published to date regarding GIRK functionality and roles in cells other than neuronal and cardiac. Evidence for GIRK subunit expression in the pancreas [23, 25, 49, 56, 58] and pancreatic islets [18, 64] of various organisms and in insulinoma [4, 11, 13, 54, 55, 57] is particularly ample.
The identity of the G protein reactive channels in β-cells is still unknown. While some evidence point to the reactivation of KATP channels [2, 8, 9, 15, 16, 19, 43, 48, 65], other findings contradict this notion [45] or suggest additional or alternative channel targets [1, 10, 21, 51, 53]. Rorsman et al. ascribed a low unitary conductance to the clonidine-activated outward current at −20 mV holding potential, which is in agreement with the strong inward rectification of GIRK [45], while the work of Smith et al. demonstrated that somatostatin activates a channel with kinetics and conductance similar to those attributed to GIRK in the MIN-6 insulinoma line [53]. These two reports argue in favor of GIRK channel activation in response to the respective stimuli in β-cells. In addition, two reports have noted similar single-channel properties of somatostatin-activated currents in pancreatic α-cells [20, 64].
In this work, we perform systematic reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence studies, and present pharmacological evidence for the functionality of the GIRK channel in pancreatic islet cells. We show that all four GIRK subunits are transcribed in the islets, and demonstrate, for the first time, that the GIRK1 and GIRK3 subunits are present in these cells at the protein level, with GIRK1 localized mostly intracellularly. In addition, we show that GIRK channels are responsible for the major part of adrenaline action on both potassium conductance and membrane potential changes in islet cells. Our data may indicate that the importance of GIRK channels is not limited to neural and cardiac systems, and suggest that defects in GIRK subunits may be involved in certain types of β-cells abnormal function.
Materials and methods
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Weizmann Institute of Science.
RT-PCR
Total RNA was extracted from a 10-week-old male NMRI mouse freshly isolated islets and from INS-1E and MIN-6 insulinoma lines using the RNeasy mini kit (Qiagen, Hilden, Germany). RT-PCR reactions were performed using the OneStep RT-PCR kit (Qiagen). Following calibration experiments using actin primer sets, 20 ng of islet RNA (equivalent to 0.5 islets) and 5 ng of each cell-line RNA were used as templates in each reaction. As positive and negative controls, cerebellum RNA (10 ng) and no RNA containing reactions were used, respectively. An additional control using exocrine pancreas RNA (100 ng) from the same mouse was also included to examine the possibility that products in the islet samples arise from exocrine tissue contamination. For GIRK1 and GIRK2c, ten times lower RNA concentrations of each specimen were used. Reaction conditions were 30 min at 50°C (55°C for GIRK1 and GIRK2c), 15 min at 94°C, [30 s at 94°C, 30 s at 55°C (58°C for GIRK1 and GIRK2c), 60 s at 72°C] for 35 cycles and 10 min at 72°C. For the GIRK3 reaction, a second, nested PCR was performed, using 1 μl of the first reaction product, primers as specified below, a ReddyMix PCR master mix (ABgene, Epsom, UK), and reaction conditions of [30 s at 94°C, 30 s at 55°C, 60 s at 72°C] for 15 cycles and 10 min in 72°C. RT-PCR product locations (from CDS start) for the different gene products were: GIRK1, 435–1,100; GIRK2a, 914–1,431; GIRK2c, 914–1,282; GIRK3, 692–973; GIRK4, 739–1270. All expected products were designed to span introns in their corresponding genomic DNA sequences to avoid genomic DNA contamination artifacts. Primer sequences were (CEx., number of coding exon; nt, primer position from coding sequence start; length, length of major expected product):
-
GIRK1: sense, CTATGGCTACCGCTACATCACCG (CEx. 1, nt 435); antisense, ATGAGAAGCATTTCTTCCTGCTC (CEx. 3, nt 1,100); length 666 bp.
-
GIRK2a: sense, AGATTGTGGTCATCCTGGAG (CEx. 2, nt 914); antisense, CCCCGACTTTAAGTAAATTAT (CEx. 4, nt 1,431); length 518 bp.
-
GIRK2c: sense, AGATTGTGGTCATCCTGGAG (CEx. 2, nt 914); antisense, GGGCCTATACTTTGGATTCA (CEx. 3, nt 1,282); length 369 bp.
-
GIRK3: sense, CCATCCGAGCCAAGCTCATC (CEx. 1, nt 611); antisense, CTGCTGGGGATGGACCAGTA (CEx. 2, nt 1,067); nested sense, GCTTTGACACGGGGGACG (CEx. 1, nt 692); nested antisense, TTTCGTGGAAGCTGGCGTAG (CEx. 2, nt 973); length 282 bp.
-
GIRK4: sense, GAGAATTCATCCCCTTGAACC (CEx. 1, nt 739); antisense, GCACACAGACTTCACATTGAGC (CEx. 2, nt 1,270); length 532 bp.
Immunofluorescence
For immunofluorescence studies of pancreatic sections, NMRI mice were killed by cervical dislocation; the pancreas was removed, fixed in 4% paraformaldehyde in PBS for 2 h at 4°C, and transferred into 30% sucrose in PBS for overnight cryoprotection at 4°C. After embedding in Tissue-TEK O.C.T compound (Sakura, Tokyo, Japan), 8 μm sections were cut using a cryostat and thaw mounted on SuperFrost Plus microscope slides (Menzel-Glaser, Germany). Blocking was done with 10% FCS (Biological Industries Israel, Beit Haemek) in TBST (150 mM NaCl, 0.1% triton X-100, and 50 mM Tris–Cl pH 7.4). Following an overnight incubation with primary antibody at 4°C, wash in TBST, and 1 h incubation with secondary antibody at room temperature, slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA) or GEL/MOUNT™ (Biømeda, Foster City, CA, USA) and covered with glass coverslips. For immunofluorescence studies of the INS-1E line, cells were grown on poly-l-lysine-coated glass coverslips, washed in PBS, and fixed in 4% PFA in PBS for 20 min. After 30 min in blocking solution (1% glycine, 2% BSA, 10% FCS, 0.1% triton X-100, 150 mM NaCl, 10 mM Tris–Cl, pH 7.5), samples were incubated for 1 h with primary antibody in blocking solution, washed three times in PBS, incubated for 1 h in 1:100 secondary antibody in blocking solution, washed again in PBS, and coverslips were placed on slides overlaid with mowiol. GIRK subunit staining was performed with rabbit polyclonal anti-GIRK subunit-specific antibodies (Alomone, Jerusalem, Israel; 1:66 from the concentration specified by the manufacturer) and cyanine Cy3 conjugated donkey anti-rabbit IgG (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). For double staining of GIRK subunits and islet hormones, guinea pig anti-human insulin (1:200), guinea pig anti-rat pancreatic polypeptide (PP; 1:500; both from LINCO Research, St. Charles, MO, USA), goat anti-human glucagon (1:100), and goat anti-human somatostatin (SS; 1:50; both from Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. As secondary antibodies in colabeling experiments, cyanine Cy5 (for costaining with insulin) or Cy2 (for costaining with other hormones) donkey anti-rabbit IgG and either cyanine Cy3 conjugated bovine anti-goat or rhodamine RX conjugated donkey anti-guinea pig IgG (all from Jackson) were used. All secondary antibodies were used in 1:200 dilutions. Activities and specificities of the α-GIRK antibodies were verified by immunostaining of subunit-specific transfected HEK293 cells (data not shown). Appropriate controls were set to ensure no cross-reactivities and cross-signaling. The samples were examined with a Zeiss LSM 510 confocal microscope.
Islet isolation and cell dispersion
Islets were isolated by enzymatic digestion. NMRI mice (6–10 weeks) were killed by cervical dislocation, and HBSS containing Liberase RI (0.25 μg ml−1; Roche, Indianapolis, IN, USA) and DNAseI (10 μg ml−1; Roche) was injected to the common duct. Following removal and incubation at 37°C, the pancreas was disrupted by a blunted Pasteur pipette, washed three times in HBSS+DNAseI, and islets were handpicked twice into HBSS and dispersed into single cells immediately. Dispersion was performed using Dispase II (0.6 U ml−1; Roche) essentially as described by Josefsen et al. [28], and cells were plated on coverslips and maintained in RPMI1640 containing 10% fetal calf serum and 1:100 Pen-Strep solution (both from Biological Industries Israel, Beit Haemek).
Electrophysiology
Patch clamp studies were performed on 1- to 3-day-old cultured cells. To maximize the β-cells fraction among recorded cells, only cells with diameter ≥11 μm were selected for clamping. Patch pipettes were fabricated from borosilicate glass capillaries and fire polished. Pipette resistance using the solutions specified below ranged from 2 to 5 MΩ. Currents were recorded using an Axopatch 200B (Axon Instruments) patch clamp amplifier. Signals were analog filtered using a 1-kHz low pass Bessel filter. For membrane potential measurements and current clamp studies under the standard whole-cell configuration, we used a bath solution containing (in mM): 140 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 11 d-glucose, 10 HEPES (pH 7.4 with NaOH). The pipette filling solution contained (in mM): 107 KCl, 1.2 MgCl2, 1 CaCl2, 5 HEPES (pH 7.2) 2 Na2ATP, 0.3 LiGTP. For current measurements in the perforated patch configuration, the bath solution contained (in mM): 140 KCl, 1.2 MgCl2, 2.6 CaCl2, 11 d-glucose, 5 HEPES (pH 7.4 with KOH) and the pipette solution was (in mM): 30 KCl, 95 K-gluconate, 1 MgCl2, 1.6 NaH2PO4, 4.8 Na2HPO4 (pH 7.2), nystatin 120 μg ml−1. To keep the cells in a native ionic environment as long as possible, seal was established in the low potassium bath solution mentioned above, and switching to high potassium was performed following the perforated patch formation usually within 5–10 min. Current–voltage (I–V) studies were performed on the same cells following the steady voltage recordings. The I–V protocol consisted of 120 ms voltage steps from −120 to +50 mV in 10 mV increments, from a holding potential of −90 mV with 1 s interval between steps. All measurements were performed at room temperature. Data acquisition and analysis were done using the pCLAMP8.2 software package (Axon Instruments). Values in text and graphs are presented as the mean±SE.
Results
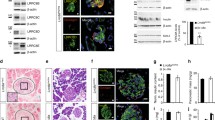
To examine the expression of GIRK channel subunits in pancreatic islet cells, we applied both RT-PCR and immunofluorescence studies. RT-PCR analysis was performed on mouse islets and two insulinoma lines, the rat-derived INS-1E [3, 35] and the mouse-derived MIN-6 [37]. Products of the expected sizes for all four GIRK subunit transcripts were found in mouse islets (Fig. 1a) and their identity confirmed by either an additional, nested reaction or by sequencing. In the case of GIRK1, where sense and antisense primers were located on coding exons 1 and 3, respectively, three products were evident in both the cerebellum and islets samples. In addition to a major band of the expected main GIRK1 transcript size, shorter faint bands were consistently obtained. Sequencing revealed that the shortest product lacks the entire GIRK1 second coding exon and may correspond to a previously described alternative GIRK1 splice variant [38, 66], while an additional band of intermediate size lacks only a part of the exon and may represent a novel, less abundant splice variant (Fig. 1b). This novel variant, termed GIRK1f, results from the splicing out of a novel intron within the second coding exon, whose boundaries are in agreement with splice site consensus sequences. GIRK1f is expected to yield a truncated protein of 270 amino acids, differing from the full GIRK1 form in its last 30 amino acids, with an N-glycosylation site at amino acids 254–257. Only the full-length product was evident in the INS-1E and, faintly, in the MIN-6 samples.
a RT-PCR analysis of GIRK subunits in mouse islets, insulinoma cell lines, and controls. Numbers in parentheses denote the expected product size for each primer set. C cerebellum, e exocrine pancreas, is islets, in INS-1E cells, m MIN-6 cells, 0 no RNA template. b Partial structures of GIRK1 (top) and GIRK2 (bottom) splice variants in islets and cerebellum. Upper and lower case letters, respectively, mark exon and intron sequences of the novel variant boundaries. Numbers below the GIRK1f structure mark the positions of the exon–intron boundaries from the consensus GIRK1 starting codon. The stop codon of the GIRK2c variant is boxed in the sequence below GIRK2c-1
A number of GIRK2 splice variants, differing in their tissue distribution and physiological characteristics, have been described to date [24, 59]. We analyzed the two major variants GIRK2a and GIRK2c [24] separately using different primer sets. Both GIRK2a and GIRK2c transcripts were detected in islets and in both insulinoma cell lines, but not in the exocrine pancreas. For GIRK2a, an additional, weaker band, 48-bp longer than the main, expected product, could be seen in the cerebellum, islet, and MIN-6 samples, but not in the INS-1E sample. Sequencing revealed that this product is probably a novel splice variant carrying an extension of exon 6a into exon 6b [59] terminated at a splice site consensus sequence, and resulting in a protein product similar to GIRK2c in that region (Fig. 1b). GIRK3 and GIRK4 transcripts were found in the islets but could not be detected in both cell lines even following a second round of PCR using the same or nested primers.
Immunofluorescence studies were performed on mouse pancreatic sections and on Ins-1E cells. In pancreatic sections, islet-specific staining was observed for all four GIRK subunits (Fig. 2 and Supplementary Figs. 1, 2, and 3). The immunofluorescent signal appeared to prevail throughout most, but not all, islet cells for all subunits, and signal intensities varied between cells. In the α-GIRK2, α-GIRK3, and α-GIRK4 samples, some cells appeared to have a clearly more intense signal than the general population. α-GIRK1 immunoreactivity was interestingly concentrated in an intracellular compartment (Fig. 3b). Antibody preabsorption with the respective immunogens led to the complete loss of the islet-specific signal for all subunits (data not shown).
a Anti-GIRK subunit staining in INS-1E cells. For GIRK2, three different fields demonstrating different intracellular localizations are shown. The bottom right field is a magnification of the squared area in the bottom left field. Bar = 10 μm (5 μm in magnification). b A magnification of a region within an islet demonstrating the perinuclear localization of GIRK1. Bar = 10 μm
The pancreatic islet is composed of four cell types. In addition to the insulin-producing β-cells, which constitute the majority of the islet cell population, three other cell types, mainly located at the periphery of the islet, are distinguished by the production of different hormones: the glucagon-producing α-cells, the somatostatin (SS)-producing δ-cells, and the pancreatic polypeptide-producing PP cells. The uneven α-GIRK staining patterns prompted us to investigate the correlation between GIRK staining and different islet cell types by double immunolabeling of each channel subunit versus each islet hormone. Observations of the staining patterns indicated a rather different expression pattern for each subunit and each cells type, and are concluded in Table 1.
Although α-GIRK1 immunoreactivity was evident in most cells, double staining with islet hormones indicated that GIRK1 expression is almost exclusively restricted to insulin-labeled cells (Fig. 2 and Supplementary Figs. 1, 2, and 3). Immunolabeling of the other subunits, however, revealed more complex patterns, and strongly labeled cells appeared to correlate with α-glucagon and α-somatostatin in different patterns: α-glucagon-labeled cells appeared to colocalize almost completely with strong α-GIRK2 staining and to a high degree with strong α-GIRK3 and α-GIRK4 staining (Supplementary Fig. 1). α-Somatostatin labeling almost completely colocalized with strong α-GIRK4 staining and partially colocalized with strong α-GIRK2 staining, but not with strong α-GIRK3 staining (Supplementary Fig. 2). α-Insulin labeling usually corresponded with a weaker α-GIRK2, α-GIRK3, and α-GIRK4 staining (Fig. 2). In α-PP-labeled cells, no labeling of any α-GIRK subunit antibodies could be observed (Supplementary Fig. 3).
Specific GIRK1 and GIRK2 labeling could also be seen in INS-1E cells (Fig. 3a). Similar to mouse islets, α-GIRK1 immunoreactivity was restricted to perinuclear compartments. GIRK2 staining was scattered throughout most cells, but stronger specific signals were occasionally detected on the plasma membrane or in intracellular structures in a pattern similar to that of α-GIRK1 immunoreactivity. Specific weak α-GIRK3 staining was seen throughout the cells. This signal was, however, sometimes difficult to discriminate from nonspecific signal. A weak α-GIRK4 signal was evident only upon strong exposure in INS-1E cells and appeared to be nonspecific as it existed also in the antigen preabsorbed control sample (Fig. 3a).
To study GIRK activity in pancreatic islet cells, we recorded whole-cell plasma membrane currents under the perforated patch configuration using 140 mM potassium in the extracellular solution at a holding potential of −90 mV. To block residual KATP currents and to eliminate a possible effect of adrenaline on KATP channels, tolbutamide (100 μM) was applied to the cells throughout the experiment. Under these conditions, current density was 11.2 ± 2.2 pA pF−1. This current was defined as I basal. The application of adrenaline (5 μM) evoked an inward current in 15 of 23 cells recorded (65%; Fig. 4a). Interestingly, adrenaline caused a considerable current inhibition in four of the eight remaining cells (data not shown). These cells were not further studied. The mean adrenaline-induced current density (I adren) in the positively responding cells was 6.6 ± 1.6 pA pF−1 (n = 15; Fig. 4b). Addition of the GIRK-selective toxin tertiapin-Q (Tpn-Q) at 100 nM had a profound inhibitory effect on both I basal and I total (i.e., I basal + I adren) within seconds in all cells recorded. Tpn-Q current density inhibition in the positively responding cells in the presence of adrenaline (total I Tpn-Q) was 8.0 ± 2.0 pA pF−1 (n = 15). After subtraction of basal I Tpn-Q, the calculated value for induced I Tpn-Q was 6.4 ± 1.8 pA pF−1. The average Tpn-Q percent inhibition of the adrenaline induced current was 102 ± 10%. Tpn-Q effects on both basal and induced currents were reversible within seconds in all cells (Fig. 4a).
Current response of dispersed β-cells to adrenaline and Tpn-Q. a Current traces of two adrenaline-responsive cells (tpn Tpn-Q). Top a cell exhibiting low basal currents, an incomplete inhibition of I adren by Tpn-Q, and no basal I Tpn-Q. Bottom a cell exhibiting both basal and induced I Tpn-Q. b I adren in positively responding cells before and during Tpn-Q application and after Tpn-Q washout (n = 15). AD adrenaline. c Rectification of dispersed islet cells responses to adrenaline and Tpn-Q. Top steady state I–V curve of the mean adrenaline-activated current (n = 3). Center steady state I–V curve of the mean total Tpn-Q inhibited current in the presence of adrenaline in cells demonstrating adrenaline-activated current (n = 3). Bottom steady state I–V curve of the mean basal Tpn-Q inhibited current (n = 5)
Tpn-Q has been shown to block other channels beside GIRK with similar potency [27, 29]. To verify the origin of I adren and I Tpn-Q, we examined their voltage dependence using a voltage step protocol. The adrenaline-activated current appeared to be strongly inwardly rectifying. However, an additional, inhibitory voltage-dependent effect, peaking at −20 to −10 mV, was evident and was sufficient to reverse the total adrenaline effect to inhibitory at −10 mV (Fig. 4c, top). Both basal and total I Tpn-Q effects were strongly inwardly rectifying. Interestingly, a voltage-dependent inhibitory effect with similar properties to that of adrenaline was seen in the case of basal, but not total, I Tpn-Q (Fig. 4c, center and bottom).
Adrenaline has been reported to hyperpolarize glucose- or sulfonylurea-treated, electrically active islet and insulinoma cells to an extent of complete cessation of action potential firing. Since Tpn-Q blocked most of the adrenaline-activated current, it seemed probable that Tpn-Q block may suffice to depolarize adrenaline-treated cells to a potential in which firing will resume. To investigate the effect of adrenaline and Tpn-Q on the islet cell membrane potential, we recorded the potential changes caused by these agents in the standard whole-cell mode. This set of experiments was performed on cells depolarized by tolbutamide, with 2 mM ATP concentration in the pipette. This ATP concentration was found to be low enough to keep most of the cells at a hyperpolarized, nonexcitatory potential in our experimental system. Following patch formation (with 11 mM glucose in the bath solution), the membrane potential gradually hyperpolarized within a few minutes until stabilizing at a value of −61.1 ± 1.4 mV (n = 35). The addition of 100 μM tolbutamide depolarized the cells to an average potential of −38.1 ± 2.2 mV and initiated action potential activity of variable amplitude, frequency, and pattern. In 20% of the cells (7/35), current injection of up to ±5 pA was required to achieve a basal membrane potential of around −40 mV. The addition of 5 μM adrenaline hyperpolarized the cell membrane in 49% (17/35) of the cells by an average of 18.8 ± 2.0 mV (from −36.9 ± 1.9 to −54.9 ± 2.2 mV; Fig. 5a, b). Electrical activity was abolished following adrenaline application in 88% (15/17) of the responsive cells.
Membrane potential response of dispersed β-cells to adrenaline and Tpn-Q. a A representative membrane potential trace of an adrenaline- and Tpn-Q-responsive cell. b Average membrane potential values of adrenaline-responsive cells during the course of the experiment from left to right (n = 17). Tol 100 μM tolbutamide, AD 5 μM adrenaline, Tpn-Q 100 nM tertiapin-Q. c A histogram depicting current and voltage adrenaline effects in adrenaline-responsive cells (n = 12)
Application of Tpn-Q (100 nM) had a depolarizing effect in 88% (15/17) of the adrenaline-reactive cells. The response to Tpn-Q averaged 12.7 ± 2.2 mV (69.7 ± 9.5%) in the adrenaline-reactive cells, bringing their average potential to −42.2 ± 2.8 mV (Fig. 5a,b). Electrical activity was resumed in 53% (8/15) of the cells in which it had been extinguished by adrenaline. In three cells, Tpn-Q effect exceeded that of adrenaline, leading to a more depolarized potential than in the absence of both substances, thus indicating the existence of a basal Tpn-Q-sensitive current at least in these cells.
To study the relations between the effects of adrenaline and Tpn-Q on the membrane potential and their effect on inward currents, we switched to the voltage clamp mode in 26 of the recorded cells (12 adrenaline-responsive and 14 nonresponsive) and measured the currents at −90 mV using a high [K+] bath solution containing tolbutamide as in the voltage clamp experiments described above. Adrenaline caused an inward current activation only in cells that hyperpolarized in response to adrenaline in the current clamp mode, with a density of 33.0 pA pF−1. Current and potential responses were correlated up to a value of 30–40 pA pF−1, which sufficed to achieve approximately 25 mV hyperpolarization, and cells that exhibited higher current activation showed no further hyperpolarization (Fig. 5c). Tpn-Q inhibition in the presence of adrenaline was 19.2 ± 5.4 pA pF−1 in the adrenaline-responsive cells, with an average percent inhibition of 86.8 ± 4.2%. Since basal I Tpn-Q values were not measured in this experiment, this value represents the total I Tpn-Q and is, therefore, an overestimation of the actual Tpn-Q-sensitive fraction.
Discussion
In this paper, we present evidence for the presence of all four GIRK channel subunits in pancreatic islets, and demonstrate that this channel is functional and responsible for most of the electrical response to adrenaline, a potent GSIS inhibitor, in dissociated islet cells. Our work is the first to provide pharmacological evidence for the involvement of GIRK in islet cell physiology. In addition, our findings concerning GIRK expression patterns in the islets differ in some details from published data.
It has been previously shown that GIRK subunit expression in the islet is selective and spatially variable. Yoshimoto et al. reported GIRK4 subunit expression in all islet cells, a non-β-cell confined GIRK2c expression and a lack of GIRK1 and GIRK3 expression [64]. Other reports, using cloning and RT-PCR strategies, provided indications for GIRK1 expression in islets and insulinoma cell lines [11, 13, 18, 54]. Low levels of GIRK3 expression in human pancreas have been detected by RT-PCR [58] but not by RNA hybridization [49], a discrepancy that may be explained by islet-restricted expression. In this paper, using both RT-PCR and immunostaining, we provide evidence for the expression of all four GIRK subunits in the islets, with partial overlap with insulin immunoreactivity at the cellular level (Figs. 1, 2, and 3b). Our observations indicate that while GIRK1 expression is restricted to β-cells, other GIRK subunits are more potently expressed in α- and δ-cells, and PP cells are apparently GIRK-free.
α-GIRK1 immunoreactivity appeared to localize to an intracellular compartment (Figs. 3 and 4a). This finding is intriguing in light of the emerging interest in the intracellular trafficking of ion channels in general and GIRK in particular [34, 36]. Existing data suggest that GIRK1 lacks ER export signal and is consequently dependent on coassembly with other subunits for proper targeting to the plasma membrane. In light of the relatively low levels of other GIRK subunits in β-cells, the only cell type that appears to express GIRK1 in the islet (Fig. 2 and Supplementary Figs. 1, 2, and 3), it may be suggested that most GIRK1 molecules are trapped in the ER due to the shortage of membrane-targeting partners. A similar intracellular pattern was obtained in our study by immunostaining of cultured rat hippocampal neurons (data not shown) and in other studies using the same antibody in rat retinal ganglion cells [6], deep dorsal horn neurons [12], a glioma cell line, and rat spinal cord astrocytes [40].
Hyperpolarization of dispersed mice islet cells in response to GSIS inhibitors has been demonstrated by a number of groups using both electrophysiological and fluorometry techniques [10, 39, 42, 45, 51]. We found the electrical response of islet cells to adrenaline inconsistent and variable from culture to culture. We failed to identify any parameter related to the islet isolation and cell dispersion procedures or to culture conditions that had a clear effect on the magnitude and prevalence of the response. Nonetheless, almost whenever a clear and robust adrenaline response was observed, it was effectively inhibited by Tpn-Q application. Tertiapin effect on the electrical response to adrenaline in mouse has been examined by Sieg et al. [51] who could not recognize any effect of the toxin in SUR-1 knockout mice. Two major differences between that study and ours may explain the discrepancy between the reports: First, Sieg et al. used 10 nM tertiapin, a concentration which is close to the K i of GIRK inhibition [26], while we used a tenfold higher concentration of a more stable toxin variant, tertiapin-Q [27]. The use of optimal blocker concentrations may be critical especially in membrane potential measurements, in which the residual nonblocked conductance may be sufficient to preserve the membrane potential close to its maximal effect. In addition, in the study of Sieg et al., adrenaline effect was compared between tertiapin-treated and untreated cells, while we applied adrenaline first and compared the membrane potential before and after Tpn-Q addition on the same cell, a procedure that avoids the complexity caused by cell to cell variance in basal membrane potential and adrenaline responsiveness. Interestingly, the reported data of Sieg et al. show a trend for a reduced magnitude of the membrane potential response to adrenaline in the presence of tertiapin, but not other tested agents. Considering the notes above, this trend may well be explained by a substantial involvement of tertiapin-sensitive currents in the mediation of the electrical response of islet cells to adrenaline.
Tertiapin has also been shown to block ROMK channels in nanomolar concentrations [26, 27]. Although ROMK is expressed in pancreatic islets [63] (Iwanir and Reuveny, unpublished results), it is unlikely that this channel mediates the response to adrenaline, as there is no evidence for its coupling to G proteins and activation by their receptor ligands. Our I–V studies indicate that the main component of the adrenaline-activated current and of the total and basal tertiapin-sensitive currents is strongly inwardly rectifying (Fig. 4c), a key feature of the GIRK channel, which discriminates it from the mildly rectifying ROMK [22]. It appears improbable also that the Tpn-Q effect resulted from basal or adrenaline-induced KATP current inhibition as our experiments were performed in saturating bath concentrations of tolbutamide. In addition, the toxin has no effect on native KATP currents in rabbit cardiac myocytes [30]. Therefore, we believe that our results strongly indicate that adrenaline-induced hyperpolarization is largely mediated through GIRK channel activation. However, in spite of the strong inward rectification of basal I Tpn-Q, we cannot rule out a possible involvement of ROMK channels in the basal Tpn-Q-sensitive currents that were clearly evident in most of the recorded cells. Alternatively, these currents may be the outcome of basal GIRK activity, whose magnitude and relative fraction from total activity is system- and condition-dependent [14, 17, 30, 41, 60, 61].
Recently, Tpn-Q was demonstrated to inhibit the voltage-dependent Ca+2-activated K+ channel (BK) in a use-dependent manner [29]. This inhibition appears irrelevant to our membrane potential and steady voltage clamp experiments, in which the Tpn-Q effect was instantaneous and occurred in a hyperpolarized potential. Nevertheless, it is probable that the voltage-dependent component of basal I Tpn-Q originates from BK channels, as the continuous application of positive potentials during the experiment may allow at least partial preactivation of the channel, rendering it sensitive to the toxin. Interestingly, a similar voltage-dependent current was inhibited by adrenaline (Fig. 4c, top). The absence of such a component in the total I Tpn-Q suggests that adrenaline and Tpn-Q inhibit the same current, which was not detected by the total I Tpn-Q measurement since it had been already inhibited by adrenaline. While BK channels are stimulated by α1- and β-adrenergic pathway activation in other tissues [7, 32, 47], they are inhibited by somatostatin in the HIT insulinoma cells [44]. Since the α2-adrenoceptor-mediated inhibitory action of adrenaline predominates in β-cells, it appears reasonable that adrenaline leads to BK inhibition in these cells.
As insulin-producers, β-cells are considered the primary cell type in the islet. Correspondingly, the role and actions of adrenaline in β-cells have received most attention compared to other islet cell types. Islet cell types differ in size, capacitance, and various electrical properties, most prominently the glucose dependency of the resting potential. For our recordings, we selected only cells ≥11 μm in diameter, practically eliminating the smaller α-cells. The capacitance and resting potential of the recorded cell population in general and of the adrenaline-responsive cells in particular were normally distributed in both the voltage and the current clamp experiments, and cells at the extremities of the distribution curves did not display extraordinary electrical properties or adrenaline/Tpn-Q responsiveness, suggesting that most, if not all, cells belong to the same, or similar, subpopulation. Since β-cells are the main cell type of the islet, and since noradrenaline has been shown to have no effect on the membrane potential of α-cells [64], it is very unlikely that the latter constitute a significant fraction of our entire sample and of the adrenaline-responsive cell population. δ- and PP cells, however, cannot be unequivocally discriminated from β-cells, and their adrenaline responsiveness has not been explored. In light of the apparent differences of GIRK subunit expression profiles between islet cell types, it is tempting to speculate that different I Tpn-Q levels and adrenaline responsiveness patterns are associated with different cell types. The absence of GIRK1 and excess of other subunits in α- and δ-cells (Supplementary Figs. 1 and 2) suggest that GIRK channels in these cells may act differently than in β-cells. It is generally agreed that non-GIRK1-containing GIRK channels have both basal and total lower conductance than the “classic” GIRK1/2 and GIRK1/4 types [49, 60]. However, the relative abundance of GIRK2, GIRK3, and GIRK4 in α- and δ-cells renders it difficult to ascribe certain behaviors to certain cell types, although we can hypothesize that poor adrenaline sensitivity, which was generally correlated with low to absent basal I Tpn-Q, is associated with PP cells. As there is no documented evidence that channel composition may effect the direction of the GIRK response to G protein activation, the inhibitory effect of adrenaline on inward currents seen in some cells is more likely to result from extrinsic regulatory mechanisms such as the previously documented inhibitory effect of PIP2 depletion or Gβ5 subunit-mediated regulation [33] rather than from channel composition. This does not exclude, however, the possibility that these cells indeed represent a different cell type, most likely δ-cells. Nevertheless, it is more than likely that β-cells constitute most of the entire sample and of the adrenaline-responsive population in both the voltage and current clamp experiments.
While previous studies failed to find a correlation between ISIs-induced hyperpolarization and ionic currents [45, 51, 53], our results clearly demonstrate the effect of adrenaline in both the current and voltage clamp modes. Moreover, by switching from current to voltage clamp in the same cell, we were able to establish a clear correlation between the effects of adrenaline on whole-cell currents and on the membrane potential. According to our results, conductance density activation of approximately 300 pS pF−1 was sufficient for a maximal adrenaline effect on the membrane potential. Much higher values were occasionally recorded, demonstrating the existence of some “reserve” channels. This may provide a sensitive mechanism to establish a full-blown physiological response following the activation of just a fraction of the receptors at low physiological adrenaline concentrations.
To summarize, in this paper, we provide evidence for GIRK channel subunit expression in pancreatic islet cells, and show that the channel is functional and responsible for most of the electrical response to the insulin secretion inhibitor adrenaline in these cells. Future experiments are needed to reveal the role of this electrical response in the inhibitory effect of adrenaline on insulin secretion, which may be complex in light of the composite effect of adrenaline in islet cells, which involves multiple parallel effector systems [50].
References
Abel KB, Lehr S, Ullrich S (1996) Adrenaline-, not somatostatin-induced hyperpolarization is accompanied by a sustained inhibition of insulin secretion in INS-1 cells. Activation of sulphonylurea K+ATP channels is not involved. Pflugers Arch 432:89–96
Ahren B, Berggren PO, Bokvist K, Rorsman P (1989) Does galanin inhibit insulin secretion by opening of the ATP-regulated K+ channel in the beta-cell? Peptides 10:453–457
Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167–178
Bond CT, Ammala C, Ashfield R, Blair TA, Gribble F, Khan RN, Lee K, Proks P, Rowe IC, Sakura H et al (1995) Cloning and functional expression of the cDNA encoding an inwardly-rectifying potassium channel expressed in pancreatic beta-cells and in the brain. FEBS Lett 367:61–66
Bunemann M, Bucheler MM, Philipp M, Lohse MJ, Hein L (2001) Activation and deactivation kinetics of alpha 2A- and alpha 2C-adrenergic receptor-activated G protein-activated inwardly rectifying K+ channel currents. J Biol Chem 276:47512–47517
Chen L, Yu YC, Zhao JW, Yang XL (2004) Inwardly rectifying potassium channels in rat retinal ganglion cells. Eur J Neurosci 20:956–964
Chung S, Soh H, Uhm D (1999) Beta-adrenergic modulation of maxi-K channels in vascular smooth muscle via Gi through a membrane-delimited pathway. Pflugers Arch 437:508–510
de Weille J, Schmid-Antomarchi H, Fosset M, Lazdunski M (1988) ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. Proc Natl Acad Sci U S A 85:1312–1316
de Weille JR, Schmid-Antomarchi H, Fosset M, Lazdunski M (1989) Regulation of ATP-sensitive K+ channels in insulinoma cells: activation by somatostatin and protein kinase C and the role of cAMP. Proc Natl Acad Sci U S A 86:2971–2975
Debuyser A, Drews G, Henquin JC (1991) Adrenaline inhibition of insulin release: role of the repolarization of the B cell membrane. Pflugers Arch 419:131–137
DePaoli AM, Bell GI, Stoffel M (1994) G protein-activated inwardly rectifying potassium channel (GIRK1/KGA) mRNA in adult rat heart and brain by in situ hybridization histochemistry. Mol Cell Neurosci 5:515–522
Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F (2003) Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci 6:274–281
Dixon AK, Gubitz AK, Ashford ML, Richardson PJ, Freeman TC (1995) Distribution of mRNA encoding the inwardly rectifying K+ channel, BIR1 in rat tissues. FEBS Lett 374:135–140
Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U (2005) The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation 112:3697–3706
Drews G, Debuyser A, Henquin JC (1994) Significance of membrane repolarization and cyclic AMP changes in mouse pancreatic B-cells for the inhibition of insulin release by galanin. Mol Cell Endocrinol 105:97–102
Dunne MJ, Bullett MJ, Li GD, Wollheim CB, Petersen OH (1989) Galanin activates nucleotide-dependent K+ channels in insulin-secreting cells via a pertussis toxin-sensitive G-protein. EMBO J 8:413–420
Ehrlich JR, Cha TJ, Zhang L, Chartier D, Villeneuve L, Hebert TE, Nattel S (2004) Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol 557:583–597
Ferrer J, Nichols CG, Makhina EN, Salkoff L, Bernstein J, Gerhard D, Wasson J, Ramanadham S, Permutt A (1995) Pancreatic islet cells express a family of inwardly rectifying K+ channel subunits which interact to form G-protein-activated channels. J Biol Chem 270:26086–26091
Fosset M, Schmid-Antomarchi H, de Weille JR, Lazdunski M (1988) Somatostatin activates glibenclamide-sensitive and ATP-regulated K+ channels in insulinoma cells via a G-protein. FEBS Lett 242:94–96
Gromada J, Hoy M, Olsen HL, Gotfredsen CF, Buschard K, Rorsman P, Bokvist K (2001) Gi2 proteins couple somatostatin receptors to low-conductance K+ channels in rat pancreatic alpha-cells. Pflugers Arch 442:19–26
Herbst M, Sasse P, Greger R, Yu H, Hescheler J, Ullrich S (2002) Membrane potential dependent modulations of calcium oscillations in insulin-secreting INS-1 cells. Cell Calcium 31:115–126
Hille B (2001) Ion channels of excitable membranes, 3rd edn. Sineauer, Sunderland, MA
Iizuka M, Kubo Y, Tsunenari I, Pan CX, Akiba I, Kono T (1995) Functional characterization and localization of a cardiac-type inwardly rectifying K+ channel. Receptors Channels 3:299–315
Inanobe A, Horio Y, Fujita A, Tanemoto M, Hibino H, Inageda K, Kurachi Y (1999) Molecular cloning and characterization of a novel splicing variant of the Kir3.2 subunit predominantly expressed in mouse testis. J Physiol 521(Pt 1):19–30
Isomoto S, Kondo C, Takahashi N, Matsumoto S, Yamada M, Takumi T, Horio Y, Kurachi Y (1996) A novel ubiquitously distributed isoform of GIRK2 (GIRK2B) enhances GIRK1 expression of the G-protein-gated K+ current in Xenopus oocytes. Biochem Biophys Res Commun 218:286–291
Jin W, Lu Z (1998) A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 37:13291–13299
Jin W, Lu Z (1999) Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 38:14286–14293
Josefsen K, Stenvang JP, Kindmark H, Berggren PO, Horn T, Kjaer T, Buschard K (1996) Fluorescence-activated cell sorted rat islet cells and studies of the insulin secretory process. J Endocrinol 149:145–154
Kanjhan R, Coulson EJ, Adams DJ, Bellingham MC (2005) Tertiapin-Q blocks recombinant and native large conductance K+ channels in a use-dependent manner. J Pharmacol Exp Ther 314:1353–1361
Kitamura H, Yokoyama M, Akita H, Matsushita K, Kurachi Y, Yamada M (2000) Tertiapin potently and selectively blocks muscarinic K(+) channels in rabbit cardiac myocytes. J Pharmacol Exp Ther 293:196–205
Kreienkamp HJ, Honck HH, Richter D (1997) Coupling of rat somatostatin receptor subtypes to a G-protein gated inwardly rectifying potassium channel (GIRK1). FEBS Lett 419:92–94
Kume H, Graziano MP, Kotlikoff MI (1992) Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A 89:11051–11055
Lei Q, Jones MB, Talley EM, Garrison JC, Bayliss DA (2003) Molecular mechanisms mediating inhibition of G protein-coupled inwardly-rectifying K+ channels. Mol Cells 15:1–9
Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY (2002) Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron 33:715–729
Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P (2004) Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145:667–678
Mirshahi T, Logothetis DE (2002) GIRK channel trafficking: different paths for different family members. Mol Interv 2:289–291
Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K (1990) Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126–132
Nelson CS, Marino JL, Allen CN (1997) Cloning and characterization of Kir3.1 (GIRK1) C-terminal alternative splice variants. Brain Res Mol Brain Res 46:185–196
Nilsson T, Arkhammar P, Rorsman P, Berggren PO (1989) Suppression of insulin release by galanin and somatostatin is mediated by a G-protein. An effect involving repolarization and reduction in cytoplasmic free Ca2+ concentration. J Biol Chem 264:973–980
Olsen ML, Sontheimer H (2004) Mislocalization of Kir channels in malignant glia. Glia 46:63–73
Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N (2002) G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron 33:87–99
Renstrom E, Ding WG, Bokvist K, Rorsman P (1996) Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron 17:513–522
Ribalet B, Eddlestone GT (1995) Characterization of the G protein coupling of a somatostatin receptor to the K+ATP channel in insulin-secreting mammalian HIT and RIN cell lines. J Physiol 485(Pt 1):73–86
Ribalet B, Eddlestone GT (1995) Characterization of the G protein coupling of SRIF and beta-adrenergic receptors to the maxi KCa channel in insulin-secreting cells. J Membr Biol 148:111–125
Rorsman P, Bokvist K, Ammala C, Arkhammar P, Berggren PO, Larsson O, Wahlander K (1991) Activation by adrenaline of a low-conductance G protein-dependent K+ channel in mouse pancreatic B cells. Nature 349:77–79
Rorsman P (1997) The pancreatic beta-cell as a fuel sensor: an electrophysiologist’s viewpoint. Diabetologia 40:487–495
Ryan JS, Tao QP, Kelly ME (1998) Adrenergic regulation of calcium-activated potassium current in cultured rabbit pigmented ciliary epithelial cells. J Physiol 511(Pt 1):145–157
Schermerhorn T, Sharp GW (2000) Norepinephrine acts on the KATP channel and produces different effects on [Ca2+]i in oscillating and non-oscillating HIT-T15 cells. Cell Calcium 27:163–173
Schoots O, Wilson JM, Ethier N, Bigras E, Hebert TE, Van Tol HH (1999) Co-expression of human Kir3 subunits can yield channels with different functional properties. Cell Signal 11:871–883
Sharp GW (1996) Mechanisms of inhibition of insulin release. Am J Physiol 271:C1781–C1799
Sieg A, Su J, Munoz A, Buchenau M, Nakazaki M, Aguilar-Bryan L, Bryan J, Ullrich S (2004) Epinephrine-induced hyperpolarization of islet cells without KATP channels. Am J Physiol Endocrinol Metab 286:E463–E471
Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA (1998) Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem 273:23321–23326
Smith PA, Sellers LA, Humphrey PP (2001) Somatostatin activates two types of inwardly rectifying K+ channels in MIN-6 cells. J Physiol 532:127–142
Stoffel M, Espinosa R 3rd, Powell KL, Philipson LH, Le Beau MM, Bell GI (1994) Human G-protein-coupled inwardly rectifying potassium channel (GIRK1) gene (KCNJ3): localization to chromosome 2 and identification of a simple tandem repeat polymorphism. Genomics 21:254–256
Stoffel M, Tokuyama Y, Trabb JB, German MS, Tsaar ML, Jan LY, Polonsky KS, Bell GI (1995) Cloning of rat KATP-2 channel and decreased expression in pancreatic islets of male Zucker diabetic fatty rats. Biochem Biophys Res Commun 212:894–899
Tanizawa Y, Matsubara A, Ueda K, Katagiri H, Kuwano A, Ferrer J, Permutt MA, Oka Y (1996) A human pancreatic islet inwardly rectifying potassium channel: cDNA cloning, determination of the genomic structure and genetic variations in Japanese NIDDM patients. Diabetologia 39:447–452
Tsaur ML, Menzel S, Lai FP, Espinosa R 3rd, Concannon P, Spielman RS, Hanis CL, Cox NJ, Le Beau MM, German MS et al (1995) Isolation of a cDNA clone encoding a KATP channel-like protein expressed in insulin-secreting cells, localization of the human gene to chromosome band 21q22.1, and linkage studies with NIDDM. Diabetes 44:592–596
Vaughn J, Wolford JK, Prochazka M, Permana PA (2000) Genomic structure and expression of human KCNJ9 (Kir3.3/GIRK3). Biochem Biophys Res Commun 274:302–309
Wei J, Hodes ME, Piva R, Feng Y, Wang Y, Ghetti B, Dlouhy SR (1998) Characterization of murine Girk2 transcript isoforms: structure and differential expression. Genomics 51:379–390
Wischmeyer E, Doring F, Spauschus A, Thomzig A, Veh R, Karschin A (1997) Subunit interactions in the assembly of neuronal Kir3.0 inwardly rectifying K+ channels. Mol Cell Neurosci 9:194–206
Wiser O, Qian X, Ehlers M, Ja WW, Roberts RW, Reuveny E, Jan YN, Jan LY (2006) Modulation of basal and receptor-induced GIRK potassium channel activity and neuronal excitability by the mammalian PINS homolog LGN. Neuron 50:561–573
Yamada M, Inanobe A, Kurachi Y (1998) G protein regulation of potassium ion channels. Pharmacol Rev 50:723–760
Yano H, Philipson LH, Kugler JL, Tokuyama Y, Davis EM, Le Beau MM, Nelson DJ, Bell GI, Takeda J (1994) Alternative splicing of human inwardly rectifying K+ channel ROMK1 mRNA. Mol Pharmacol 45:854–860
Yoshimoto Y, Fukuyama Y, Horio Y, Inanobe A, Gotoh M, Kurachi Y (1999) Somatostatin induces hyperpolarization in pancreatic islet alpha cells by activating a G protein-gated K+ channel. FEBS Lett 444:265–269
Zhao Y, Fang Q, Straub SG, Sharp GW (2008) Both Gi and Go heterotrimeric G proteins are required to exert the full effect of norepinephrine on the beta-cell KATP channel. J Biol Chem 283:5306–5316
Zhu L, Wu X, Wu MB, Chan KW, Logothetis DE, Thornhill WB (2001) Cloning and characterization of G protein-gated inward rectifier K+ channel (GIRK1) isoforms from heart and brain. J Mol Neurosci 16:21–32
Acknowledgements
We would like to thank Dr. Colin Nichols and Dr. Mike Walker for their help, and Dr. Claes Wollheim and Dr. Jun-Ichi Miyazaki for their permission to use the INS-1E and MIN-6 lines, respectively. This work was supported in part by the Minerva Foundation, the Israeli Science Foundation (ISF grant 128/05), and the Y. Leon Benoziyo Institute for Molecular Medicine.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Co-localization of GIRK subunits (red) and glucagon (green) in mouse pancreatic islets. Bar = 50 m (GIF 1.02 MB)
Supplementary Fig. 2
Co-localization of GIRK subunits (red) and somatostatin (green) in mouse pancreatic islets. Bar = 50 m (GIF 915 kb)
Supplementary Fig. 3
Co-localization of GIRK subunits (red) and pancreatic polypeptide (green) in mouse pancreatic islets. Bar = 50 m (GIF 0.97 MB)
Rights and permissions
About this article
Cite this article
Iwanir, S., Reuveny, E. Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels. Pflugers Arch - Eur J Physiol 456, 1097–1108 (2008). https://doi.org/10.1007/s00424-008-0479-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-008-0479-4