Abstract
Cellular responses induced by stress are essential for the survival of cells under adverse conditions. These responses, resulting in cell adaptation to the stress, are accomplished by a variety of processes at the molecular level. After an alteration in homeostatic conditions, intracellular signalling processes link the sensing mechanism to adaptive or compensatory changes in gene expression. The ability of cells to adapt to hyperosmotic stress involves early responses in which ions move across cell membranes and late responses characterized by increased synthesis of either membrane transporters essential for uptake of organic osmolytes or of enzymes involved in their synthesis. The goal of these responses is to return the cell to its normal size and maintain cellular homeostasis. The enhanced synthesis of molecular chaperones, such as heat shock proteins, is another important component of the adaptive process that contributes to cell survival. Some responses are common to different stresses, whereas others are specific. In the first part of the review, we illustrate the characteristic and specific features of adaptive response to hypertonicity; we then describe similarities to and differences from other cellular stresses, such as genotoxic agents, nutrient starvation and heat shock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
General pattern of adaptive responses of cells to hypertonicity
Many types of mammalian cells can survive a moderately hypertonic environment due to a specific adaptation process that results in the cellular accumulation of compatible osmolytes [25, 26]. This adaptation process, summarized in Fig. 1, involves early responses, occurring over milliseconds to minutes, and later responses, requiring hours to days. The virtually instantaneous reduction in cell volume due to the osmotic efflux of water induced by acute hypertonic stress is rapidly corrected by what is referred to as regulatory volume increase (RVI). This early process is mediated by pre-existing ion transport systems, including the Na+–K+–2Cl− co-transporter (NKCC), the Na+/H+ exchanger and the Cl−/HCO3− exchanger [86, 104]. These increases in the intracellular concentrations of potassium, sodium and chloride ions and the accompanying influx of water cause RVI. The later phase is characterized by increased production of heat shock proteins (HSPs) and either the synthesis or the uptake and cellular accumulation of compatible osmolytes. In mammalian cells, the latter includes neutral amino acids or their derivatives, polyols such as sorbitol and myo-inositol, and methylamines such as betaine. The usual explanation of this phenomenon is the need to replace the early cellular accumulation of inorganic ions with small organic molecules that do not affect cell function even at relatively high intracellular concentrations [159]. Accumulation of compatible osmolytes within the cell thus maintains intracellular water homeostasis without impairing normal biochemical functions such as protein synthesis. For example, overall cellular protein synthesis typically halves during the first 30 min exposure to hypertonicity but then recovers as cells accumulate compatible osmolytes and lose potassium [124, 127]. In a cell-free protein synthesis system, both initiation and elongation are inhibited by high concentrations of inorganic ions but not by compatible osmolytes [22]. These compatible osmolytes thus enable cells to survive under hypertonic conditions, protecting them from apoptosis and modulating the adaptive response [5, 78, 100].
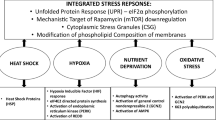
Schematic illustration of the molecular mechanisms involved in the adaptation of mammalian cells to hypertonic stress. Hypertonicity causes cell shrinkage leading to increases in intracellular ionic strength and induces DNA breaks. RVI is initially mediated by ions uptake; then, cell counteracts ionic strength by substituting inorganic ions with amino acids and compatible osmolytes and by synthesizing HSP70. When RVI is impaired or full adaptation is prevented by deprivation of compatible osmolytes, apoptotic cell death occurs. See the text for a detailed description
The adaptive responses to hypertonicity have been studied mainly in kidney-derived epithelial cells, as these cells are physiologically exposed to marked variations in osmolarity during their normal function in vivo [17, 117]. However, similar responses have been detected in several other cell models exposed to hypertonicity such as chondrocytes [39], macrophages [40], endothelial cells [127], mesothelial cells [100], nucleo pulposus cells [146], astrocytes [119] and neurons [97]. The maintenance of these responses to hypertonicity, which are quite expensive in terms of gene information, gene expression and metabolic input, suggests a physiological role. In this context, it should be noted that articular cartilage is subjected to mechanical stresses that modulate extracellular ionic and osmotic conditions [111, 148]. During periods of prolonged mechanical loading, chondrocytes are exposed to an increasingly hyperosmotic environment because fluid is extruded, but cations are preferentially retained by proteoglycans increasing the osmotic pressure within the matrix. In a recent study, direct measurement of tissue osmolality revealed that lymphoid tissues are hyperosmolar (30 mosmol/kg higher than serum), indicating the importance of the osmotic stress response pathway to the function of lymphocytes in vivo during an immune response [63]. In endothelial cells, such a role remains obscure, although it is worth noting that endothelial cells are constantly exposed to the shear and stress of blood flow as well as modest alterations in blood osmolarity. It is also likely that certain pathological conditions, such as diabetic hyperglycaemia, hyperosmolar coma or severe dehydration, might require fast osmotic responses. Such osmotic adaptation might be particularly important to endothelial cells that mediate the exchange of water, small solutes, such as ions and nutrients, and even macromolecules between plasma and interstitial fluid.
In this cell model, we reported [5, 127] that initial cell shrinkage is followed by RVI concomitant with an increase in intracellular potassium content. Then, the activity of amino acid transport System A increases due to a transcriptional regulation of SNAT-2 gene [4, 8], accompanied by an accumulation of amino acids and a gradual decline of cellular K+ content [127]. The subsequent decline in System A activity was paralleled by an induction of the transporters of compatible osmolytes (betaine, myo-inositol and taurine) [5, 127].
Recent observations have suggested that creatine supplementation affords protection against hypertonicity in muscle and endothelial cells [9] by acting as a compatible osmolyte.
The biochemical and molecular events characterizing the hyperosmotic cellular response are quite specific and are related to cellular homeostasis. However, different stressors can induce similar cellular responses, and some of the mechanisms involved in the adaptation are shared. This aspect, summarized in Fig. 2, is discussed below, together with further details of the responses to hypertonicity.
Early responses
Initiation of responses
Despite a large number of studies on the osmotic stress response, the signals involved in the initiation of the adaptive response pathways have yet to be elucidated. As NKCC can also be activated by iso-osmotic cell shrinkage in (Na+–K+)-free medium [80] decrease in cell volume, rather than increase in external osmolarity or ionic strength, it seems to trigger initial ion uptake. Similar iso-osmotic cell shrinkage also activates the uptake of amino acids [125], again indicating that cell shrinkage is the trigger. How this trigger is sensed, however, is not well understood. Probably more than one volume-sensing mechanism, such as change in membrane tension or cytoskeletal architecture, increase in ionic strength or macromolecular crowding, are involved in the initiation of different signalling pathways. Changes in intracellular concentrations of specific ions (e.g. calcium) and activation of phosphorylation/dephosphorylation reactions could also be involved [86, 107, 141].
Because initial activation of ion transport occurs within minutes, it is likely to stem from post-translational modification, not increased synthesis of transporters, and phosphorylation now seems to be the key. Lytle et al. [96] showed that cell shrinkage induced both phosphorylation and activation of NKCC. Phosphorylation of NKCC1 by SPAK (STE20/SPS1-related proline/alanine-rich kinase) activates the cotransporter [53, 129]. It has been reported recently that reduced expression of WNK1 (with no lysine protein kinase-1) or OSR1 (oxidative stress-responsive kinase-1) causes a significant reduction in NKCC activity in Hela cells in the presence of sorbitol, keeping with the notion that these kinases are involved in the activation of NKCC in response to hyperosmotic stress [11]. On the other hand, recent work [43, 44] ruled out a role of myosin light chain phosphorylation in the osmotic regulation of NKCC, as suggested by earlier work [2, 42, 79].
The osmotic stress response pathway has been well characterized in the yeast Saccharomyces cerevisiae [65]. In this pathway, two osmotic sensor proteins, Sl1 and Sho1, are linked to Hog-1, the p38 MAPK homolog high-osmolality glycerol kinase, which regulates downstream transcription factors for osmostress-regulated genes [131]. In contrast, in mammalian cells, the adaptive hyperosmotic stress response involves multiple signalling pathways. p38 MAPK is an essential component, but other kinases such as ERK, JNK, PKC, PKA and ATM are activated in response to hypertonicity [139]. Hypertonic stress also activates cell surface EGF receptor tyrosine kinase [92] as well as cytosolic tyrosine kinases such as SRC, FYN and YES [34].
DNA damage
High intracellular ionic strength induced by hyperosmolarity causes DNA damage in the form of double-strand breaks [85]. However, in contrast to responses induced by many genotoxic agents, such damage is not repaired, and adapted cells restore their proliferation rate despite the presence of many double-strand DNA breaks [49–51]. It has been proposed that such DNA damage could be the sensor of hypertonic stress that initiates adaptive signalling cascades.
All cells have DNA damage–response pathways, which can repair DNA accurately and prevent accumulation of mutations. This is essential in preventing diseases caused by mutations induced by ionizing radiation, UV radiation, chemical carcinogens, reactive oxygen species or alkylating agents. DNA damage rapidly arrests the cell cycle, and DNA repair, specific to the type of DNA damage, is activated. Cell cycle arrest generally persists until the DNA is repaired. Failure to repair the DNA damage activates apoptosis, which eliminates potentially malignant cells. The exact nature of the response depends on the type and extent of the DNA damage [164].
Exposure of renal inner medullary cells to high levels of NaCl induces DNA breaks [50, 85]. Such DNA damage caused by hypertonicity triggers a transient cell cycle arrest at any stage of the cell cycle [47, 48]. Activation of G2/M arrest is similar to that induced by UV radiation [24] and is mediated by p38 kinase [48] and GADD45 protein [99]. In common with the response elicited by many genotoxic agents, p53 protein is involved in the G1 arrest [47]. Expression of growth arrest DNA damage (GADD) genes (GADD45 and GADD153) is induced by genotoxic agents that cause DNA damage and also by nutrient depletion. Potential GADD45 functions during environmental stress include promotion of cell cycle arrest, apoptosis, chromatin remodeling and DNA repair. Recent results demonstrate that induction of GADD45 isoforms inhibit mitosis and promotes G2/M arrest during moderate hyperosmotic stress [99]. A similar role of GADD45 in cell cycle checkpoint pathways responsible for G2/M arrest has also been demonstrated in human keratinocytes exposed to UV radiation and alkylating agents, where it inhibits the activity of the Cdc2-cyclin B1 complex [161]. It has been shown that essential amino acid deprivation induces GADD153 expression by both transcriptional and post-transcriptional mechanisms [23]. Moreover, glutamine deprivation has been found to cause rapid elevation of GADD45 and GADD153 via marked stabilization of mRNA levels in human breast cell lines [1].
In contrast to the responses evoked by many genotoxic agents, the cell cycle arrest induced by hypertonicity does not activate the DNA repair machinery. Cells adapted to high NaCl restore their proliferation rate despite the presence of numerous double-strand DNA breaks [49, 50]. Thus, both in cell culture (kidney derived mIMCD3, COS-7, HEK293 cells and mouse embryonic fibroblasts) and in vivo (inner medullary cells in mice), high NaCl causes persistent DNA damage that is not repaired unless physiological NaCl levels are restored. The activation of several components of the canonical DNA damage response, such as Mre11 exonuclease, Chk1 and histone H2AX, is impaired and results in the inhibition of DNA repair. High NaCl causes Mre11 exonuclease to move from the nucleus to the cytoplasm; Chk1 and histone H2AX, which become phosphorylated immediately after induction of DNA breaks by genotoxic agents, are not phosphorylated in response to hypertonicity [49, 50]. No increase in γH2AX foci formation (an indicator of DNA damage) was detected in hypertonic saline-treated human amnion FL cells [165]. However, neutral comet assay conducted on these cells also produced negative results. These data suggest that hypertonic saline itself may not induce significant DNA damage, at least in this cell model, and raise the question of whether DNA breaks are a hallmark of hypertonic stress. These discrepancies could be due to the difference in cell model (origin, cells adapted to high NaCl vs cell acutely treated) and/or to the different extent of NaCl elevation.
Nevertheless, although high NaCl inhibits some mechanisms of the DNA repair, it up-regulates some other components, such as GADD45 [99], GADD153 [84], p53 [46], Ku86 [52] and ATM [69] proteins, known to be involved in the response to DNA damage. Some of these proteins might have a different role in hypertonic stress condition because the persistence of DNA damage might be required for cell adaptation to hypertonicity and cell survival. DNA breaks might thus represent the primary signal responsible for initiating the adaptive signalling cascades.
ATM kinase is activated early during the DNA damage response to ionizing and UV radiation by autophosphorylation on S1981, and this activation is not strictly dependent on direct binding to DNA strand breaks but may stem from a disruption of chromatin structure [14]. Hypertonicity also activates ATM by phosphorylation on S1981 [69], and the role of this activation during hypertonic stress has been reported recently. ATM is involved in the activation [69] and nuclear translocation [163] of tonicity-responsive enhancer-binding protein (TonEBP). ATM activity is necessary although not sufficient for full hypertonicity-induced TonEBP transcriptional competence. In contrast, the activation of ATM by other DNA damage agents does not activate TonEBP [69], suggesting that additional signals elicited by hypertonic stress but not by ionizing and ultraviolet radiations are required.
Dmitrieva et al. [52] have recently demonstrated that Ku protein (a factor playing a central role promoting DNA end-joining in the repair of DNA double-strand breaks) is not involved in DNA repair; but in cells adapted to high NaCl, it helps maintain chromosomal integrity despite the continuous presence of DNA breaks. Indeed, cells deficient in Ku protein have impaired proliferation, aberrant mitosis and increased chromatin fragmentation on exposure to high NaCl.
Stress-mediated activation of p53, a well-known tumour suppressor, usually results in cell cycle delay until damage is repaired, enhancing cell survival or inducing apoptosis, which removes highly damaged cells. In hyperosmotic conditions, p53 protein rapidly increases, being phosphorylated on S15 and becoming transcriptionally active [46], although in this situation, the role of p53 is poorly defined. Accumulation and activation of p53 in proliferating mIMCD3 cells induced by hypertonic treatment restricts DNA replication and, in contrast to other stresses, reduces apoptosis [46, 47]. Despite these observations, Cai et al. [31] failed to find a significant role for p53 in normal renal inner medullary cells under conditions like those in vivo. Similarly, in NIH 3T3 cells exposed to acute elevation of osmolarity, p53 is involved in the sequence of signalling events in the cell shrinkage-induced caspase-3 activation [59], but a clear-cut role of p53 as inducer of the intrinsic apoptotic cascade remains to be established. It should be noted that the release of cytochrome c from mitochondria, which is involved in the activation of caspase-9, an activator of caspase-3, was not evaluated in this paper and was not detected by other authors [36, 105].
Cell shrinkage and induction of apoptosis
A peculiar variant of iso-osmotic cell shrinkage, apoptotic volume decrease, is a hallmark of the apoptotic mode of programmed cell death and is considered an integral part of the apoptotic pathway [21, 98]. Apoptosis can be triggered under hypertonicity when the mechanisms involved in cell adaptive responses are impaired by rapid and intense elevation of the osmolarity. Under severe hypertonic conditions [7, 105] or when a full adaptation is prevented by deprivation of compatible osmolytes [5, 78], the cells die by apoptosis. Moreover, a moderate osmotic stress is sufficient to induce apoptosis in thymocytes and lymphoid cells that lack an active RVI response and do not compensate for an initial reduction in cell volume after exposure to hypertonicity [20]. These findings suggest that apoptosis is associated with an early cell shrinkage when an adequate compensatory RVI cannot be triggered. The persistence of cell shrinkage plays a critical role in activating the apoptotic process, although the underlying mechanisms are unclear. Conversely, in some cell types, transport proteins normally activated by shrinkage and contributing to RVI are inhibited during apoptosis [87, 158]. An example is given by Fas (CD95)-receptor stimulation that leads to the inhibition of Na+/H+ exchanger with associated cell shrinkage and DNA fragmentation in Jurkat T-lymphocytes [87]. A model of apoptosis dependent on cell shrinkage has been described at the molecular level in leukaemia-derived cells subjected to acute deprivation of glutamine, a major compatible osmolyte involved in the regulation of cell volume [60]. In particular, the rapid and persistent cell shrinkage associated with glutamine deprivation has been shown to activate the extrinsic apoptotic pathway by promoting the ligand-independent multimerization of surface Fas (CD95) receptors. A comparable mechanism might play a role in the promotion of the reported ligand-independent clustering of TNF receptors responsible for the activation of the apoptotic pathway under hypertonic stress [58, 88].
Induction of osmolyte transport systems
Amino acids
The amino acid transport known as System A [120] is regulated in a variety of ways [27] including hyperosmotic stress [124] and amino acid starvation [103]. Cell incubation in isosmotic, amino acid-free saline, a condition known to reduce the intracellular amino acids pool, is followed by gradual and progressive cell shrinkage [54] with an increase in ionic strength within cells [55]. In contrast with the immediate cellular response to hyperosmotic shrinkage, which takes place within seconds and involves increases in the intracellular concentrations of charged ions, cell shrinkage induced by amino acid starvation is not recovered even after prolonged exposure. However, cells activate the system A transport system, and when the extracellular medium is supplemented with amino acids, cell volume rapidly recovers [54].
Complementary DNAs coding for amino acid transporters with operative properties typical of System A have now been cloned independently by three groups [133, 142, 160]. The transporter, originally named ATA-2, SAT2, SA1, is called SNAT2 (sodium-dependent neutral amino acid transporter-2) [101] and belongs to the SLC38 family. An increase in the mRNA for SNAT2 has been demonstrated during exposure to hypertonic stress and amino acid deprivation [4, 5, 8, 56, 57], indicating that transcriptional regulation underlies the induction of System A by both stresses. Although no direct regulation of the SNAT2 gene by activation of TonE (see “Compatible osmolytes” below) during hypertonic stress has been demonstrated, an amino acid response element in the first intron of the SNAT2 gene has been identified [122]. There is only one report indicating that, in transgenic T blasts, loss of NFAT5 (TonEBP) function decreases the amount of SNAT2 mRNA under hypertonic condition [144]. In human fibroblast, inhibition of SNAT2 expression by RNA interference prevents not only the accumulation of amino acids but also recovery of cell volume [18]. It should be noted, however, that the RVI response in these cells is associated with an only slight increase in cell potassium content, suggesting that ion transport systems exert little effect on RVI [38].
Investigations have been performed to define the signal transduction pathways involved in the response of SNAT2 gene to amino acid depletion as well as to hypertonic stress. The involvement of ERK1/2 in the signalling pathways activated by both amino acid starvation and hypertonicity has been demonstrated in human fibroblasts [54]. López-Fontanals et al. [95] showed that in CHO-K1 cells, the kinase pathways for these two stresses differ, that ERK and JNK were required only for the response to amino acid depletion, not for the response to hypertonicity, whereas p38 seemed to be involved only in the osmotic response. It should be noted, however, that these authors failed to detect SNAT2 mRNA in CHO-K1 cells exposed to hypertonicity, in contrast to results obtained by others with the same [8] or different kinds of cells [4, 5, 56, 57, 113, 143, 144]. Consistent with the idea that two different signalling pathways are involved is the recent finding that induction of the mRNA by amino acid starvation involves phosphorylation of eIF2a (eukaryotic initiation factor 2a), whereas the response to hypertonic stress is independent of eIF2a [61].
Other cellular stresses also affect amino acid transport System A. During the early phase of a hyperthermic treatment, 3T3 cells exhibit an increase in the activity of specific amino acid transporters mediated by an activation or relocation of pre-existing transport proteins [128]. In contrast with hypertonic stress [145], this induction does not require mRNA and protein synthesis. Similar results have been obtained in L6 muscle cells exposed to sodium arsenite or to heat shock [102]. Both of these stresses enhance System A activity within minutes, which is too soon to be accounted for by transcriptional regulation. The role of an increase in the uptake of amino acids in response to heat shock is not clear, although it may be related to the concomitant swelling observed in the early phase of heat shock [128].
Cyclic strain, an experimental condition that mimics the change in vessel diameter of the major arteries as result of pulsatile blood flow, stimulates l-proline uptake in vascular smooth muscle cells. In common with hypertonic treatment, this stress increases the Vmax for proline uptake, induces the expression of SNAT2 mRNA, and depends on protein synthesis [134].
Compatible osmolytes
When osmotic stress persists for a longer period of time, osmolytes that are more compatible than amino acids are required. During prolonged exposure of cells to hypertonicity, cell survival depends on the synthesis either of a compatible osmolyte itself (e.g. sorbitol) or of BGT1, SMIT and TAUT transporters and intracellular accumulation of betaine, taurine and myo-inositol [26]. Indeed, cells do not adapt and die by apoptosis when hypertonic medium has been depleted of these compatible osmolytes despite the persistence of high intracellular amino acids level [5].
A major role in this response to hyperosmolarity is played by TonEBP, a member of the NFAT family of transcription factors [12, 106, 156]. The activation of TonEBP leading to the induction of osmoprotective genes by binding to Ton-E (tonicity responsive enhancer) is achieved by different pathways that regulate phosphorylation and nuclear translocation [37, 147], transactivation [89] and induction [155]. The full activation of the key factor TonEPB requires the coordinated activity of Fyn, PKA, p38 and ATM and possibly other factors. It is not clear, however, if phosphorylation plays a key role in the regulation of the activity of TonEBP [71]. Recently, it was shown that MDCK cells expressing kinase-active MEKK3 showed increased BGT1 mRNA, and TonE-mediated reporter gene expression correlated with up-regulation of p38α and ERK5 [121]. Another mechanism induced by hypertonicity to stimulate TonEBP activity requires the dissociation of RNA helicase A, which normally exerts an inhibitory effect on TonEBP [35]. Finally, it is noteworthy that the activation of osmoprotective genes can be achieved independently of TonEBP activation, as reported in rat brain [97]. Depending on the neuronal cell type and on the gene considered, TonEBP may be insufficient or unnecessary for the hyperosmotic response. Amino acid depletion, like hypertonicity, has been found to give rise to a nuclear redistribution of TonEBP and a stimulation of myo-inositol transport in human fibroblasts [55].
Compatible osmolytes are not only important for cell volume homeostasis but also for cell protection during other stressful conditions: They can act as chemical chaperones and stabilize native protein structures and function [41, 132, 154]. Exposure to ultraviolet B or A radiation increased osmolyte uptake in human keratinocytes [153] by affecting BGT-1, SMIT and TAUT-T mRNAs levels. In common with hyperosmotic conditions, the increase in osmolytes uptake reflects an increased synthesis of transporter proteins rather than the activation of pre-existing transporters. This increase in osmolytes uptake by UV is thus a part of the defence strategy of keratinocytes to the detrimental effects of UV irradiation.
It has been proposed that desiccation, another stress which, in common with hypertonicity, leads to efflux of intracellular water, also induces similar cellular responses. A human cell line derived from embryonic kidney is capable of responding to desiccation stress via rapid activation of JNK and p38MAPK, but there is no induction of aldose reductase, BGT-1 or SMIT genes [67, 68].
Heat shock response
Induction of heat shock proteins
In addition to accumulating osmolytes, cells are also protected from hypertonicity by expressing HSPs. These molecular chaperones are induced during the first hours of hypertonic treatment to counteract the detrimental effects of elevated intracellular ionic strength and to protect intracellular macromolecules against unfolding and aggregation [33, 126]. HSP70 also exerts anti-apoptotic effects under stressful conditions [45, 62, 110], which include hypertonic stress [90, 118]. The list of stressors that induce HSPs is large and includes various acute and chronic conditions such as elevated temperature, heavy metals, chemical toxicants, infections, oxidative stress [108, 109] as well as hypertonicity [33, 126]. The HSP70 family represents the most highly conserved of the HSPs and includes both constitutively expressed and inducible members [94].
The transcriptional response to heat shock is considered a paradigm for inducible gene expression in eukaryotic cells. It is mediated by the binding of the heat shock factor1 (HSF1) to the heat shock elements (HSEs) in the promoter of HSP genes. HSF1 is present in unstressed cells as an inactive monomeric form complexed with HSP90, P23 and an immunophilin [19]. HSF1 activation involves trimerisation, acquisition of DNA-binding activity and transcriptional competence. Recently, it has been reported that HSF1 activation by heat shock is also mediated by a ribonucleoprotein complex containing translation elongation factor eEF1A and a non-coding RNA called HSR1 [137]. Activation of HSF1 is a multistep process. HSF1 binding to DNA is insufficient to induce transcription, and complete transcriptional activity requires hyperphosphorylation of HSF1 [66]. Although heat shock and most other stresses convert HSF1 to the fully active trimer capable of gene transcription, other stresses activate HSF1 to an intermediate trimeric state, which is able to bind DNA but is transcriptionally inert.
A transient and brief exposure of cells to a hypertonic medium (hypertonic shock) leads to the activation of HSF1 as a function of the osmotic strength of the medium [3, 32]. However, unlike the response to heat shock, the activation of HSF1 by a hypertonic shock is not followed by increased transcription of HSP70 gene or by the accumulation of HSP70 mRNA or protein in either 3T3 [3] or Hela cells [32]. Similarly, sodium salicylate treatment activates HSF1 without gene transcription [72, 73]. In contrast to hypertonic shock, however, prolonged exposure to a moderately hypertonic medium induces accumulation of the HSP70 mRNA and protein after a few hours [33, 115, 126]. The mechanism of this enhanced expression of the HSP70 during such hypertonic treatment is not completely understood. In 3T3 cells exposed to prolonged hypertonic treatment, HSF1 activation and HSP70 gene transcription are not required for increased HSP70 expression. Instead, ActD chase experiments suggest that post-transcriptional stabilization contributes to the accumulation of HSP70 mRNA. In contrast, the increase in mRNA level of SNAT2, another gene induced by hypertonicity with similar kinetics, is regulated mainly at the level of transcription [6].
The expression of HSPs in response to other stressful conditions via stabilization of the induced mRNAs, rather than by transcriptional activation of heat shock genes, has been reported. In human peripheral blood monocytes, phorbol esters induced accumulation of mRNA and an increased expression of HSP90 and HSP70 that are not blocked by the transcriptional inhibitor ActD [70]. In chondrocytes and other cells, continuous high hydrostatic pressure causes HSP70 accumulation via HSP70 mRNA stabilization without transcriptional induction of the HSP70 gene [74, 75]. Post-translational regulation of HSP70 in several kinds of cells exposed to electromagnetic fields at low frequency has also been described recently [10]. This stress leads to the accumulation of inducible HSP70 by increasing its stability independently of HSP70 gene transcription and the synthesis of new mRNA and protein.
As described above, TonEBP plays a central role in the cellular response to hypertonicity, increasing the transcription of osmoprotective genes that leads to the accumulation of compatible osmolytes. Interestingly, TonEBP, whose mRNA is stabilized by hypertonicity [30], also regulates a HSP70 gene named HSP70-2 in mouse inner medullary epithelial cells (mIMCD) cells [157] and in human embryonic kidney epithelial cells [64]. HSP70 mRNA is significantly decreased in Hela cells where TonEBP expression was silenced by RNA interference [112]. Analysis of HSP70 mRNA expression is complicated because two different HSP70 genes express identical proteins but have completely different sequences in the untranslated region [150], and the names given in human, mouse and rat differ in a confusing fashion, even in the same species [162]. For example, the above-cited mouse HSP70-2 gene is also called hsp70.1, and mouse HSP70-1 is also called hsp70.3 or Hsp70A1. Woo et al. [157] reported that in response to hypertonicity, HSP70-2 mRNA, but not HSP70-1, was induced. This is in agreement, not in conflict as indicated by Huang and Tunnacliffe [67], with the findings of Shim et al. [140] that heat shock induces both hsp70.1 and hsp70.3 mRNA, whereas osmotic stress induces expression of hsp70.1. In hsp70.1-deficient mouse embryonic fibroblasts, an increased susceptibility to osmotic stress and apoptosis has been described [90, 140]. In p2mIME cells, however, a linear or single step increase in osmolarity elevates both hsp70.1 and hsp70.3 mRNAs [29]. Because only hsp70.1/HSP70-2 contains three TonE sites and hsp70.3/HSP70-1 does not, alternative mechanisms not implicating TonEBP activation could be involved in the induction of HSP70 mRNA under hypertonicity.
Osp94 has been identified as a new member of the HSP110/SSE subfamily of HSPs and is induced by hyperosmotic stress [81, 149], heat shock and cadmium in cultured mIMCD3 cells [135]. The 5′-flanking region of the Osp94 gene has functional Ton-E and HSE enhancer elements that respectively respond independently to hypertonicity and heat stress [82]. Treatment of mIMCD3 cells with MAPK inhibitors showed a marked decrease in Osp94 mRNA level induced by hypertonic stress. In contrast, the induction of Osp94 mRNA by heat shock was insensitive to these inhibitors. Similarly, when the cells were pretreated with MG132, a proteasome inhibitor, the increased level of Osp94 mRNA caused by either hypertonic or heat-shock stress was abolished.
Cross tolerance to stresses
Acquired thermal tolerance is the ability of cells to tolerate a severe heat shock, too severe to be tolerated normally, after they have been “conditioned” by preliminary exposure to a mild heat shock [83, 93, 123]. Exposure to a mild form of one stress can also confer tolerance to a severe form of a different stress. This “cross stress tolerance” has been demonstrated after pre-conditioning cells with heat shock to several other stresses, including ATP depletion [76, 151, 152], ultraviolet radiation [15], gamma radiation [77] and ischemia-reperfusion injury [91, 130]. Although it might not be the sole factor involved [13], the increased cellular expression and retention of HSPs induced by the mild heat shock provides the obvious explanation for such acquired tolerance [123]. Several studies have demonstrated that chaperone-mediated cytoprotection can be largely attributed to the suppression of apoptosis [45, 62, 90, 110, 118]. Induction of the expression of HSPs is the obvious common response to most stresses, but complete adaptation of cultured cells to an increase in osmolarity clearly involves other biochemical processes with an ordered sequence of changes occurring over a period of about 24 h. Cells must accumulate compatible osmolytes and/or HSP70 to tolerate subsequent, more severe hypertonicity. Adaptation to mild hypertonicity renders endothelial cells tolerant to subsequent otherwise lethal hypertonic stress [7]. This osmotolerance is associated with the cellular accumulation of osmolytes such as amino acids, betaine and myo-inositol and does not correlate with HSP70 expression. In contrast, HSP72 plays a major role in MDCK cells adapted to high NaCl when they are challenged with hyperosmotic solutions of urea, a potent protein-destabilizing agent, which freely enters cells [114]. HSPs might have a particularly important protective role under these conditions, and several reports have described a primary role of HSPs in the acquisition of urea resistance [16, 115, 116]. Stepwise or gradual adaptation from isotonic conditions might also enable the cells to survive even more severe hypertonic stress. Cai et al. [28] showed that almost 90% of mIMCD3 remained viable, when their culture medium was slowly changed, over a period of 20 h from 0.6 to 1.6 osmol (kg H2O)-1, compared with only 30% survival when the change was abrupt. A more gradual increase in osmolarity provides more time to accumulate compatible osmolytes and to enhance the expression of HSP70 [29].
Endothelial cells adapted to hyperosmolarity become tolerant to subsequent otherwise lethal heat shock [7]. Similarly Santos et al. [136] showed that mIMCD3 cells rendered osmotically tolerant by gradual adaptation to hypertonic media are also more resistant to a thermal stress and to a variety of other stressful conditions (hydrogen peroxide, therapeutic drugs and heavy metal compounds). Preliminary heat shock increases the survival of mIMCD3 cells during their subsequent exposure to hypertonicity [135]. However, preliminary exposure of endothelial cells to mild heat shock does not provide protection against subsequent hyperosmotic challenge, suggesting that the extent of such cross tolerance is limited, at least in endothelial cells [7]. There is no evidence that heat shock can induce the expression of the transporters for amino acids or compatible osmolytes such as betaine. It does not induce mRNA for ATA2 in mIMCD3 cells [113] or for BGT1 in MDCK cells [138].
Conclusions and perspectives
The general mechanisms involved in cellular adaptation to hyperosmolarity have been known for some time, although new insights are emerging. Sensing hypertonicity in mammalian cells has been variously attributed to changes in intracellular cell volume, ionic strength and molecular crowding. Additional perturbations must now be considered, including DNA damage. In contrast to the repair-response to DNA damage caused by genotoxic agents, DNA double-strand breaks induced by hypertonicity are not repaired and do not impair cell proliferation and survival. During the early phase of DNA repair, ATM, a protein kinase involved in this process, has a new role leading to the activation of TonEBP. More studies are needed to elucidate the role of DNA damage in the adaptive response to hypertonicity, and many questions remain to be answered. What causes DNA breaks? Are DNA breaks to be considered a general hallmark caused by hypertonicity? Is the appearance of DNA breaks dependent on the nature of the solute employed (NaCl vs sucrose) and function of the final osmolarity imposed? How can cells ignore their DNA damage? How is apoptosis suppressed?
Although a fundamental role of TonEBP in cells under hypertonic stress is well established, important questions remain regarding the identity of its activators and the associated signalling pathways. Whether activation of TonEBP is required for SNAT2 transcription in response to hypertonicity is still unclear, as is the identity of the other transcription factors or regulatory elements involved.
We ran into difficulties during the comparison of the responses evoked by the stressors considered in this review because some stressful conditions are applied for a short time (heat shock and UV radiation), whereas others (hypertonicity and amino acids deprivation) are imposed continuously. Supporting these considerations, a hypertonic shock induces HSF1 activation but differently from the heat shock does not induce gene transcription; in contrast, during hypertonic treatment, HSF1 is not involved in the HSP70 transcription, TonEBP regulates a HSP70 gene named HSP70-2 by binding to TonE elements, and post-transcriptional stabilization contributes to the accumulation of HSP70 mRNA. Moreover, the same stress applied with different intensity (e.g. different osmolarities) may induce divergent responses. Cellular shrinkage, depending on the cell type, length and intensity of stress, may lead to opposite responses: RVI associated with survival or lack of RVI with concomitant appearance of apoptosis. Persistence of cell shrinkage plays a critical role in activating the apoptotic process, although the mechanisms involved in the transition between the survival pathway and the decision for apoptosis remain to be elucidated.
References
Abcouwer SF, Schwarz C, Meguid RA (1999) Glutamine deprivation induces the expression of GADD45 and GADD153 primarily by mRNA stabilization. J Biol Chem 274:28645–28651
Akar F, Jiang G, Paul RJ, O’Neill WC (2001) Contractile regulation of the Na(+)–K(+)–2Cl(−) cotransporter in vascular smooth muscle. Am J Physiol 281:C579–C584
Alfieri R, Petronini PG, Urbani S, Borghetti AF (1996) Activation of heat-shock transcription factor 1 by hypertonic shock in 3T3 cells. Biochem J 319:601–606
Alfieri RR, Petronini PG, Bonelli MA, Caccamo AE, Cavazzoni A, Borghetti AF, Wheeler KP (2001) Osmotic regulation of ATA2 mRNA expression and amino acid transport system A activity. Biochem Biophys Res Commun 283:174–178
Alfieri RR, Cavazzoni A, Petronini PG, Bonelli MA, Caccamo AE, Borghetti AF, Wheeler KP (2002) Compatible osmolytes modulate the responses of porcine endothelial cells to hypertonicity and protect them from apoptosis. J Physiol 540:499–508
Alfieri RR, Bonelli MA, Petronini PG, Borghetti AF (2002) Stabilization of hsp70 mRNA on prolonged cell exposure to hypertonicity. Biochim Biophys Acta 1592:135–140
Alfieri RR, Petronini PG, Bonelli MA, Desenzani S, Cavazzoni A, Borghetti AF, Wheeler KP (2004) Roles of compatible osmolytes and heat shock protein 70 in the induction of tolerance to stresses in porcine endothelial cells. J Physiol 555:757–767
Alfieri RR, Bonelli MA, Petronini PG, Desenzani S, Cavazzoni A, Borghetti AF, Wheeler KP (2005) Hypertonic stress and amino acid deprivation both increase expression of mRNA for amino acid transport system A. J Gen Physiol 125:37–39
Alfieri RR, Bonelli MA, Cavazzoni A, Brigotti M, Fumarola C, Sestili P, Mozzoni P, De Palma G, Mutti A, Carnicelli D, Vacondio F, Silva C, Borghetti AF, Wheeler KP, Petronini PG (2006) Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol 576:391–401
Alfieri RR, Bonelli MA, Pedrazzi G, Desenzani S, Ghillani M, Fumarola C, Ghibelli L, Borghetti AF, Petronini PG (2006) Increased levels of inducible HSP70 in cells exposed to electromagnetic fields. Radiat Res 165:95–104
Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH (2006) WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA 103:10883–10888
Aramburu J, Drews-Elger K, Estrada-Gelonch A, Minguillon J, Morancho B, Santiago V, Lopez-Rodriguez C (2006) Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol 72(11):1597–1604
Bader SB, Price BD, Mannheim-Rodman LA, Calderwood SK (1992) Inhibition of heat shock gene expression does not block the development of thermotolerance. J Cell Physiol 151:56–62
Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499–506
Barbe MF, Tytell M, Gower DJ, Welch WJ (1988) Hyperthermia protects against light damage in the rat retina. Science 241:1817–1820
Beck FX, Grunbein R, Lugmayr K, Neuhofer W (2000) Heat shock proteins and the cellular response to osmotic stress. Cell Physiol Biochem 10:303–306
Beck FX, Neuhofer W (2005) Response of renal medullary cells to osmotic stress. Contrib Nephrol 148:21–34
Bevilacqua E, Bussolati O, Dall’Asta V, Gaccioli F, Sala R, Gazzola GC, Franchi-Gazzola R (2005) SNAT2 silencing prevents the osmotic induction of transport system A and hinders cell recovery from hypertonic stress. FEBS Lett 579:3376–3380
Bharadwaj S, Ali A, Ovsenek N (1999) Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 in vivo. Mol Cell Biol 19:8033–8041
Bortner CD, Cidlowski JA (1996) Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. Am J Physiol 271:C950–C961
Bortner CD, Cidlowski JA (1998) A necessary role for cell shrinkage in apoptosis. Biochem Pharmacol 56:1549–1559
Brigotti M, Petronini PG, Carnicelli D, Alfieri RR, Bonelli MA, Borghetti AF, Wheeler KP (2003) Effects of osmolarity, ions and compatible osmolytes on cell-free protein synthesis. Biochem J 369:369–374
Bruhat A, Jousse C, Wang XZ, Ron D, Ferrara M, Fafournoux P (1997) Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem 272:17588–17593
Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ Jr (2001) Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102–107
Burg MB (1995) Molecular basis of osmotic regulation. Am J Physiol 268:F983–F996
Burg MB, Kwon HM, Kultz D (1997) Regulation of gene expression by hypertonicity. Ann Rev Physiol 59:437–455
Bussolati O, Dall’Asta V, Franchi-Gazzola R, Sala R, Rotoli BM, Visigalli R, Casado J, Lopez-Fontanals M, Pastor-Anglada M, Gazzola GC (2001) The role of system A for neutral amino acid transport in the regulation of cell volume. Mol Membr Biol 18:27–38
Cai Q, Michea L, Andrews P, Zhang Z, Rocha G, Dmitrieva N, Burg MB (2002) Rate of increase of osmolarity determines osmotic tolerance of mouse inner medullary epithelial cells. Am J Physiol 283:F792–F798
Cai Q, Ferraris JD, Burg MB (2004) Greater tolerance of renal medullary cells for a slow increase in osmolality is associated with enhanced expression of HSP70 and other osmoprotective genes. Am J Physiol 286:F58–F67
Cai Q, Ferraris JD, Burg MB (2005) High NaCl increases TonEBP/OREBP mRNA and protein by stabilizing its mRNA. Am J Physiol 289:F803–F807
Cai Q, Dmitrieva NI, Ferraris JD, Michea LF, Salvador JM, Hollander MC, Fornace AJ Jr, Fenton RA, Burg MB (2006) Effects of expression of p53 and Gadd45 on osmotic tolerance of renal inner medullary cells. Am J Physiol 291:F341–F349
Caruccio L, Bae S, Liu AY-C, Chen KY (1997) The heat-shock transcription factor HSF1 is rapidly activated by either hyper- or hypoosmotic stress in mammalian cells. Biochem J 327:341–347
Cohen DM, Wasserman JC, Gullans SR (1991) Immediate early gene and HSP70 expression in hyperosmotic stress in MDCK cells. Am J Physiol 261:C594–C601
Cohen DM (2005) SRC family in cell volume regulation. Am J Physiol 288:C483–C493
Colla E, Lee SD, Sheen MR, Woo SK, Kwon HM (2006) TonEBP is inhibited by RNA helicase A via interaction involving the E’F loop. Biochem J 3931:411–419
Copp J, Wiley S, Ward MW, van der Geer P (2005) Hypertonic shock inhibits growth factor receptor signaling, induces caspase-3 activation, and causes reversible fragmentation of the mitochondrial network. Am J Physiol 288:C403–C415
Dahl SC, Handler JS, Kwon HM (2001) Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol 280:C248–C253
Dall’Asta V, Rossi PA, Bussolati O, Gazzola GC (1994) Response of human fibroblasts to hypertonic stress. Cell shrinkage is counteracted by an enhanced active transport of neutral amino acids. J Biol Chem 269:10485–10491
De Angelis E, Petronini PG, Borghetti P, Borghetti AF, Wheeler KP (1999) Induction of betaine-γ-aminobutyric acid transport activity in porcine chondrocytes exposed to hypertonicity. J Physiol 518:187–194
Denkert C, Warskulat U, Hensel F, Haussinger D (1998) Osmolyte strategy in human monocytes and macrophages: involvement of p38MAPK in hyperosmotic induction of betaine and myo-inositol transporters. Arch Biochem Biophys 354:172–180
Diamant S, Eliahu N, Rosenthal D, Goloubinoff P (2001) Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem 276:39586–39591
Di Ciano-Oliveira C, Sirokmany G, Szaszi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A (2003) Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am J Physiol 285:C555–C566
Di Ciano-Oliveira C, Lodyga M, Fan L, Szaszi K, Hosoya H, Rotstein OD, Kapus A (2005) Is myosin light-chain phosphorylation a regulatory signal for the osmotic activation of the Na+–K+–2Cl− cotransporter? Am J Physiol 289:C68–C81
Di Ciano-Oliveira C, Thirone AC, Szaszi K, Kapus A (2006) Osmotic stress and the cytoskeleton: the R(h)ole of Rho GTPases. Acta Physiol 187:257–272
Didelot C, Schmitt E, Brunet M, Maingret L, Parcellier A, Garrido C (2006) Heat shock proteins: endogenous modulators of apoptotic cell death. Handb Exp Pharmacol 172:171–198
Dmitrieva N, Kultz D, Michea L, Ferraris J, Burg MB (2000) Protection of renal inner medullary epithelial cells from apoptosis by hypertonic stress-induced p53 activation. J Biol Chem 275:18243–18247
Dmitrieva NI, Michea L, Burg MB (2001) p53 protects renal inner medullary cells from hypertonic stress by restricting DNA replication. Am J Physiol 281:F522–F530
Dmitrieva NI, Bulavin DV, Fornace AJ Jr, Burg MB (2002) Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. Proc Natl Acad Sci USA 99:184–189
Dmitrieva NI, Bulavin DV, Burg MB (2003) High NaCl causes Mre11 to leave the nucleus, disrupting DNA damage signaling and repair. Am J Physiol 285:F266–F274
Dmitrieva NI, Cai Q, Burg MB (2004) Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc Natl Acad Sci USA 101:2317–2322
Dmitrieva NI, Burg MB (2005) Hypertonic stress response. Mutat Res 569:65–74
Dmitrieva NI, Celeste A, Nussenzweig A, Burg MB (2005) Ku86 preserves chromatin integrity in cells adapted to high NaCl. Proc Natl Acad Sci USA 102:10730–10735
Dowd BF, Forbush B (2003) PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na–K–Cl cotransporter (NKCC1). J Biol Chem 278:27347–27353
Franchi-Gazzola R, Visigalli R, Bussolati O, Dall’Asta V, Gazzola GC (1999) Adaptive increase of amino acid transport system A requires ERK1/2 activation. J Biol Chem 274:28922–28928
Franchi-Gazzola R, Visigalli R, Dall’Asta V, Sala R, Woo SK, Kwon HM, Gazzola GC, Bussolati O (2001) Amino acid depletion activates TonEBP and sodium-coupled inositol transport. Am J Physiol 280:C1465–C1474
Franchi-Gazzola R, Sala R, Bussolati O, Visigalli R, Dall’Asta V, Ganapathy V, Gazzola GC (2001) The adaptive regulation of amino acid transport system A is associated to changes in ATA2 expression. FEBS Lett 490:11–14
Franchi-Gazzola R, Gaccioli F, Bevilacqua E, Visigalli R, Dall’Asta V, Sala R, Varoqui H, Erickson JD, Gazzola GC, Bussolati O (2004) The synthesis of SNAT2 transporters is required for the hypertonic stimulation of system A transport activity. Biochim Biophys Acta 1667:157–166
Franco DL, Nojek IM, Molinero L, Coso OA, Costas MA (2002) Osmotic stress sensitizes naturally resistant cells to TNF-alpha-induced apoptosis. Cell Death Differ 9:1090–1098
Friis MB, Friborg CR, Schneider L, Nielsen MB, Lambert IH, Christensen ST, Hoffmann EK (2005) Cell shrinkage as a signal to apoptosis in NIH 3T3 fibroblasts. J Physiol 567:427–443
Fumarola C, Zerbini A, Guidotti GG (2001) Glutamine deprivation-mediated cell shrinkage induces ligand-independent CD95 receptor signaling and apoptosis. Cell Death Differ 8:1004–1013
Gaccioli F, Huang CC, Wang C, Bevilacqua E, Franchi-Gazzola R, Gazzola GC, Bussolati O, Snider MD, Hatzoglou M (2006) Amino acid starvation induces the SNAT2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2 alpha phosphorylation and cap-independent translation. J Biol Chem 281:17929–17940
Garrido C, Gurbuxani S, Ravagnan L, Kroemer G (2001) Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 286:433–442
Go WY, Liu X, Roti MA, Liu F, Ho SN (2004) NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101:10673–10678
Heo JI, Lee MS, Kim JH, Lee JS, Kim J, Park JB, Lee JY, Han JA, Kim JI (2006) The role of tonicity responsive enhancer sites in the transcriptional regulation of human hsp70-2 in response to hypertonic stress. Exp Mol Med 38:295–301
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372
Holmberg CI, Hietakangas V, Mikhailov A, Rantanen JO, Kallio M, Meinander A, Hellman J, Morrice N, MacKintosh C, Morimoto RI, Eriksson JE, Sistonen L (2001) Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J 20:3800–3810
Huang Z, Tunnacliffe A (2004) Response of human cells to desiccation: comparison with hyperosmotic stress response. J Physiol 558:181–191
Huang Z, Tunnacliffe A (2005) Gene induction by desiccation stress in human cell cultures. FEBS Lett 579:4973–4977
Irarrazabal CE, Liu JC, Burg MB, Ferraris JD (2004) ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 101:8809–8814
Jacquier-Sarlin MR, Jornot L, Polla BS (1995) Differential expression and regulation of hsp70 and hsp90 by phorbol esters and heat shock. J Biol Chem 270:14094–14099
Jeon US, Kim JA, Sheen MR, Kwon HM (2006) How tonicity regulates genes: story of TonEBP transcriptional activator. Acta Physiol 187:241–247
Jurivich DA, Sistonen L, Kroes RA, Morimoto RI (1992) Effect of sodium salicylate on the human heat shock response. Science 255:1243–1245
Jurivich DA, Pachetti C, Qiu L, Welk JF (1995) Salicylate triggers heat shock factor differently than heat. J Biol Chem 270:24489–24495
Kaarniranta K, Elo M, Sironen R, Lammi MJ, Goldring MB, Eriksson JE, Sistonen L, Helminen HJ (1998) Hsp70 accumulation in chondrocytic cells exposed to high continuous hydrostatic pressure coincides with mRNA stabilization rather than transcriptional activation. Proc Natl Acad Sci USA 95:2319–2324
Kaarniranta K, Holmberg CI, Helminen HJ, Eriksson JE, Sistonen L, Lammi MJ (2000) Protein synthesis is required for stabilization of hsp70 mRNA upon exposure to both hydrostatic pressurization and elevated temperature. FEBS Lett 475:283–286
Kabakov AE, Budagova KR, Latchman DS, Kampinga HH (2002) Stressful preconditioning and HSP70 overexpression attenuate proteotoxicity of cellular ATP depletion. Am J Physiol 283:C521–C534
Kabakov AE, Malyutina YV, Latchman DS (2006) Hsf1-mediated stress response can transiently enhance cellular radioresistance. Radiat Res 165:410–423
Kitamura H, Yamauchi A, Nakanishi T, Takamitsu Y, Sugiura T, Akagi A, Moriyama T, Horio M, Imai E (1997) Effects of inhibition of myo-inositol transport on MDCK cells under hypertonic environment. Am J Physiol 272:F267–F272
Klein JD, O’Neill WC (1995) Volume-sensitive myosin phosphorylation in vascular endothelial cells: correlation with Na–K–2Cl cotransport. Am J Physiol 269:C1524–C1531
Klein JD, Lamitina ST, O’Neill WC (1999) JNK is a volume-sensitive kinase that phosphorylates the Na–K–2Cl cotransporter in vitro. Am J Physiol 277:C425–C431
Kojima R, Randall J, Brenner BM, Gullans SR (1996) Osmotic stress protein 94 (Osp94). A new member of the Hsp110/SSE gene subfamily. J Biol Chem 271:12327–12332
Kojima R, Randall JD, Ito E, Manshio H, Suzuki Y, Gullans SR (2004) Regulation of expression of the stress response gene, Osp94: identification of the tonicity response element and intracellular signalling pathways. Biochem J 380:783–794
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J App Physiol 92:2177–2186
Kultz D, Madhany S, Burg MB (1998) Hyperosmolality causes growth arrest of murine kidney cells. Induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2. J Biol Chem 273:13645–13651
Kultz D, Chakravarty D (2001) Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc Natl Acad Sci USA 98:1999–2004
Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306
Lang F, Madlung J, Bock J, Lukewille U, Kaltenbach S, Lang KS, Belka C, Wagner CA, Lang HJ, Gulbins E, Lepple-Wienhues A (2000) Inhibition of Jurkat-T-lymphocyte Na+/H+-exchanger by CD95(Fas/Apo-1)-receptor stimulation. Pflugers Arch 440:902–907
Lang KS, Fillon S, Schneider D, Rammensee HG, Lang F (2002) Stimulation of TNF alpha expression by hyperosmotic stress. Pflugers Arch 443:798–803
Lee SD, Colla E, Sheen MR, Na KY, Kwon HM (2003) Multiple domains of TonEBP cooperate to stimulate transcription in response to hypertonicity. J Biol Chem 278:47571–47577
Lee JS, Lee JJ, Seo JS (2005) HSP70 deficiency results in activation of c-Jun N-terminal Kinase, extracellular signal-regulated kinase, and caspase-3 in hyperosmolarity-induced apoptosis. J Biol Chem 280:6634–6641
Lepore DA, Knight KR, Anderson RL, Morrison WA (2001) Role of priming stresses and Hsp70 in protection from ischemia-reperfusion injury in cardiac and skeletal muscle. Cell Stress Chaperones 6:93–96
Lezama R, Diaz-Tellez A, Ramos-Mandujano G, Oropeza L, Pasantes-Morales H (2005) Epidermal growth factor receptor is a common element in the signaling pathways activated by cell volume changes in isosmotic, hyposmotic or hyperosmotic conditions. Neurochem Res 30:1589–1597
Li GC, Werb Z (1982) Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster ovary cells. Proc Natl Acad Sci USA 79:3218–3222
Lindquist S, Craig EA (1988) The heat shock proteins. Annu Rev Genet 22:631–677
López-Fontanals M, Rodríguez-Mulero S, Casado FJ, Dérijard B, Pastor-Anglada M (2003) The osmoregulatory and the amino acid-regulated responses of system A are mediated by different signal transduction pathways. J Gen Physiol 122:5–16
Lytle C, McManus T (2002) Coordinate modulation of Na–K–2Cl cotransport and K–Cl cotransport by cell volume and chloride. Am J Physiol 283:C1422–C1431
Maallem S, Berod A, Mutin M, Kwon HM, Tappaz ML (2006) Large discrepancies in cellular distribution of the tonicity-induced expression of osmoprotective genes and their regulatory transcription factor TonEBP in rat brain. Neuroscience 142:355–368
Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y (2000) Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA 97:9487–9492
Mak SK, Kultz D (2004) Gadd45 proteins induce G2/M arrest and modulate apoptosis in kidney cells exposed to hyperosmotic stress. J Biol Chem 279:39075–39084
Matsuoka Y, Yamauchi A, Nakanishi T, Sugiura T, Kitamura H, Horio M, Takamitsu Y, Ando A, Imai E, Hori M (1999) Response to hypertonicity in mesothelial cells: role of Na+/myo-inositol co-transporter. Nephrol Dial Transplant 14:1217–1223
Mackenzie B, Erickson JD (2004) Sodium-coupled neutral amino acid (system N/A) transporters of the SLC38 gene family. Pflügers Arch 447:784–795
McDowell HE, Eyers PA, Hundal HS (1998) Regulation of system A amino acid transport in L6 rat skeletal muscle cells by insulin, chemical and hyperthermic stress. FEBS Lett 441:15–19
McGivan JD, Pastor-Anglada M (1994) Regulatory and molecular aspects of mammalian amino acid transport. Biochem J 299:321–334
McManus ML, Churchwell KB, Strange K (1995) Regulation of cell volume in health and disease. N Engl J Med 333:1260–1266
Michea L, Combs C, Andrews P, Dmitrieva N, Burg MB (2002) Mitochondrial dysfunction is an early event in high-NaCl-induced apoptosis of mIMCD3 cells. Am J Physiol 282:F981–F990
Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM (1999) Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA 96:2538–2542
Mongin AA, Orlov SN (2001) Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology 8:77–88
Morimoto RI, Tissieres A, Georgopoulos C (1990) The stress response, function of the proteins and perspectives. In: Morimoto RI, Tissieres A, Georgopoulos C (eds) Stress proteins in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 1–36
Morimoto RI, Milarski KL (1990) Expression and function of vertebrate hsp70 genes. In: Morimoto RI, Tissieres A, Georgopoulos C (eds) Stress proteins in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 323–359
Morishima N (2005) Control of cell fate by Hsp70: more than an evanescent meeting. J Biochem 137:449–453
Mow VC, Wang CC, Hung CT (1999) The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthr Cartil 7:41–58
Na KY, Woo SK, Lee SD, Kwon HM (2003) Silencing of TonEBP/NFAT5 transcriptional activator by RNA interference. J Am Soc Nephrol 14:283–288
Nahm O, Woo SK, Handler JS, Kwon M (2002) Involvement of multiple kinase pathways in stimulation of gene transcription by hypertonicity. Am J Physiol 282:C49–C58
Neuhofer W, Müller E, Burger-Kentischer A, Frack ML, Thurau K, Beck FX (1998) Pretreatment with hypertonic NaCl protects MDCK cells against high urea concentrations. Pflugers Arch 435:407–414
Neuhofer W, Muller E, Burger-Kentischer A, Fraek ML, Thurau K, Beck FX (1999) Inhibition of NaCl-induced heat shock protein 72 expression renders MDCK cells susceptible to high urea concentrations. Pflugers Arch 437:611–616
Neuhofer W, Fraek ML, Ouyang N, Beck FX (2005) Differential expression of heat shock protein 27 and 70 in renal papillary collecting duct and interstitial cells—implications for urea resistance. J Physiol 564:715–722
Neuhofer W, Beck FX (2006) Survival in hostile environments: strategies of renal medullary cells. Physiology 21:171–180
Nylandsted J, Jaattela M, Hoffmann EK, Pedersen SF (2004) Heat shock protein 70 inhibits shrinkage-induced programmed cell death via mechanisms independent of effects on cell volume-regulatory membrane transport proteins. Pflugers Arch 449:175–185
Olsen M, Sarup A, Larsson OM, Schousboe A (2005) Effect of hyperosmotic conditions on the expression of the betaine-GABA-transporter (BGT-1) in cultured mouse astrocytes. Neurochem Res 30:855–865
Oxender DL, Christensen HN (1963) Distinct mediating systems for the transport of neutral amino acids by the Ehrlich cell. J Biol Chem 238:3686–3699
Padda R, Wamsley-Davis AM, Gustin MC, Ross R, Yu C, Sheikh-Hamad D (2006) MEKK3-mediated signaling to p38 kinase and TonE in hypertonically stressed kidney cells. Am J Physiol 291:F874–F881
Palii SS, Thiaville MM, Pan YX, Zhong C, Kilberg MS (2006) Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) system A transporter gene. Biochem J 395:517–527
Parsell DA, Taulien J, Lindquist S (1993) The role of heat-shock proteins in thermotolerance. Philos Trans R Soc Lond B Biol Sci 339:279–285
Petronini PG, Tramacere R, Kay JE, Borghetti AF (1986) Adaptive response of cultured fibroblasts to hyperosmolarity. Exp Cell Res 165:180–190
Petronini PG, Tramacere M, Wheeler KP, Borghetti AF (1990) Induction of amino acid transport activity in chick embryo fibroblasts by replacement of extracellular sodium chloride with disaccharide. Biochim Biophys Acta 1053:144–150
Petronini PG, Alfieri RR, De Angelis E, Campanini C, Borghetti AF, Wheeler KP (1993) Different HSP70 expression and cell survival during adaptive responses of 3T3 and transformed 3T3 cells to osmotic stress. Brit J Cancer 67:493–499
Petronini PG, Alfieri RR, Losio MN, Caccamo AE, Cavazzoni A, Bonelli MA, Borghetti AF, Wheeler KP (2000) Induction of BGT1 and amino acid system A transport activities in endothelial cells exposed to hyperosmolarity. Am J Physiol 279:R1580–R1589
Petronini PG, Caccamo AE, Alfieri RR, Bonelli MA, Borghetti AF (2001) The effect of heat shock on amino acid transport and cell volume in 3T3 cells. Amino Acids 20:363–380
Piechotta K, Lu J, Delpire E (2002) Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277:50812–50819
Plumier JC, Currie RW (1996) Heat shock-induced myocardial protection against ischemic injury: a role for Hsp70? Cell Stress Chaperones 1:13–17
Proft M, Mas G, de Nadal E, Vendrell A, Noriega N, Struhl K, Posas F (2006) The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol Cell 23:241–250
Qu Y, Bolen CL, Bolen DW (1998) Osmolyte-driven contraction of a random coil protein. Proc Natl Acad Sci USA 95:9268–9273
Reimer RJ, Chaudhry FA, Gray AT, Edwards RH (2000) Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc Natl Acad Sci USA 97:7715–7720
Reyna SV, Ensenat D, Johnson FK, Wang H, Schafer AI, Durante W (2004) Cyclic strain stimulates l-proline transport in vascular smooth muscle cells. Am J Hypertens 17:712–717
Santos BC, Chevaile A, Kojima R, Gullans SR (1998) Characterization of the Hsp110/SSE gene family response to hyperosmolality and other stresses. Am J Physiol 274:F1054–F1061
Santos BC, Pullman JM, Chevaile A, Welch WJ, Gullans SR (2003) Chronic hyperosmolarity mediates constitutive expression of molecular chaperones and resistance to injury. Am J Physiol 284:F564–F574
Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E (2006) RNA-mediated response to heat shock in mammalian cells. Nature 440:556–560
Sheikh-Hamad D, Garcia-Perez A, Ferraris JD, Peters EM, Burg MB (1994) Induction of gene expression by heat shock versus osmotic stress. Am J Physiol 267:F28–F34
Sheikh-Hamad D, Gustin MC (2004) MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am J Physiol 287:F1102–F1110
Shim EH, Kim JI, Bang ES, Heo JS, Lee JS, Kim EY, Lee JE, Park WY, Kim SH, Kim HS, Smithies O, Jang JJ, Jin DI, Seo JS (2002) Targeted disruption of hsp70.1 sensitizes to osmotic stress. EMBO Rep 3:857–861
Strange K (2004) Cellular volume homeostasis. Adv Physiol Educ 28:155–159
Sugawara M, Nakanishi T, Fei Y-J, Huang W, Ganapathy ME, Leibach FH, Ganapathy V (2000) Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J Biol Chem 275:16473–16477
Takanaga H, Tokuda N, Ohtsuki S, Hosoya KI, Terasaki T (2002) ATA2 is predominantly expressed as system A at the blood–brain barrier and acts as brain-to-blood efflux transport for l-proline. Mol Pharmacol 61:1289–1296
Trama J, Go WY, Ho SN (2002) The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol 169:5477–5488
Tramacere M, Petronini PG, Severini A, Borghetti AF (1984) Osmoregulation of amino acid transport activity in cultured fibroblasts. Exp Cell Res 151:70–79
Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV (2006) TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem 281:25416–25424
Tong EH, Guo JJ, Huang AL, Liu H, Hu CD, Chung SS, Ko BC (2006) Regulation of nucleocytoplasmic trafficking of transcription factor OREBP/TonEBP/NFAT5. J Biol Chem 281:23870–23879
Urban JP, Hall AC, Gehl KA (1993) Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol 154:262–270
Valkova N, Kultz D (2006) Constitutive and inducible stress proteins dominate the proteome of the murine inner medullary collecting duct-3 (mIMCD3) cell line. Biochim Biophys Acta 1764:1007–1020
Walter L, Rauh F, Gunther E (1994) Comparative analysis of the three major histocompatibility complex-linked heat shock protein 70 (Hsp70) genes of the rat. Immunogenetics 40:325–330
Wang YH, Borkan SC (1996) Prior heat stress enhances survival of renal epithelial cells after ATP depletion. Am J Physiol 270:F1057–F1065
Wang YH, Knowlton AA, Li FH, Borkan SC (2002) Hsp72 expression enhances survival in adenosine triphosphate-depleted renal epithelial cells. Cell Stress Chaperones 7:137–145
Warskulat U, Reinen A, Grether-Beck S, Krutmann J, Haussinger D (2004) The osmolyte strategy of normal human keratinocytes in maintaining cell homeostasis. J Invest Dermatol 123:516–521
Welch WJ, Brown CR (1996) Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1:109–115
Woo SK, Dahl SC, Handler JS, Kwon HM (2000) Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. Am J Physiol 278:F1006–F1012
Woo SK, Lee SD, Kwon HM (2002a) TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch 444:579–585
Woo SK, Lee SD, Na KY, Park WK, Kwon HM (2002b) TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol 22:5753–5760
Wu KL, Khan S, Lakhe-Reddy S, Wang L, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR (2003) Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol 284:F829–F839
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD (2000) A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem 275:22790–22797
Zhan Q, Antinore MJ, Wang XW, Carrier F, Smith ML, Harris CC, Fornace AJ Jr (1999) Association with Cdc2 and inhibition of Cdc2/cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 18:2892–2900
Zhang Z, Ferraris JD, Brooks HL, Brisc I, Burg MB (2003) Expression of osmotic stress-related genes in tissues of normal and hyposmotic rats. Am J Physiol 285:F688–F693
Zhang Z, Ferraris JD, Irarrazabal CE, Dmitrieva NI, Park JH, Burg MB (2005) Ataxia telangiectasia-mutated, a DNA damage-inducible kinase, contributes to high NaCl-induced nuclear localization of transcription factor TonEBP/OREBP. Am J Physiol 289:F506–F511
Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408:433–439
Zhou C, Li Z, Diao H, Yu Y, Zhu W, Dai Y, Chen FF, Yang J (2006) DNA damage evaluated by gammaH2AX foci formation by a selective group of chemical/physical stressors. Mutat Res 604:8–18
Acknowledgments
The authors thank Dr. K.P. Wheeler and Dr. A.F. Borghetti for carefully reading and for the constructive criticism of this manuscript.
We apologize to the colleagues whose works have not been cited in the present review because of space limitations and our inability to find their work in the literature search.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alfieri, R.R., Petronini, P.G. Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch - Eur J Physiol 454, 173–185 (2007). https://doi.org/10.1007/s00424-006-0195-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0195-x