Abstract
The systemic induction of cytokines and prostaglandins plays a key role in the development of fever. However, whether fever is triggered by local injection of lipopolysaccharide (LPS) and the involvement of locally produced prostaglandins in periodontal tissue has never been assessed. Thus, we tested the hypothesis that the trigeminal nerve is a neuronal pathway that signals the brain during acute periodontitis, and this response involves prostaglandin induction. Rats were given a gingival intra-pouch injection of sterile saline or Escherichia coli lipopolysaccharide, at doses of 10 and 100 μg/kg. Some animals were pre-treated with the local anesthetic mepivacaine or had the peripheral branches of the trigeminal nerves transected. Another group of animals were pre-treated (locally or systemically) with the nonselective inhibitor of cyclooxygenases diclofenac. Body core temperature (T b) was measured by means of biotelemetry before and after injections. LPS elicited a dose-dependent increase in T b, which was abolished by mepivacaine, bilateral transection of the peripheral branches of the trigeminal nerve, or local treatment with diclofenac. The results indicate that there is an activation of periodontal nerves to induce fever by LPS. It also shows that local formation of prostaglandins plays a role in fever development. Moreover, immunohistochemistry detected c-fos expression in the subnucleus caudalis of spinal trigeminal nucleus and in the preoptic area of the hypothalamus 2 and 3 h after LPS injection, further confirming the role of trigeminal nerve signaling brain in acute periodontitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fever is a multimediated process that is part of the acute-phase reaction to systemic inflammation, being characterized by an elevation in body core temperature (T b) [21, 28]. It is currently accepted that fever results from de novo synthesis of protein mediators, especially the cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1 β), IL-6, and of mediators derived from lipid metabolism, which is the case for prostaglandin E2 (PGE2) [3]. These signals from the periphery may gain access to the brain by different routes: (a) saturable transendothelial transport [1, 2]; (b) via afferent fibers that travel mostly through the vagus nerve and make their first synapse in the nucleus of the solitary tract [4, 27]; (c) via circumventricular organs, such as the organum vasculosum laminae terminalis and the subfornical organ, which lack a blood–brain barrier [4, 40]; and (d) via interaction with cells located in the blood–brain interface, i.e., endothelial [8, 42] and perivascular [13, 33] cells. The activation of these afferent pathways ultimately increases the level of PGE2 in the brain [3] which has been reported to be, at least in part, produced by endothelial cells [12, 14, 32, 45]. By interacting with EP3 receptors [25, 43] and consequently decreasing the intracellular level of cyclic adenosine monophosphate in preoptic neurons [38, 39], PGE2 triggers an appropriate thermoeffector response to increase T b.
However, fever and other brain-mediated sickness responses may also occur in response to localized inflammation [29]. This response seems to occur in the absence of a substantial raise in the circulating levels of cytokines [29], when stimulation of peripheral sensory nerves transport signals of the activated immune system to the brain [29]. To study this phenomenon, an experimental fever model has been employed in which lipopolysaccharide (LPS) is administered not systemically but locally into a subcutaneous air pouch in the back of rodents (sometimes combined with a subcutaneous implanted Teflon chamber) that does not lead to LPS appearance in the circulation [31]. This approach, which has never been applied in periodontium, contrasts to the intraperitoneal (i.p.) or intravenous injection of LPS, which nicely mimics systemic infection.
The somatosensory innervations of the face and oral cavity in mammals are provided by ophthalmic, maxillary, and mandibular divisions of the trigeminal nerve [7, 22]. There is strong evidence supporting that the anatomical and physiological consequences of nerve injuries of the trigeminal system differ from those seen after peripheral nerve injury [17]. In addition, craniofacial pain also shows some differences from those involving spinal nerves [10, 15, 36]. Nevertheless, the possible differences between the roles played by inflammatory mediators after injury of peripheral and trigeminal nerve system have not been assessed. Therefore, the aim of the present study was to test the hypothesis that LPS induces fever when injected locally in the periodontal tissue by activating afferent trigeminal nerve and that the well-established mediator PGE2 takes part in this response. We also assessed c-fos expression in the spinal trigeminal nucleus (including the subnucleus caudalis, interpolaris, and oralis) and in the preoptic area (POA) of hypothalamus 2 and 3 h after LPS injection to further confirm the role of trigeminal nerve on signaling the brain in acute periodontitis.
Materials and methods
Animals
The experiments were performed on male Wistar rats weighing 280–310 g at the time of the experiments. All rats were maintained in individual plastic cages, with controlled ambient temperature (25.0±1.0°C) and a 12:12 h light–dark cycle (lights on at 6:00 A.M.). The animals had free access to water and food. The experiments were performed between 8:00 A.M. and 4:00 P.M. The study was conducted in compliance with the guidelines of the American Physiological Society and with the approval of the University of São Paulo Animal Care and Use Committee (Protocol 04.1.350.53.8).
Surgery
All surgical procedures were performed in rats anesthetized with 2,2,2-tribromoethanol (250 mg/kg, i.p.) and protected with antibiotic [benzylpenicillin 160,000 IU/kg, streptomycin 33.3 mg/kg, and dihydrostreptomycin 33.3 mg/kg, intramuscular (i.m.)]. All animals were chronically implanted with a gingival catheter (4.5 cm of polyethylene tubing PE10) connected to 1 cm of a PE-50 (Clay Adams, Parsippany, NJ, USA) for injection of drugs in the periodontal protection tissue. The catheter was inserted in the vestibular anterior maxilla. A small volume of air (∼5 μl) was injected through a 0.2-μm filter to delineate a pouch. The catheter was tunneled subcutaneously and exteriorized through the back of the neck to be connected to a needle under conscious, freely moving conditions at the moment of the injection. In a control group involving diclofenac treatment (protocol 4), the animals had a second catheter inserted, as described above, but in the palate. In animals designated for experiments involving T b measurements, a miniature battery-operated temperature-sensitive transmitter (model ER-4000, Mini-Mitter, Sunriver, OR, USA) was implanted through a medial laparotomy. Another group of animals had the peripheral branches of the trigeminal nerve bilaterally transected. This procedure involved a 1-cm incision in the skin, above the zygomatic branch, to locate the infra-orbital foramen and expose and cut bilaterally all the branches of the trigeminal nerve that emerge from the infra-orbital foramen. These branches included: (1) the anterior superior alveolar branches that supply innervation to the incisive superior teeth and the mucous membrane, (2) the external nasal branches that supply innervation to the skin of the nose and muzzle, and (3) the superior labial branches that supply innervation to the upper lip and the mucous membrane of the mouth [19]. The wound was cleansed and closed with sutures. Sham operations were performed in the same way but without cutting the nerves. The experiments involving nerve transection were performed 7 days after the surgery. For all other procedures, the experiments were performed 3 days after the surgery.
Body temperature measurements
For all protocols, T b was measured by biotelemetry (Mini-Mitter, Sunriver, OR, USA) at 5-min intervals and plotted at 15-min intervals, during a period of 30 min before and 5 h after the treatments. Data were acquired and fed to a computer by using The Vital View software (Mini-Mitter, Sunriver, OR, USA).
Drugs
LPS derived from Escherichia coli (O111:B4 Sigma, St. Louis, MO, USA) was dissolved in sterile 0.9% sodium chloride (saline). LPS was injected locally at a dose of 10 and 100 μg/kg. The cyclooxygenases (COX) nonselective inhibitor diclofenac-sodium (Calbiochem, La Jolla, CA, USA) was dissolved in 95% sterile saline and 5% ethanol and injected at a dose of 5 mg/kg. The local anesthetic mepivacaine chloride was purchased as 3% solution (30 mg/ml) dissolved in pyrogen-free saline (MEPISV-DFL, Jacarepaguá-Rio de Janeiro, Brazil) and it was injected at a dose of 1.2 mg/kg. The doses were chosen on the basis of pilot experiments from our laboratory and of a previous study [30].
Histological procedures:
transcardiac perfusion and brain sectioning The animals were anesthetized intraperitoneally with a mixture of ketamine (Ketamina Agener, Embu-Guaçu, São Paulo, Brazil) and xylazine (Dopaser, Calier S.A., Barcelona, Spain) at a dose of 20 mg/kg, i.m., ketamine and 2 mg/kg, i.m., xylazine. To confirm anesthesia depth, the withdrawal reflex to tail pinch was assessed. Once anesthetized, they were perfused transcardially with phosphate-buffered saline (PBS, 200 ml, pH 7.4) followed by freshly prepared ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (200 ml, pH 7.4). The brains were rapidly removed and soaked in the same fixative solution for 4 h (4°C) and then cryoprotected by overnight soaking in 30% sucrose–phosphate buffer. They were frozen in dry ice, mounted onto a cryostat (Frigocut 2800, Leica, Wetzlar, Germany), and cut into 40-μm coronal sections, which were collected in a cryoprotectant solution (0.05 M sodium phosphate buffer, pH 7.3, 30% ethyleneglicol, and 20% glycerol) and stored at −20°C.
Fos immunohistochemistry
Every third section was processed for Fos immunohistochemistry. The free-floating sections were washed in PBS (0.01 M, pH 7.4) and incubated in PBS containing 1% hydrogen peroxide for 10 min to inactivate endogenous peroxidase activity. After several rinses in PBS for 30 min, the sections were placed in 5% normal goat serum (Vector, Burlingame, CA, USA) for 45 min and then incubated for 24 h at 4°C with polyclonal anti-c-fos serum generated in rabbits (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The serum was diluted (1:1,000) in PBS containing 1% bovine serum albumin and 0.5% Triton X-100. After being rinsed in PBS, the sections were incubated for 1.5 h at room temperature with biotinylated goat anti-rabbit IgG (1:400, Vector). They were subsequently washed in PBS and placed for 30 min in avidin–biotin peroxidase complex (ABC kit, Vectastain, Vector, Burlingame, CA, USA). The labeled neurons were revealed by a 5- to 10-min incubation with 0.05% 3,3′-diaminobenzidine tetrachloride and 0.1% hydrogen peroxide. The polyclonal anti-c-fos antibody was omitted in negative controls. The sections were mounted on gelatin-coated slides, dehydrated through an ascending ethanol series, cleared with xylene, and coverslipped with Entellan. As the antibody used in this study recognizes c-fos and Fos-related proteins, the immunoreactive neurons are described as Fos-like immunoreactive (Fos-IR).
Fos immunohistochemistry analysis
The sections were analyzed by light microscopy and the labeled neurons were registered with the use of an image analysis system (Keiss KS 300). For quantification, one brain section of each nucleus (subnucleus caudalis of spinal trigeminal nucleus and preoptic area of hypothalamus) was selected for unilateral counts for each rat. The sampling method for Fos counting was based on a study by Magdalena et al. [23]. The sections were selected based on the atlas of Paxinos and Watson [26] and the section that had the largest number of FOS-IR cells were counted unilaterally, using a computerized image analysis system (NIH System, Image J). Adobe Photoshop (San Jose, CA, USA) was used to enhance the contrast of the images.
Experimental protocols
Periodontal injection procedure
All injections into the periodontium were made with the use of a 10- or 25-μl Hamilton syringe. LPS was injected at a volume of 10 μl/kg (∼3 μl/animal); LPS solutions of 1 and 10 mg/ml were used to achieve final doses of 10 and 100 μg/kg, respectively. Mepivacaine and diclofenac were injected at a volume of 40 μl/kg (∼12 μl/animal); mepivacaine and diclofenac solutions of 30 and 125 mg/ml were used to achieve final doses of 1.2 and 5 mg/kg, respectively. Before the injection of each drug, a volume of ∼3 μl of air was injected through the catheter to delineate a pouch.
-
1.
Local injection of LPS and fever: This experiment aimed at testing whether LPS (10 or 100 μg/kg) would cause fever when injected locally, via the gingival catheter, into the periodontal protection tissue. The control rats were injected with pyrogen-free saline (vehicle) instead of LPS. T b was measured during a period of 5 h after injection.
-
2.
Determination of the effect of the mepivacaine on LPS fever: To verify the possible effect of local anesthetic on LPS-induced fever, the animals were injected via the gingival catheter, as described above, with mepivacaine (1.2 mg/kg). At 10 min after the treatment with mepivacaine, the animals received a periodontal injection of LPS (100 μg/kg). The control animals were injected with vehicle (saline) instead of mepivacaine. To verify if mepivacaine blocks fever by acting locally or systemically, another group of animals received an i.p. injection of the same dose of mepivacaine (1.2 mg/ml, injected at a volume of 1 ml/kg) 10 min before the periodontal injection of LPS (100 μg/kg).
-
3.
Determination of the effect of bilateral transection of the peripheral branches of the trigeminal nerve: To confirm the participation of the trigeminal nerve on LPS-induced fever, the animals were submitted to a bilateral transection of the peripheral branches of the trigeminal nerve, and a week after the surgery they were injected with LPS into the periodontium (100 μg/kg). Sham-operated animals were also injected with LPS (100 μg/kg).
-
4.
Determination of the effect of the diclofenac on LPS fever: To verify the possible effect of local prostaglandins on LPS fever, the animals were injected, via the gingival catheter, with diclofenac (5 mg/kg) into the periodontium. At 10 min after the treatment with diclofenac, the animals received a periodontal injection of LPS (100 μg/kg). The control animals received periodontal injections of vehicle (5% ethanol in saline) followed by LPS (100 μg/kg). The diclofenac dose was used based on a previous study [29] and because, when preliminary doses of diclofenac were tested, the thermoregulatory response to the dose of 5 mg/kg was the most consistent and repeatable in our experimental protocols. To further confirm if the effect of diclofenac is due to a local blockade of prostaglandin synthesis, another group of animals received an injection of the same dose of diclofenac (5 mg/kg) via the catheter implanted in the palate at 10 min before the periodontal injection of LPS (100 μg/kg).
-
5.
Verification and quantification of the expression of immunoreactive Fos protein in the spinal trigeminal nucleus (caudal, interpolaris, and oralis subnuclei) and in the preoptic area of hypothalamus after LPS administration on the periodontium: To verify the expression of c-fos in the spinal trigeminal nucleus and in the preoptic area, a dose of 100 μg/kg LPS was administered (via catheter) into the periodontium of rats and three different times of killing were used: at 1, 2, and 3 h after injection. The brain sections were processed for immunohistochemistry and the immunoreactive neurons were quantified.
Statistical analysis
All values are reported as means±SEM. The thermal response to administration of LPS alone or along with pharmacological treatments or vehicles was compared by two-way analysis of variance (ANOVA) for repeated measures, followed by Duncan’s post hoc test or by t test to compare each point. The values were considered significantly different when p<0.05. Thermal indices were used in Figs. 1, 2, 3, and 4 to improve data analysis. They were calculated for each rat, as areas under the delta T b curves (°C h). The delta T b curves were calculated by subtracting the temperature values after the treatments by its own initial T b. Statistical analyses were performed on these data using ANOVA followed by Duncan’s post hoc test. Values of p<0.05 were considered significant. The number of Fos-IR neurons was compared by one-way analysis of variance for repeated measures, followed by Duncan’s post hoc test, with p<0.05 indicating a significant difference.
a Body core temperature responses to saline and LPS (10 and 100 μg/kg) injected into the periodontal protection tissue of rats. In sub-panels 1–4, the number of animals per group is shown in parentheses. b Thermal indices after saline and 10 and 100 μg/kg LPS treatments. The values are means±SEM (*p< 0.05 compared with saline-treated group)
Determination of the effect of the mepivacaine on LPS fever. a Body core temperature response to injection of local or i.p. administration of mepivacaine (1.2 mg/kg) 10 min before LPS (100 μg/kg) injection into the periodontal protection tissue of rats. The control animals were injected with vehicle and LPS. b Thermal indices summarize these results. The values are means±SEM (*p<0.05 compared with LPS and vehicle-treated group)
a Effect of maxillary trigeminal nerves transection on the LPS-induced febrile response. LPS (100 μg/kg) was injected into the periodontal protection tissue of both groups of rats (nerve-transected and sham-operated). b Thermal indices after LPS treatment in sham-operated or nerve-transected rats (*p<0.05 compared with sham-operated group)
a Determination of the effect of the diclofenac on LPS fever. Body core temperature response to local injection of diclofenac (5 mg/kg) and LPS (100 μg/kg) into the periodontal protection tissue of rats. The control animals were injected with vehicle and LPS. In addition, some rats were treated with diclofenac (5 mg/kg) in the palate before LPS (100 μg/kg) was injected in the periodontal protection tissue. b Thermal indices summarize these results. The values are means±SEM (*p<0.05 compared with LPS and vehicle-treated group)
Results
Figure 1 shows the effect of injection of LPS into the periodontal tissues on T b. The low dose (10 μg/kg) of LPS elicited a slowly developing, biphasic fever, whereas the high dose (100 μg/kg) of LPS caused a long-lasting fever compared with saline (F 38,247=6.96, p<0.05).
The effect of mepivacaine on the LPS-induced fever is shown in Fig. 2. Pretreatment with local mepivacaine significantly reduced LPS-induced fever (F 36,324=2.26, p<0.05), mainly during the period from 2 to 4 h. An intraperitoneal injection of mepivacaine did not alter LPS-induced fever, indicating that the effect of mepivacaine in LPS-induced fever is not due to a systemic action of the anesthetic.
To investigate the participation of afferent neural signals, LPS-induced fever was studied in a group with bilateral peripheral trigeminal nerve transections. The febrile response in animals with transected trigeminal nerves was blunted (F 19,285=9.61, p<0.05) compared with that of sham-operated animals treated with LPS (Fig. 3). This result is also an evidence that the LPS-induced fever is mediated locally and by means of stimulation of trigeminal nerve fibers.
To verify the role of locally produced prostaglandins on the LPS-induced fever, the animals were pre-treated with the prostaglandin synthesis inhibitor diclofenac. The pre-injection of diclofenac into the periodontal tissues resulted in a significant reduction of the febrile response (F 36,270=5.20, p< 0.05) compared with the animals treated with vehicle and LPS. Diclofenac injected in the palate did not also affect LPS-induced fever, indicating that the effect of diclofenac was due to a local blockade of prostaglandin synthesis. These data are depicted in Fig. 4.
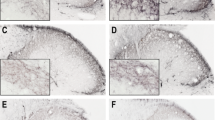
In the LPS-treated group, Fos-positive neurons were found bilaterally in the subnucleus caudalis of spinal trigeminal nucleus and in the preoptic area of hypothalamus 2 and 3 h after injections (Fig. 5). In the control group (treated with saline), the number of Fos-positive neurons was significantly lower than that observed in the LPS-treated group (p<0.05) in the three different times of killing (Figs. 5 and 6). In the group killed 1 h after treatment, very few Fos-positive neurons were found in the subnucleus caudalis and in the POA in both groups (p>0.05). Furthermore, in the oralis and interpolaris subnuclei, the labeled neurons were absent (data not shown). As to the time course, it was observed that, 3 h after LPS, the number of Fos-positive neurons was significantly greater than at 2 h (p<0.05) in the subnucleus caudalis of the spinal trigeminal nucleus and in the POA (Fig. 6). The negative control did not show any labeled neuron.
Photomicrographs of subnucleus caudalis of spinal trigeminal nucleus (a) and preoptic area of hypothalamus (b) showing Fos-IR neurons after single saline (1) or LPS (100 μg/kg) injection on periodontium in different times of killing: 2 (3) and 3 h (4) after injection. The negative control group (2) did not show any labeled neuron. The coronal schematics in the right panels show the localization of the areas in light gray. ac, anterior comissure; LV, lateral ventricle; ox, optic chiasm; POA, preoptic area; 3V, third ventricle; Sp5, spinal trigeminal tract; Sp5C, caudal part of spinal trigeminal nucleus; py, pyramidal tract
Effect of periodontium LPS injection (100 μg/kg) on c-fos expression, in three different times of killing: 1, 2 and 3 h after injection. The control animals were treated with sterile saline. Bars represent the average±SEM (n=4 animals per group) of the number of c-fos IR neurons. a Subnucleus caudalis of spinal trigeminal nucleus. b preoptic area of hypothalamus (*p<0.05 compared with saline-treated group). nd, not detectable. The negative control group did not show any labeled neuron.
Discussion
In the present study, we have locally injected LPS into the periodontal protection tissue to verify the possible induction of fever mediated by the involvement of periodontal afferent neural signals. Our results indicate that local LPS injection causes a dose-dependent febrile response. To verify the involvement of the trigeminal nerve as an afferent signal to induce the febrile response, we used a local anesthetic (mepivacaine) as well as trigeminal nerve transection. Both treatments caused a significant reduction of the LPS-induced fever, which strongly supports a role of neural signals from the oral cavity that can be activated by inflammatory stimuli which is transmitted to the brain to induce fever. Next, we investigated such a role in more detail. To this end, we combined the LPS treatment with the local injection of COX inhibitor, diclofenac. Our data show that the LPS-induced fever resulted from a prostaglandin-dependent mechanism, as the COX inhibitor virtually abolished fever. In addition, by investigating the expression of c-fos, we showed neuronal activation in the subnucleus caudalis, where the trigeminal nerve makes its first synapse, and in neurons of the POA known to be the thermointegrative site of the central nervous system [3, 4, 21, 24, 38] in response to local injection of LPS in the peridontium.
The effect of the local anesthetic mepivacaine (attenuation of the LPS-induced fever) seems not to be due to a systemic action of mepivacaine, as i.p. injections of the anesthetic did not alter the febrile response (Fig. 2). However, it is known that local anesthetics can also block a variety of ion channels [16] not only on nerve cells but also on inflammatory cells [37], thus affecting the immune function [34]. In spite of this observation, there is a strong argument indicating that the effect of anesthetics on immune function may not be directly related to the observed reduced febrile response. In a previous study, where LPS was injected locally into the back of guinea pigs [30], it was shown that although local anesthetics blocked the febrile response, IL-6 was unimpaired by the treatment with local anesthetics [30]. Further supporting this evidence is the fact that IL-6 has been reported to be the only cytokine which escapes in considerable amounts from the site of local inflammation into the systemic circulation [9]. In addition, we also applied a model of transection of the peripheral branches of the trigeminal nerve, and the results were similar to the mepivacaine treatment, further supporting the role of trigeminal nerve as an afferent signaling pathway from local inflammation. To our knowledge, this is the first time that this model of transection of the peripheral branches of the trigeminal nerve was used to disrupt this route of immune-to-brain communication. The surgical procedures were made with respect to the anatomical structures and did not affect the drinking and eating behavior or the baseline T c of rats.
It is interesting to note that a clear febrile response was observed after LPS was injected in the periodontal tissue despite the fact that, as a general rule, chronic periodontitis is not accompanied by fever. This inconsistency may be due to the fact that our model reproduces an acute periodontitis and not a chronic one. In fact, during acute periodontitis (periodontal abscesses, for instance), fever is usually observed in patients [18, 20, 35].
The brain plays a central role in coordinating several responses to systemic inflammation, including fever. It transduces the febrigenic signals from the periphery into appropriate adjustments of thermoeffector activity to ultimately increase T b. It has been recently proposed that blood-borne mediators of the febrile response would not be the only route to activate febrile mechanisms but that, rather, afferent fibers traveling through the vagus nerve would also participate [4, 27]. Moreover, the activation of cutaneous afferent nerves by immune signals has been also suggested to play a role in the manifestation of the febrile response [29–31]. The present study adds the participation of afferent neural signals originating in the mouth and transmitted to the brain by the trigeminal nerve to this scenario. It may be true that, after activation of any of these afferent pathways, ultimately an increased level of prostaglandin E2 (the proximal mediator of fever) in the POA may take place, which is consistent with our data about c-fos activation in the POA (Figs. 5 and 6).
The c-fos expression technique has been extensively used as a marker of neuronal activity, induced by a number of stimuli including nociceptive stimuli in the oral cavity caused by tooth movement [23] and LPS-induced pulpar inflammation [11]. It is interesting to note that, in the latter study, c-fos expression was restricted to the caudal subnucleus of the spinal trigeminal nucleus, which is very similar to our findings (Fig. 5). Several studies have shown that the caudal part of the spinal trigeminal nucleus is an important site that relays nociceptive input from orofacial structures [36] including the dentition [6].
The increase in T b started about 1.5 h after LPS administration (Fig. 1), whereas c-fos expression was not observed at 1 h, but at 2 and 3 h after the endotoxin challenge (Figs. 5 and 6). These results are in agreement with Valles et al. [44] who suggest that c-fos response to endotoxin appears to be slower and longer lasting than that of other kinds of stressors, with maximum increases in c-fos expression between 2 and 4 h.
The neurochemical and neurophysiological changes induced by injury to the nerves of the trigeminal system are clearly distinct from those seen after lesions of other peripheral nerves, as recently reviewed by Fried et al. [17]. For instance, the onset of spontaneous nociception in the former case is more delayed (but can be sustained for relatively long periods) and is not associated with ganglionic sympathetic terminals [5, 41]. Nevertheless, current knowledge about the specific mechanisms underlying trigeminal mediated information, not only of inflammatory but also of neuropathic origin, is still remarkably sparse. In this regard, the present study provides neuropharmacological insight into some of the mechanisms involved in the febrile response after LPS injection in the protection periodontal tissue of the rat, which is innervated by the trigeminal system. The data provided in this paper reveal a significant contribution of periodontal nerves and of local COX activation associated with the LPS-induced fever.
After being infected with pathogens or challenged with pathogenic molecules such as LPS, the animals display a number of behavioral alterations, namely, sickness behavior that may include anorexia, adipsia, lethargy, and reduced sociability [21] besides fever. In the past decades, progress has been made in understanding the mechanisms of fever, which is considered as a hallmark of disease [21]. The vagus nerve has been more recently reported to play a key role in the mediation of sickness behavior, being an important sensorial afferent [3]. The present study provides evidence that periodontal afferent nerves may be activated by immune signals to mediate the febrile response and possibly other components of the sickness behavior.
In conclusion, although differences between the trigeminal system in relation to other peripheral nerves in mediating both inflammatory and neuropathic information do exist [17], the stimulation of nerves and the participation of COX to elicit fever after local LPS injection in the rat periodontal tissue seem not to differ from those elicited by LPS in the body.
References
Banks WA, Farr SA, Morley JE (2002–2003) Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation 10:319–327
Banks WA, Kastin AJ, Durham DA (1989) Bidirectional transport of interleukin-1 alpha across the blood–brain barrier. Brain Res Bull 23:433–437
Blatteis CM, Sehic E (1997) Fever: how may circulating pyrogens signal the brain. News Physiol Sci 12:1–9
Blatteis CM, Sehic E, Li S (2000) Pyrogen sensing and signaling: old views and new concepts. Clin Infect Dis 31:168–177
Bongenhielm U, Boissonade FM, Westermark A, Robinson PP, Fried K (1999) Sympathetic nerve sprouting fails to occur in the trigeminal ganglion after peripheral nerve injury in the rat. Pain 82:283–288
Byers MR, Chudler EH, Iadarola MJ (2000) Chronic tooth pulp inflammation causes transient and persistent expression of Fos in dynorphin-rich regions of rat brainstem. Brain Res 861:191–207
Byers MR, Dong WK (1989) Comparison of trigeminal receptor location and structure in the periodontal ligament of different types of teeth from the rat, cat, and monkey. J Comp Neurol 279:117–127
Cao C, Matsumura K, Yamagata K, Watanabe Y (1996) Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 beta: a possible site of prostaglandin synthesis responsible for fever. Brain Res 733:263–272
Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN (2000) Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol 526:653–661
Cechetto DF, Standaert DG, Saper CB (1985) Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 240:153–160
Chattipakorn SC, Sigurdsson A, Light AR, Narhi M, Maixner W (2002) Trigeminal c-fos expression and behavioral responses to pulpal inflammation in ferrets. Pain 99:61–69
Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson P, Ericsson-Dahlstrand A (2001) Inflamatory response: pathway across the blood–brain barrier. Nature 410:430–431
Elmiquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, Saper CB (1997) Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol 381:119–129
Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A (2003) Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci 6:1137–1138
Feil K, Herbert H (1995) Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker–Fuse nuclei. J Comp Neurol 353:506–528
Fozzard HÁ, Lee PJ, Lipkind GM (2005) Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr Pharm Des 11:2671–2686
Fried K, Bongenhielm U, Boissonade FM, Robinson PP (2001) Nerve injury-induced pain in the trigeminal system. Neuroscientist 7:155–165
Fukai K, Kato M, Mikami I, Otaki K, Hasegawa A, Seki N, Takagi M, Katagiri M (1989) Case report of periodontal disease with periodic neutropenia. Nippon Shishubyo Gakkai Kaishi 31:1242–1253
Greene EC (ed) (1959) Nervous system. Anatomy of the rat. Hafner, New York, pp 115–141
Katz J, Weinnstein E, Barak S, Livneh A (1992) Dental disease in the differential diagnosis of fever of unknown origin. Ann Dent 51:3–5
Kluger MJ (1991) Fever: role of pyrogens and cryogens. Physiol Rev 71:93–127
Lazarov NE (2001) Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol 66:19–59
Magdalena CM, Navarro VP, Park DM, Stuani MBS, Rocha MJA (2004) c-fos expression in the rat brain nuclei following incisor tooth movement. J Dent Res 83:50–54
Nagashima K, Nakai S, Tanaka M, Kanosue K (2000) Neuronal circuitries involved in thermoregulation. Auton Neurosci 85:18–25
Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB (2003) Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol 551:945–954
Paxinos G, Watson, C (1998) The rat brain in stereotaxic coordinates. Academic
Romanovsky AA (2000) Thermoregulatory manifestations of systemic inflammation: lessons from vagotomy. Auton Neurosci 85:39–48
Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF (2005) Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 10:2193–2216
Ross G, Hubschle T, Pehl U, Braun HA, Voigt K, Gerstberger R, Roth J (2003) Fever induction by localized subcutaneous inflammation in guinea pigs: the role of cytokines and prostaglandins. J Appl Physiol 94:1395–1402
Ross G, Roth J, Störr B, Voigt K, Zeisberger E (2000) Afferent nerves are involved in the febrile response to injection of LPS into artificial subcutaneous chamber in guinea pigs. Physiol Behav 71:305–313
Roth J, Storr B, Martin D, Voigt K, Zeisberger E (2000) The role of local induction of tumor necrosis factor by LPS within a subcutaneous air pouch in the febrile response in guinea pigs. Neuroimmunomodulation 7:169–176
Saha S, Engstrom L, Mackerlova L, Jakobsson PJ, Blomqvist A (2005) Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol 288(5):R1100–R1107
Schiltz JC, Sawchenko PE (2003) Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci 8:1321–1329
Schmidt W, Schmidt H, Bauer H, Gebhard MM, Martin E (1997) Influence of lidocaine on endotoxin-induced leukocyte–endothelial cell adhesion and macromolecular leakage in vivo. Anesthesiology 87:617–624
Schroder H (1979) Therapeutic and diagnostic problems of generalized acute suppurative periodontitis marginalis profunda. Dtsch Zahnärztl Z 34(6):506–510
Sessle BJ (1986) Recent developments in pain research: central mechanisms of orofacial pain and its control. J Endod 12:435–444
Sinclair R, Eriksson AS, Gretzer C, Cassuto J, thomsen P (1993) Inhibitory effects of amide local anesthetics on stimulus induced human leukocyte metabolic activation, LTB4 release and IL-1 secretion. Acta Anaesthesiol Scand 37:159–165
Steiner AA, Antunes-Rodrigues J, Branco LG (2002) Role of preoptic second messenger systems (cAMP and cGMP) in the febrile response. Brain Res 944(1–2):135–145
Steiner AA, Reste G, Branco LG (2003) Role of the brain heme oxygenase–carbon monoxide pathway in stress fever in rats. Neurosci Lett 341(3):193–196
Takahashi Y, Smith P, Ferguson A, Pittman QJ (1997) Circumventricular organs and fever. Am J Physiol 273:R1690–R1695
Tal M, Devor M (1992) Ectopic discharge in injured nerves: comparison of trigeminal and somatic afferents. Brain Res 579(1):148–151
Tilders FJ, Derijk RH, Vandam AM, Vincent VA, Schotanus K, Persoons JH (1994) Activation of the hypothalamus–pituitary–adrenal axis by bacterial endotoxins: routes and intermediate signals. Psychoneuroendocrinology 19:209–232
Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S (1998) Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature 395(6699):281–284
Valles A, Marti O, Armario A (2005) Mapping the areas sensitive to long-term endotoxin tolerance in the rat brain: a c-fos mRNA study. J Neurochem 93:1177–1188
Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S (2001) Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci 21:2669–2677
Acknowledgements
We thank Gustavo Michel Batista de Souza and Lidiane de Cássia Anastácio for excellent technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), PRONEX, and USP. Lidiane de Cássia Anastácia and Gustavo Michel Batista de Souza are recipients of FAPESP technician scholarships. Valeria P. Navarro is a recipient of a FAPESP graduate scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navarro, V.P., Iyomasa, M.M., Leite-Panissi, C.R.A. et al. New role of the trigeminal nerve as a neuronal pathway signaling brain in acute periodontitis: participation of local prostaglandins. Pflugers Arch - Eur J Physiol 453, 73–82 (2006). https://doi.org/10.1007/s00424-006-0113-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0113-2