Abstract
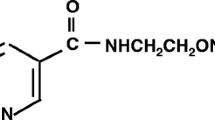
AICAR (5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide) is an adenosine analog which improves the recovery of the heart after ischemia. In some tissues AICAR enters cells and stimulates AMP-activated protein kinase (AMPK). We explored the mechanism of cardioprotection in isolated rat hearts. We confirmed that AICAR (0.5 mM) applied 10 min prior to a 30-min period of ischemia and present throughout ischemia and reperfusion caused a substantial improvement in the recovery of developed pressure on reperfusion. However, adenosine (100 μM) produced no improvement, suggesting that the mechanism of action of AICAR was not increased endogenous adenosine production. Measurements of intracellular sodium concentration ([Na+]i) showed that AICAR prevented the rapid rise of [Na+]i, which normally occurs on reperfusion. Inhibitors of the cardiac sodium–hydrogen exchanger (NHE1) also protect the heart from ischemic damage and also prevent the rapid rise of [Na+]i on reperfusion, suggesting that AICAR might cause the inhibition of NHE1. We tested this possibility on isolated rat ventricular myocytes in which the recovery of pHi after NH4Cl exposure provides a measure of NHE1 activity. AICAR (0.5 μmM) inhibited NHE1 activity in response to an acid load by about 80%. To test whether the AICAR-induced inhibition of NHE1 arose through adenosine, we used the adenosine receptor blocker 8-sulfophenyltheophylline (8-SPT) and found that it had no measureable effect. To test whether the AICAR-induced inhibition of NHE1 might occur through the activation of AMPK, we measured the activity of two isoforms of AMPK. Surprisingly, activity was reduced, whereas in many other tissues AICAR increases AMPK activity. Furthermore, this effect of AMPK was blocked by 8-SPT, suggesting that the inhibition of AMPK arose through an adenosine-receptor-related pathway. We conclude that AICAR inhibits NHE1 through an unidentified pathway. This inhibition may make a contribution to the cardioprotective effects of AICAR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
AICAR [5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide; 5-aminoimidazole-4-carboxamide (AICA) riboside; acadesine] is an analog of adenosine that can enter cardiac cells and has a number of effects on cell metabolism (for review see Cook and Karmazyn [8] and Mullane [33]). Its principal effects are to inhibit adenosine kinase and adenosine deaminase with the net effect that adenosine production is increased, though only in the ischemic or metabolically inhibited heart. In some cell types AICAR is phosphorylated to AICA ribotide (ZMP) (an analog of AMP) and this analog is capable of stimulating AMP-activated protein kinase (AMPK) [9, 17]. ZMP may also enter the de novo pathway for adenosine synthesis and inhibit AMP deaminase, and both pathways may contribute to the increase in adenosine and/or ATP levels. However, there is a dispute as to whether ZMP is produced in the heart, with several studies showing effects attributed to AMP kinase activation [27, 37], but one study showing no production of ZMP in the heart in contrast to the liver [18].
The earliest studies of AICAR suggested that it might increase ATP [40] and led to investigations of whether AICAR might protect the heart from ischemia. For instance, Galinanes et al. [13, 14] showed that AICAR provided protection against ischemic damage in the rat heart both in an acute ischemia/reperfusion model and in a transplantation model involving prolonged ischemia. These and other successful animal experiments have led to a series of clinical trials on the value of AICAR on the incidence of ischemic damage to hearts following coronary bypass grafting. Individual trials produced only minor benefits, which were not statistically significant [31], but a meta-analysis of five such trials showed that AICAR reduced both the frequency of myocardial infarcts and early death after coronary bypass surgery [28].
Although AICAR exerts beneficial effects on recovery from ischemia, the mechanisms involved remain uncertain. The early suggestion that the benefits arose from improved levels of ATP have not been confirmed [14, 30, 34]. Instead, it has been proposed that the benefits arise from the increased production of adenosine [16] and are secondary to the cardioprotective effects of adenosine. In support of this theory, it has been shown that the protective effects of AICAR were blocked by the adenosine receptor blocker 8-sulfophenyltheophylline (8-SPT) [21, 33, 46]. While this may be the mechanism of cardioprotection in some species, in the rat heart there is a dispute as to whether adenosine is cardioprotective, with studies both demonstrating [12, 26] and failing to demonstrate [6, 25] cardioprotection (for review, see Ganote and Armstrong [15]). Thus, it is possible that other mechanisms of action of AICAR may be important in the rat heart.
In the present study, we reinvestigated the actions of AICAR in a model of ischemic/reperfusion damage in the isolated rat heart, with the aim of determining the mechanism of cardioprotection.
Materials and methods
The experiments were performed on hearts from female Sprague–Dawley rats and were approved by the Animal Ethical Committee of the University of Sydney. Rats (200–250 g) were anesthetized with pentobarbitone (100 mg/kg) and the hearts were excised and perfused at a constant flow rate of 10 ml/min (12–15 ml/min/g wet weight) with Tyrode’s solution at 37°C. The perfusate had the following composition (in mM): NaCl 119, KCl 4, NaH2PO4 1.2, MgSO4 1.2, NaHCO3 25, CaCl2 1, and glucose 11. The solutions were equilibrated with 95% O2/5% CO2 to give a pH of 7.4. AICAR was obtained from Toronto Research Chemicals, Canada; other drugs used in this study (adenosine and 8-SPT) were obtained from Sigma-Aldrich. All drugs were dissolved in a concentrated stock solution in water immediately prior to use.
Langendorff-perfused hearts
Hearts were continuously stimulated at 2 Hz after the sinoatrial node was excised and the atrio-ventricular node was crushed. The low rate of stimulation was chosen to minimize the consequences of the low O2 content of the perfusate, which lacks hemoglobin [1]. Isovolumic left ventricular developed pressure (LVDP) was monitored with a balloon in the left ventricle. Ischemia was produced by stopping perfusion inflow to the heart while the heart was maintained at 37°C. The standard period of ischemia was 30 min and was followed by 30 min of reperfusion.
AICAR was added to the perfusate 10 min before the period of ischemia and the same perfusate was used for reperfusion—thus, AICAR was present before, during, and after the 30 min of ischemia. In preliminary experiments, we determined that the beneficial effects of AICAR on recovery from ischemia were near-maximal at 0.5 mM, and this concentration was subsequently used in the main study. Adenosine was administered using this protocol and using a separate protocol in which adenosine was only present for between 15 and 5 min before the start of the ischemia.
During ischemia, the LVDP declined rapidly and a contracture developed. We measured the peak of this ischemic contracture (mm Hg) and estimated the onset time from the time after the start of ischemia for the contracture to reach 10% of its maximum. Recovery from ischemia was assessed by magnitude of the LVDP measured after 30 min of reperfusion and was expressed as percent of the preischemia LVDP. Recovery was also assessed from the magnitude of the reperfusion contracture measured as the increase in diastolic pressure from the end of ischemia to the peak during reperfusion (mm Hg).
Intracellular sodium measurements
[Na+]i was measured using the fluorescent indicator sodium-binding benzofuran isophthalate (SBFI) loaded in its membrane-permeable acetoxymethyl (AM) ester form. The hearts were placed in a chamber mounted on the stage of an inverted microscope modified for fluorescence measurements. Briefly, after measuring the autofluorescence, the hearts were loaded for 40–60 min by perfusion with SBFI–AM. After loading, the hearts were illuminated by rapidly alternating light at 340 and 380 nm using a spinning wheel device, and fluorescence was recorded at 530 nm. The fluorescence signals from SBFI were converted to [Na+]i using established calibration methods; correction was made for the changes in autofluorescence that occur during ischemia. We have previously established that this method measures [Na+]i in the epicardium and myocardium to a depth of about 0.1 to 0.2 mm. Full details of the methods for the use of SBFI have been published [35]. The [Na+]i was measured at the end of the control period and at the end of the 30-min ischemia, and the rise in [Na+]i during ischemia was calculated as the difference. On reperfusion, [Na+]i rises rapidly, reaching a peak at about 5 min, e.g., Fig. 2a. When present, we measured the peak [Na+]i; when this peak was absent, e.g., Fig. 2b, the [Na+]i after 5 min of reperfusion was measured. The change in [Na+]i on reperfusion was the difference between [Na+]i at the end of ischemia and the value after 5 min of reperfusion.
Isolated ventricular cells
Ventricular myocytes were used for the measurements of sodium–hydrogen exchanger (NHE1) activity. Myocytes were isolated from Langendorff-perfused rat hearts using standard methods with low extracellular calcium and collagenase [5]. The cells were loaded with a pH-sensitive fluorescence dye, seminaphthorhodafluors–AM (5 μM for 20 min at 22–24°C). Healthy cells were selected for experimentation based on morphology (distinctive rod shape, well-defined cell edges, and clear striations) and function (absence of spontaneous contractions and brisk response to electrical stimulation in Ca2+-containing solution). The isolated cells were used at room temperature (23°C) and in a nominally bicarbonate-free solution (buffered with 4-2-hydroxyethyl-1-piperazineethanesulfonic acid). Under these conditions it is known that NHE1 is the most important pathway causing recovery from an acid load [24]. Healthy cells were exposed to NH4Cl (20 mM) for 2 min, which causes an alkalosis. Upon removal of NH4Cl, the cells rapidly become acidotic, and the rate of recovery from acidosis is a measure of NHE1 activity [24]. NHE1 activity was measured by the rate of change of pHi between 6.8 and 6.9 and multiplying this value by the pH buffering of the cell [3] (for details, see Leem et al. [24]). The value of proton efflux (JH) obtained by this method was typically 0.5–1.0 mM/min. The effects of various drugs on NHE1 activity were determined by comparing JH in the same cell for a control exposure to NH4Cl and a second exposure in the presence of the drug. Typically, the exposures to NH4Cl were 20 min apart, and preliminary experiments showed that the repeated determination of JH at 20-min intervals gave constant results. In preliminary experiments, we tested the effects of 50, 100, 500, 1,000, and 2,000 μM AICAR on JH and found that 500, 1,000, and 2,000 μM all inhibited NHE1 by ≥75%, and we therefore used 500 or 1,000 μM for subsequent experiments.
Activity of AMP kinase
Rat hearts were perfused for 20 min with standard Tyrode’s solution and then perfused with various drugs for 10 min. The hearts were then rapidly frozen in liquid N2 and homogenized in the following buffer: 50 mM Tris–HCl, pH 7.5, 1 mM EDTA, 1 mM ethylene glycol bis(2-aminoethyl ether)-N,N,N′N′-tetraacetic acid, 50 mM NaF, 5 mM Na pyrophosphate, 10% glycerol, 1% Triton-X-100, 10 μg/ml trypsin inhibitor, 2 μg/ml aprotinin, 1 mM benzamidine, and 1 mM phenylmethylsulphonylfluoride. The homogenate was briefly centrifuged to remove cell debris, and the supernatant was immunoprecipitated with AMPK-isoform-specific antibodies. These antibodies were prepared locally (for details, see Chen et al. [7]). The AMPK activities in the immune complexes were measured in the presence of 200 μM AMP. Activities were calculated as picomoles of phosphate incorporated into the SAMS peptide [ACCα (73–87)A77] per minute per milligram of total protein subjected to immunoprecipitation (for details, see Chen et al. [7]).
Statistics
All data are expressed as mean±SEM. The comparison between treatment groups was made by one-way ANOVA using the Student–Newman–Keuls correction for multiple comparisons. Statistical significance was taken as P<0.05. The statistical significances of selected comparisons are given in the text and in the figures.
Results
Langendorff-perfused hearts
Figure 1a shows representative results of left ventricular developed pressure from a rat heart subjected to 30 min of ischemia followed by reperfusion. In control hearts (n=10), an ischemic contracture developed after 18±3 min and reached a magnitude of 56±5 mm Hg. The LVDP recovered to 12±2 mm Hg after 30 min reperfusion, which was 13±2% of the control (preischemic) LVDP. The reperfusion contracture was 68±8 mmHg. When AICAR (0.5 mM) was applied to the perfused heart (Fig. 1b), it generally had little effect on the LVDP during the control period, apart from a small transient fall probably caused by a transient temperature difference associated with the solution change. In AICAR-treated hearts (n=6), there was no significant effect of AICAR on the LVDP after 10 min of control perfusion. In the AICAR-treated hearts the onset of the ischemic contracture was not significantly different from that of the controls (19±4 min), but the magnitude of the ischemic contracture (79±6 mm Hg) was significantly larger than that of the controls (P<0.02). The recovery of LVDP after 30 min of reperfusion was greatly improved to 65±10 mm Hg or 70±11% of the control value (P<0.001), and the reperfusion contracture (measured from the end of ischemia to the peak during reperfusion) was reduced to 33±11 mm Hg (P<0.02). Thus, AICAR produces a large improvement in postischemic recovery in agreement with many previous reports.
LVDP in the rat heat during exposure to 30 min of ischemia at 37°C. a Control heart. Note large additional contracture during early reperfusion and failure of developed pressure to recover on reperfusion. b Heart treated with 0.5 mM AICAR for 10 min preceding ischemia. The AICAR was present during ischemia and in the reperfusion solution. Note the large recovery of developed pressure. c Collected data of the magnitude of developed pressure after ischemia. Data for adenosine (100 μM) are also included (see “Results”). Bars show mean±SEM. ** significantly larger than control, P<0.01, ANOVA. Control hearts n=10; AICAR-treated hearts n=6; adenosine-treated hearts n=3
Given the proposal that AICAR might improve postischemic performance by increasing adenosine production [16], we reinvestigated the effects of adenosine on performance postischemia/reperfusion. In three hearts, 100 μM adenosine was applied for 10 min before ischemia and remained present during ischemia and reperfusion. In these hearts, the recovery of developed pressure was not significantly different from that of the control hearts (11±6 mm Hg or 12±5% of control), and the reperfusion contracture was also unchanged (61±6 mm Hg). We also applied adenosine only in the “preconditioning period,” i.e., for 10 min from 15 to 5 min before the ischemia. In three experiments, the LVDP on recovery from ischemia was not significantly different from that of control (15±6 mm Hg), and the reperfusion contracture was also not significantly different from that of control (59±10 mm Hg). There is a dispute in the literature about whether adenosine improves ischemic recovery in the rat heart (see “Discussion”), but our data are similar to those of others, which show no effect of adenosine on ischemic recovery [6, 25].
In a separate series of experiments, we measured the intracellular sodium concentration ([Na+]i) during ischemia and reperfusion. Under control conditions (Fig. 2a), there was only a small rise of [Na+]i during 30 min of ischemia but a pronounced rise of [Na+]i during the first few minutes of reperfusion. We have previously shown that inhibitors of NHE1 abolish the rise of [Na+]i on reperfusion, establishing that the influx of Na+ arises from the activity of NHE1 as the protons accumulated during ischemia are removed [35, 42, 45]. In control experiments (n=6), the increase of [Na+]i during ischemia was 3.1±0.9 mM and the increase of [Na+]i during the early part of reperfusion was 11.9±2.5 mM. When AICAR was applied throughout ischemia and reperfusion (Fig. 2b), the increase of [Na+]i during ischemia was still present but the rise on reperfusion was abolished. In the AICAR-treated hearts (n=5), the increase of [Na+]i during ischemia was 2.5±0.9 mM, which was not significantly different from the control value (P>0.5), but the increase of [Na+]i on reperfusion was absent (increase −1.2±1.3 mM), which was significantly different from the control value (P<0.005).
Intracellular sodium in the rat heart during 30 min of exposure to ischemia. a Control heart. Note that the [Na+]i rises only slightly during ischemia but shows a pronounced rise during reperfusion. b Heart treated before, during, and after ischemia with AICAR (0.5 mM). Note that the reperfusion-induced rise in [Na+]i is absent. c Collected data on the rise of [Na+]i during ischemia (first two bars) and the rise of [Na+]i during reperfusion (second two bars). Bars show mean±SEM. * significantly greater than zero, P<0.05, paired t test; ! significantly less than control, P<0.005, unpaired t test. Control hearts n=6; AICAR-treated hearts n=5
Isolated ventricular myocytes
The rise of [Na+]i on reperfusion is caused by the activity of NHE1-removing protons and causes Na+ influx [35, 42, 45]. NHE1 inhibitors eliminate this rise and, because AICAR also inhibited this rise, we tested the hypothesis that AICAR might inhibit NHE1. We therefore measured the activity of NHE1 in the presence and absence of AICAR using standard acid loading techniques in isolated rat ventricular myocytes. Figure 3a shows representative records of intracellular pHi during the application of NH4Cl and the acidosis which occurs on its removal. The recovery of pHi from this acidosis is largely caused by the activity of NHE1, as is established in Fig. 3a when the NHE1 inhibitor cariporide (20 μM) was applied. Note that this largely abolished pHi recovery; however, normal recovery occurs rapidly when the inhibitor is removed (not shown). Figure 3a shows the effect of AICAR (0.5 mM). Note that it also largely inhibits NHE1 activity. Figure 3b shows group data and statistics for the activity of NHE1, expressed as the percent of control JH during recovery from the acid load. NHE1 activity was inhibited by 20 μM cariporide to 8±2% of the control level (n=6), while 0.5 mM AICAR inhibited NHE1 activity to 21±2% (n=7). These effects of AICAR were reversible and repeatable.
Intracellular pH measurements to determine NHE1 activity. a NH4Cl (20 mM) applied for 2 min in each record (open rectangle); only labeled in record 1 for clarity. The rate of recovery from the acidosis on removal of NH4Cl depends on NHE1 activity. 1 Control record. 2 In the presence of cariporide (20 μM). 3 In the presence of AICAR (0.5 mM). b Collected data showing NHE1 activity (measured as JH as a percentage of control; for details, see “Materials and methods”). Bars show mean±SEM. ** significantly less than control, P<0.00, paired t test. Controls n=13, cariporide-treated cells n=6, and AICAR-treated cells n=7
Given that AICAR inhibits NHE1, we attempted to define the mechanism. Several studies have shown that the cardioprotective effects of AICAR can be prevented by adenosine receptor blockers such as 8-SPT, suggesting that adenosine release and binding to its receptor is an intermediate in the process [21, 33, 46]. In the above studies, AICAR and 8-SPT were infused into intact animals, leading to plasma levels of around 20 and 5 μM, respectively. In the isolated rat heart, the near-maximal cardioprotective effects of AICAR and the inhibition of NHE1 were both observed at around 0.5–1.0 mM, so we tested whether 50 μM 8-SPT was capable of inhibiting the effects. Figure 4a shows data from isolated rat ventricular cells demonstrating (in panel 2) that AICAR inhibits NHE1 (compare panels 1 and 2), and further, that 8-SPT has no effect on this inhibition (compare panels 2 and 3). These results are confirmed in the group data (Fig. 4b), which show that 50 μM 8-SPT had no effect on NHE1 activity alone, nor did it affect the inhibition caused by 1 mM AICAR. We conclude that blocking adenosine receptors does not affect the inhibition of NHE1 activity caused by AICAR.
Effect of AICAR on NHE1 activity. a 1 Intracellular pH in response to 2 min application of 20 mM NH4Cl. Period of NH4Cl shown by open rectangle. 2 Identical to 1 but in the presence of 1 mM AICAR. Note that AICAR slows the rate of recovery of pHi after an acidosis. 3 Identical to 1 but in the presence 50 μM 8-SPT and 1 mM AICAR. b Collected data showing NHE1 activity (measured as JH as a percentage of control; for details, see “Materials and methods”). Bars show mean±SEM. ** significantly reduced compared to control, P<0.01, ANOVA, n=5 for each group
AMP-activated kinase
AICAR has been widely used for its ability to enter the cell, become phosphorylated to ZMP, and stimulate AMPK [7, 17, 37]. Because AICAR inhibited NHE1, we considered the possibility that it might activate AMPK and sought to test this by direct measurements. AMPK activities were measured in AMPK-isoform-specific antibody immunoprecipitate complexes and we confirmed earlier reports [29] that both ischemia and anoxia (O2 replaced by N2) increased AMPK activity (data not shown). However, AICAR treatment inhibited rather than increased both alpha1 and alpha2 AMPK activity (Fig. 5a,b; control hearts n=8; AICAR-treated hearts n=6). A previous report by Longus et al. [27] also failed to show a stimulation of AMPK in the heart during AICAR exposure. We also measured phosphorylation of the Thr-172 site on AMPK and found it decreased by 30% in the presence of AICAR (data not shown), confirming that AMPK activity was reduced by AICAR. Because 8-SPT is capable of reversing some actions of AICAR, we tested whether it influenced the inhibition of AMPK activity produced by AICAR. Figure 5 shows that for both α1 and α2 AMPK activity, 8-SPT (50 μM) did not alter AMPK activity but prevented the inhibitory action of AICAR (AICAR+8-SPT-treated hearts n=4; 8-SPT-treated hearts n=4).
Activity of AMPK measured by incorporation of phosphate into the SAMS peptide (see “Materials and methods”). a Activity of the α1-isoform of AMPK. Bars show mean±SEM. ** significantly less than control, P<0.002, ANOVA, n=8 (control), n=6 (AICAR), n=4 (AICAR+8-SPT), and n=4 (8-SPT). b Activity of the α2-isoform of AMPK. Bars show mean±SEM. * significantly less than control, P<0.02, ANOVA, n=8 (control), n=6 (AICAR), n=4 (AICAR+8-SPT), and n=4 (8-SPT)
Discussion
AICAR inhibits the NHE1, which contributes to cardioprotection in the isolated rat heart
The recovery of intracellular pH (pHi) after an intracellular acid load in a \({\text{HCO}}^{{\text{ - }}}_{{\text{3}}}\) -free solution is generally attributed to the NHE1 [3, 24]. In the heart, NHE1 is the main isoform [20], and it is selectively inhibited by cariporide [38]. Thus, the fact that 20 μM cariporide inhibits proton extrusion to 8±2% of the control level (Fig. 3) confirms that NHE1 is the main mechanism of proton extrusion under these circumstances. We show that AICAR (0.5 mM) reduces proton extrusion to 21±2%, which provides good evidence that AICAR blocks NHE1. We have not explored the concentration sensitivity of AICAR on NHE1 activity extensively, but our preliminary data, which covered a wide range of AICAR concentration, suggests that 50% inhibition occurs at around 50 μM (data not shown).
The data from the isolated perfused hearts is also consistent with this interpretation. The rise of [Na+]i during ischemia and/or reperfusion is thought to be caused by the activity of NHE1, which contributes to the removal of the accumulated protons by exchanging them for extracellular Na+ [20]. It is known, for instance, that in the presence of NHE1-blockers, the rise of [Na+]i on reperfusion is decreased [2, 35, 45], the recovery of pHi is slowed [43], and the mechanical recovery of the heart is greatly improved [19, 45]. The improvement of mechanical recovery by blockers of NHE1 is generally attributed to the “coupled exchanger” theory, which proposes that, during reperfusion, Na+ entry by NHE1 will reduce the inward driving force for Na+. As a consequence, the sodium/calcium exchanger causes a large influx of Ca2+ [20]. The consequent rise of [Ca2+]i is thought to activate proteases and enter mitochondria, leading to the cell damage [4]. In the present study we show that AICAR both improves recovery from ischemia and eliminates the rise of [Na+]i on reperfusion. Coupled with the evidence from ventricular cells that AICAR blocks NHE1, it is clear that AICAR is capable of contributing to cardioprotection by this mechanism.
A number of studies have demonstrated an inverse correlation between Na+ influx on reperfusion and degree of mechanical recovery on reperfusion [32, 41, 44]. Our previous study [44] used a model of ischemia identical to that of the present study and showed a strong correlation between peak [Na+]i on reperfusion and percent of recovery of LVDP under five different experimental conditions (see Fig. 4 in Xiao and Allen [44]). The strength of this correlation suggests that the level of [Na+]i can explain most of the variation in the degree of recovery. If we plot the [Na+]i on reperfusion in AICAR (8.7±0.4 mM) against the recovery of LVDP (70±11%), it lies very close to the line of best fit. This provides further quantitative support for the argument that the inhibition of NHE1 by AICAR makes a large contribution to the cardioprotection that is observed in our experiments.
AICAR also produced an increase in the magnitude of the ischemic contracture. The ischemic contracture is a rigor contracture occurring when ATP reaches low values [11]. Longus et al. [27] have shown that AICAR stimulates glycogenolysis by a non-AMPK-dependent pathway, so, conceivably, more rapid consumption of glycogen during the early exposure to AICAR causes a larger depletion of ATP and a larger rigor.
Does AICAR produce cardioprotection clinically by blocking NHE1?
In a clinical setting, AICAR is administered at doses which result in serum concentrations between 10–20 μM [31], but the mechanism of the observed cardioprotection remains uncertain. At a concentration of 500 μM, we observed around 80% NHE1 inhibition and, assuming a Ki of 50 μM, one would predict only a 20–30% inhibition of the exchanger at the concentration used in clinical trials. This suggests that if the sensitivity of the NHE1 to AICAR were the same in humans as it is in the isolated rat heart, then the contribution of NHE1 inhibition by AICAR in clinical trials would be modest at best.
Mechanism of action of AICAR on NHE1 activity
The regulation of the activity of NHEs is a complex and poorly understood topic [39]. The protein has a large intracellular tail with multiple sites for phosphorylation and two Ca/calmodulin binding sites. Many kinases have been implicated in NHE1 activation, e.g., protein kinase C, protein kinase D, mitogen-activated protein kinase, p90rsk, and p38 kinase. There are also several protein binding sites believed to be involved in the regulation of activity [36]. The pharmacological inhibitors of NHE1, amiloride and its derivatives and cariporide and it derivatives, contain a pyrazinoylguanidine or a benzoyl guandinium group [22, 39]. AICAR does not have such a structure and is probably not inhibiting NHE1 by a direct pharmacological interaction.
Given the suggestion that adenosine production might be involved in the mechanism of AICAR, we tested whether exogenous adenosine produced comparable effects to AICAR. We observed no effects of adenosine on the recovery from ischemia. The literature on this topic is quite variable. Several studies have found that in the rat, in contrast to the rabbit and the dog, adenosine provides no protection from ischemic damage [6, 25], while other studies have observed adenosine protection in the rat [12, 26]. One possible explanation for the failure of exogenous adenosine would be the breakdown and/or rapid uptake of adenosine by endothelial cells [23]. However, against this possibility is the observation that 8-SPT, a nonspecific blocker of adenosine receptors, is incapable of blocking preconditioning in the rat [25] (for recent review, see Ganote and Armstrong [15]). Based on our data, we think it unlikely that endogenous adenosine is involved in the effects of AICAR, but clearly, further study is needed.
Given our observation that AICAR inhibits NHE1, we also tested the possibility that adenosine might be involved in this action. We used the nonspecific adenosine inhibitor 8-SPT, which blocks both A1 and A2 adenosine receptors with a Ki of around 5 μM [46]. Previous studies have confirmed that 5 or 50 μM 8-SPT effectively blocks adenosine-dependent effects [33, 36]. In the present study we found that 50 μM 8-SPT alone did not affect NHE1 activity and did not affect the inhibitory action of AICAR on NHE1 activity. We conclude that the inhibition of NHE1 by AICAR is not caused by the release of adenosine acting through adenosine receptors.
Because AICAR in many cell types activates AMPK, we were interested in the possibility that the activation of AMPK by AICAR could underlie the inhibition of NHE1 [9, 17]. It is known that ischemia activates cardiac AMPK [29], and we confirmed this finding (data not shown). However, surprisingly, AICAR inhibited AMPK activity by around 60–70% (Fig. 5). Given the suggestion that adenosine receptors underlie some of the actions of AICAR, we tested whether this action was inhibited by 8-SPT, and the results show that, in the presence of 8-SPT, the inhibition of AMPK by AICAR was abolished. This result suggests that a pathway downstream of the adenosine receptor regulates AMPK activity, a concept that is supported by a recent study showing that extracellular adenosine can activate AMPK [10]. But this pathway seems unlikely to be involved in the inhibition of NHE1 or in the cardiprotection because neither of these pathways appears to involve adenosine.
Conclusions
AICAR (500 μM) inhibits NHE1, and in the isolated rat heart this inhibition contributes substantially to the cardioprotection exerted by AICAR following a period of ischemia. In human trials of AICAR as a cardioprotective agent, the concentrations used are substantially smaller (10–20 μM) and the inhibition of NHE1 seems unlikely to be more than a minor component of the mechanism of action. The mechanism of AICAR in inhibiting NHE1 is not clear, but it does not appear to operate through the release of adenosine or through the activation of AMPK.
References
Allen DG, Xiao XH (2001) Letter to the editor; Na+ entry during ischemia, reperfusion and preconditioning. Cardiovasc Res 50:164–166
An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ et al (2001) Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol Heart Circ Physiol 281:H2398–H2409
Avkiran M, Yokoyama H (2000) Adenosine A(1) receptor stimulation inhibits alpha(1)-adrenergic activation of the cardiac sarcolemmal Na(+)/H(+) exchanger. Br J Pharmacol 131:659–662
Bolli R, Marban E (1999) Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79:609–634
Cairns SP, Westerblad H, Allen DG (1993) Changes in myoplasmic pH and calcium concentration during exposure to lactate in isolated rat ventricular myocytes. J Physiol 464:561–574
Cave AC, Collis CS, Downey JM, Hearse DJ (1993) Improved functional recovery by ischaemic preconditioning is not mediated by adenosine in the globally ischaemic isolated rat heart. Cardiovasc Res 27:663–668
Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA et al (1999) Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett 460:343–348
Cook MA, Karmazyn M (1996) Cardioprotective actions of adenosine and adenosine analogs. In: Karmazyn M (ed) Myocardial ischemia: mechanisms, reperfusion, protection. Birkäuser, Basel, pp 325–344
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565
da Silva CG, Jarzyna R, Specht A, Kaczmarek E (2006) Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res 98:e39–e47
Elliott AC, Smith GL, Eisner DA, Allen DG (1992) Metabolic changes during ischaemia and their role in contractile failure in isolated ferret hearts. J Physiol 454:467–490
Fralix TA, Murphy E, London RE, Steenbergen C (1993) Protective effects of adenosine in the perfused rat heart: changes in metabolism and intracellular ion homeostasis. Am J Physiol 264:C986–C994
Galinanes M, Bullough D, Mullane KM, Hearse DJ (1992) Sustained protection by acadesine against ischemia- and reperfusion-induced injury. Studies in the transplanted rat heart. Circulation 86:589–597
Galinanes M, Mullane KM, Bullough D, Hearse DJ (1992) Acadesine and myocardial protection. Studies of time of administration and dose-response relations in the rat. Circulation 86:598–608
Ganote CE, Armstrong SC (2000) Adenosine and preconditioning in the rat heart. Cardiovasc Res 45:134–140
Gruber HE, Hoffer ME, McAllister DR, Laikind PK, Lane TA, Schmid-Schoenbein GW et al (1989) Increased adenosine concentration in blood from ischemic myocardium by AICA riboside. Effects on flow, granulocytes, and injury. Circulation 80:1400–1411
Henin N, Vincent MF, Van den Berghe G (1996) Stimulation of rat liver AMP-activated protein kinase by AMP analogues. Biochim Biophys Acta 1290:197–203
Javaux F, Vincent MF, Wagner DR, Van den Berghe G (1995) Cell-type specificity of inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside. Lack of effect in rabbit cardiomyocytes and human erythrocytes, and inhibition in FTO-2B rat hepatoma cells. Biochem J 305(Pt 3):913–919
Karmazyn M (1988) Amiloride enhances postischemic ventricular recovery: possible role of Na+–H+ exchange. Am J Physiol 255:H608–H615
Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K (1999) The myocardial Na+–H+ exchange: structure, regulation, and its role in heart disease. Circ Res 85:777–786
Kitakaze M, Takashima S, Minamino T, Node K, Shinozaki Y, Mori H et al (1999) Improvement by 5-amino-4-imidazole carboxamide riboside of the contractile dysfunction that follows brief periods of ischemia through increases in ecto-5-nucleotidase activity and adenosine release in canine hearts. Jpn Circ J 63:542–553
Kleyman TR, Cragoe EJ (1988) Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105:1–21
Lasley RD, Hegge JO, Noble MA, Mentzer RM Jr (1998) Comparison of interstitial fluid and coronary venous adenosine levels in in vivo porcine myocardium. J Mol Cell Cardiol 30:1137–1147
Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD (1999) Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol 517:159–180
Li Y, Kloner RA (1993) The cardioprotective effects of ischemic ‘preconditioning’ are not mediated by adenosine receptors in rat hearts. Circulation 87:1642–1648
Liem DA, te Lintel HM, Manintveld OC, Boomsma F, Verdouw PD, Duncker DJ (2005) Myocardium tolerant to an adenosine-dependent ischemic preconditioning stimulus can still be protected by stimuli that employ alternative signaling pathways. Am J Physiol Heart Circ Physiol 288:H1165–H1172
Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF (2003) 5-Aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol Regul Integr Comp Physiol 284:R936–R944
Mangano DT (1997) Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA 277:325–332
Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF et al (2000) Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10:1247–1255
Mauser M, Hoffmeister HM, Nienaber C, Schaper W (1985) Influence of ribose, adenosine, and “AICAR” on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circ Res 56:220–230
Menasche P, Jamieson WR, Flameng W, Davies MK (1995) Acadesine: a new drug that may improve myocardial protection in coronary artery bypass grafting. Results of the first international multicenter study. Multinational Acadesine Study Group. J Thorac Cardiovasc Surg 110:1096–1106
Meng HP, Lonsberry BB, Pierce GN (1991) Influence of perfusate pH on the postischemic recovery of cardiac contractile function: involvement of sodium-hydrogen exchange. J Pharmacol Exp Ther 258:772–777
Mullane K (1993) Acadesine: the prototype adenosine regulating agent for reducing myocardial ischaemic injury. Cardiovasc Res 27:43–47
Nakai T, Kano S, Satoh K, Hoshi K, Ichihara K (1996) Effects of adenine nucleotide analogues on myocardial dysfunction during reperfusion after ischemia in dogs. J Cardiovasc Pharmacol 28:264–270
Park CO, Xiao XH, Allen DG (1999) Changes in intracellular sodium and pH in the rat heart during ischaemia: role of the Na+/H+ exchanger. Am J Physiol 276:H1581–H1590
Peart J, Matherne GP, Cerniway RJ, Headrick JP (2001) Cardioprotection with adenosine metabolism inhibitors in ischemic-reperfused mouse heart. Cardiovasc Res 52:120–129
Russell RR III, Bergeron R, Shulman GI, Young LH (1999) Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 277:H643–H649
Scholz W, Albus U, Counillon L, Gogelein H, Lang HJ, Linz W et al (1995) Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res 29:260–268
Slepkov E, Fliegel L (2002) Structure and function of the NHE1 isoform of the Na+/H+ exchanger. Biochem Cell Biol 80:499–508
Swain JL, Hines JJ, Sabina RL, Holmes EW (1982) Accelerated repletion of ATP and GTP pools in postischemic canine myocardium using a precursor of purine de novo synthesis. Circ Res 51:102–105
Tani M, Neely JR (1989) Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+–Na+ and Na+–Ca2+ exchange. Circ Res 65:1045–1056
Xiao XH, Allen DG (1999) Role of the Na+/H+ exchanger during ischemia and preconditioning in the isolated rat heart. Circ Res 85:723–730
Xiao X, Allen DG (2000) Activity of the Na+/H+ exchanger is critical to reperfusion damage and preconditioning in the isolated rat heart. Cardiovasc Res 48:244–253
Xiao XH, Allen DG (2002) The role of endogenous angiotensin II in ischemia, reperfusion and preconditioning of the isolated rat heart. Pflügers Arch 445:643–650
Xiao XH, Allen DG (2003) The cardioprotective effects of Na+/H+ exchange inhibition and mitochondrial KATP channel activation are additive in the isolated rat heart. Pflugers Arch 447:272–279
Zhao ZQ, Williams MW, Sato H, Hudspeth DA, McGee DS, Vinten-Johansen J et al (1995) Acadesine reduces myocardial infarct size by an adenosine mediated mechanism. Cardiovasc Res 29:495–505
Acknowledgement
This study was supported by the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moopanar, T.R., Xiao, XH., Jiang, L. et al. AICAR inhibits the Na+/H+ exchanger in rat hearts—possible contribution to cardioprotection. Pflugers Arch - Eur J Physiol 453, 147–156 (2006). https://doi.org/10.1007/s00424-006-0124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0124-z