Abstract
The ClC family of chloride channels and transporters includes several members in which mutations have been associated with human disease. Clearly, an understanding of the structure–function relationships of these proteins will be critical in defining the molecular mechanisms underlying disease pathogenesis. The X-ray crystal structure of prokaryotic ClC proteins provides an exquisite template with which to model molecular aspects of eukaryotic ClC protein function. The dimeric structure of these proteins highlights the pivotal importance of intermolecular interactions in the modulation of channel/transporter activity, while mutagenesis studies implicate a crucial role for intrinsic interdomain interactions in regulated function. In this review, we will initially focus on the channel forming members of this family and discuss interactions within homodimeric channel complexes important for gating. Finally, with regard to both channel and transporter family members, we will discuss the multiple heteromeric interactions which occur with cytosolic proteins, and the putative functional impact of these interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on primary sequence similarity, eukaryotic ClC proteins have been clustered into three main subgroups [1]. ClC-1, ClC-2, and ClC-Ka,b have been clustered with ClC-0, the prototypical eukaryotic ClC channel, to form the first grouping. ClC-3 (A/B), ClC-4, and ClC-5 comprise a separate cluster, as do ClC-6 and ClC-7. Interestingly, members of the ClC-3 cluster, namely ClC-4 and ClC-5, have only recently been reported to function as chloride/proton antiporters upon exogenous expression in both Xenopus oocytes and mammalian tsA201 cells [2, 3]. Currently, the function of ClC-6 and ClC-7 as channels and/or transporters has not been resolved. Mutations in several family members have been linked to human disease (ClC-1, ClC-2, ClC-K, ClC-5, and ClC-7), highlighting their physiological significance [4–9]. In agreement with their localization, mutations in ClC-1, ClC-2, and ClC-Ka are thought to be pathogenic by altering chloride flux across the plasma membrane, resulting in changes in membrane potential or directional transepithelial transport. ClC-5 and its closely related family members ClC-4 and ClC-3, are predominantly expressed in intracellular organelles; consistent with this localization, mutations in ClC-5 are thought to cause disease by altering chloride flux and acidification of endosomes, in the proximal tubule of the kidney [10]. Finally, from the third grouping, mutations in ClC-7 are thought to cause osteopetrosis by disrupting acidification of the resorption lacuna [8].
An understanding of the structural basis for ClC protein function is key in defining the mechanism of action and molecular basis for disease. Except for ClC-K (which partners with the membrane protein Barttin [11]), it is thought that ClC proteins can confer function as a channel or transporter independently, without the participation of ancillary proteins. In fact, this was shown convincingly in studies of purified ClC-0, wherein native conduction and gating were conferred by reconstitution of purified ClC-0 into planar lipid bilayers [12]. The publication of the X-ray crystal structure of two prokaryotic members of this family galvanized the field by revealing a dimeric structure that possesses two pores through which chloride can permeate [13, 14].
Recently, functional analysis was conducted for the prokaryotic ClC for which the crystal structure was generated [15]. This prokaryotic family member functions as a chloride/proton antiporter, providing an elegant template for molecular modeling of eukaryotic ClC family members with similar function, ClC-4, ClC-5, and probably ClC-3 [2, 3, 15]. Interestingly, the crystal structure has proven its fidelity in predicting the molecular basis for key features of members of the ClC-0 cluster of chloride channels as well, i.e., the chloride permeation path and the outer and inner channel vestibules [16, 17]. Therefore, as initially suggested by Accardi and Miller, there are likely to be only subtle structural differences accounting for the different function of these ClC family members and there is value in using the prokaryotic protein structure to model certain molecular aspects of ClC channel function [15].
The X-ray crystal structure supported the “double-barreled” shot gun model of ClC channel gating originally proposed by Miller on the basis of the biophysical properties of the reconstituted ClC-0 channel activity [12]. This model proposes that conformational changes in a common gate, presumably conferred by a shared structural element of the dimer, precedes channel opening and subsequently each “barrel” or protopore can open and close independently. Together, the structure- and function-based models argue for a critical role for protein–protein interactions in gating of the channel. Not surprisingly, the physiological impact of ClC proteins also depends on their interaction with other proteins. Cell and tissue-based studies argue for an additional role in heteromeric interactions in the regulation of ClC activity and regulatory proteins have been identified which act to modify ClC protein localization and/or function.
In this review, we will focus on the role of intermolecular and intramolecular interactions in the regulation of ClC channels and transporters. Initially, we will discuss those interactions that contribute to chloride channel function within the ClC-0 cluster of ClC proteins. Finally, we will discuss examples of interactions with members throughout the ClC family of channels and transporters that form heteromeric complexes of functional significance.
Intermolecular and intramolecular interactions contribute to ClC channel gating
Protopore gating
The structural models of prokaryotic ClC proteins suggest that the minimal functional unit of eukaryotic ClC channels is dimeric [13] (Fig. 1). Together with previous biophysical studies, this structural model suggests that association of two ClC polypeptides confers function even though each individual monomer possesses a protopore through which chloride ion permeates. Dimeric association is thought to create a common gate, which undergoes “slow” conformational change to permit channel activation. Subsequent to the release of this common “slow” inactivation gate, independent “fast” gating of each protopore occurs. Gating of each protopore has been modeled as a toggle mechanism involving subtle side chain movements of a single glutamate residue residing within the chloride permeation path [14]. Protopore gating is thought to be regulated by protonation of this key glutamate residue and the relative occupancy of binding sites for chloride in the permeation path and the channel vestibules. This residue is also implicated in the proton transport function of the antiporters [2, 3], suggesting that both chloride ions and protons share at least a limited region within same conduction pathway. However, there are suggestions in the literature that the molecular basis for “fast” gating in the microsecond to millisecond time frame may also involve structures in addition to the glutamate in the pore. Eukaryotic ClCs possess extensive cytosolic termini for which no structural information exists [1]. Site-directed mutation of a residue close to the carboxy terminus of the ClC-0 (A783P), led to a change in voltage-dependent gating, although the relative contribution of the “fast” and common gate was not established in this study [18]. Similarly, Hebeisen and Fahlke reported that chloride currents mediated by ClC-1 variants lacking regions in the carboxy terminus, exhibited alterations in both the “fast” and common gate. These authors attribute these gating abnormalities to structural changes in the external channel vestibule and anion binding to this region [19]. These studies clearly suggest that gating may require complex interactions between the membrane domain and the carboxy terminus. This idea is reinforced in the discussion of the common gate in the following paragraphs.
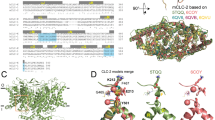
Cartoon of the putative structure of eukaryotic ClC proteins. A rectangle has been drawn around the dimeric membrane domain based on the structure of ecClC solved by Dutzler and colleagues [13]. The structures of the cytosolic regions of eukaryotic ClC channel/transporters are unknown. However, the carboxy terminus of each monomer within the dimer possesses two CBS domains and structures of proteins bearing a pair of CBS domains have been solved. Therefore, in this cartoon, we included two pictures of the structure of a hypothetical protein from Thermoplasma acidophilum (PDB code 1PMV) to represent the two carboxy termini of the ClC dimer. A circle encloses each carboxy terminus. This figure was generated using PYMOL
The common gate
The common or “slow” gating mechanism, occurring on the millisecond to second time frame in ClC-0, exhibits voltage, chloride, and pH dependence. The molecular basis for the common gate remains unclear and is thought to involve relatively substantive conformational changes, as reflected in its large temperature dependence [20]. Conceptually, it is tempting to hypothesize that the structural basis for common gating requires conformational changes at interfacial regions within the channel dimer (see Fig. 1). As revealed in the crystal structure of prokaryotic ClCs, there is an extensive interface between the two polypeptides of the dimer within the membrane. Further, there is empirical evidence to suggest that this interface may indeed play a key role in orchestrating the overall activity of the protein. In the eukaryotic proteins ClC-1 and ClC-5, disease-causing mutations can be mapped along the putative interface between the polypeptides in the membrane [21, 22]. Most of these mutations lead to severe diseases or exert a dominant effect in heterozygotes supporting the argument that these mutations perturb the common gating mechanism.
In addition to a potential role for residues located at the dimer interface in mediating the common gate function, residues within each protopore and lying close to the permeation path have also been implicated in this activity. Mutation of a cysteine (C212 in ClC-0), which lies in close proximity to the protopore glutamate residue described above and potentially buried deep within the membrane, leads to disruption of the “slow” gate and abolition of zinc-mediated inhibition [23]. These findings suggest possible molecular and functional interactions between the common and protopore modes of ClC channel gating.
Heterodimers
The potential for ClC channels to form heteromeric complexes with other family members has yet to be fully explored. As previously mentioned, ClC proteins are dimeric with an extensive interface between monomers within the membrane [13]. However, to date, the minimal molecular requirements for dimerization are unknown. There is compelling evidence to suggest that heterodimeric association of different ClC channels can occur within the ClC-0 cluster of channels. Lorenz and colleagues were the first to report that the co-expression of two different family members ClC-1 and ClC-2, led to the appearance of a distinct chloride conductance, exhibiting hybrid gating properties [24]. Subsequently, this same research group showed that the construction of concatamers of ClC-0 and ClC-2 led to the appearance of double-barreled channel activity, with one protopore mediating a ClC-0 like unitary conductance of 10 pS and another protopore with a ClC-2 like unitary conductance of approximately 3 pS [25]. These findings indicate that disparate family members within this cluster can functionally associate and further, this interaction may influence gating rather than protopore conductance.
Cytosolic domains
Electrophysiological studies of splice variants and truncated ClC channels lacking regions within the amino and/or carboxy termini support the notion that the cytoplasmic domains also contribute to the “slow” gate of these channels. Partial deletion (residues 16–61) of the amino terminus of ClC-2, a member of the ClC-0 cluster of ClC proteins, led to modification of the properties of the hyperpolarization activated “slow” gate of ClC-2 [26, 27]. Similarly, a splice variant of guinea pig ClC-2, lacking a small region within the amino terminus (residues 77–86) exhibits altered gating kinetics [28]. The chloride and pH dependency of the amino terminus truncation mutants are also altered relative to the wild-type proteins. These findings suggest that the amino terminus contributes to the molecular basis for gating. However, it is not clear whether the participation of the amino terminus is mediated through interactions with other regions of the channel protein [26] or with distinct, ancillary proteins [29].
A primary role for the carboxy terminus of ClC channels in mediating the common gate function has emerged in recent mutagenesis studies [30, 31]. As in the preceding analyses of the amino terminus, most experiments were conducted in members of the ClC-0 cluster of ClC channels. The carboxy terminus possesses a pair of putative cystathione beta-synthase (CBS) regions, CBS1 and CBS2. The duplication of CBS domains is found in most proteins bearing these regions and several crystal structures show that these regions associate to form a dimer [32]. As in other proteins possessing these domains, their dimerization is not essential for, but rather appears to enhance protein expression and function at the cell surface via an unknown mechanism [30, 32–35]. Maduke and colleagues showed that expression of the membrane domain of ClC-0 alone fails to confer channel function whereas native function can be conferred by co-expression of the membrane domain plus CBS1 with the carboxy terminal CBS2 domain [18]. Estevez and colleagues also employed a “split molecule” strategy, co-expressing the entire ClC-1 protein up to the carboxy terminus of CBS1 with the remaining full length carboxy terminus or truncated versions to determine which segments are required to confer voltage-dependent “slow” gating [30]. Co-expression of this truncated protein with the remaining carboxy terminus including CBS2 does enhance functional expression of ClC-1 [30]. The co-expressed carboxy terminus included an extensive intervening sequence, CBS2 and the extreme carboxy terminus. The second CBS domain (CBS2) appears dispensable for channel activity, as CBS1 can substitute for CBS2 in this study and an in-frame deletion of CBS2 did not markedly affect functional expression. However, a short region immediately preceding CBS2 is required for channel activity [30]. These findings suggest that CBS domain dimerization may provide an optimal molecular platform for presentation of key regions in the intervening sequence between the CBS domains to complementary interacting regions in the channel protein. Consistent with this idea, single site mutations predicted to disrupt CBS domain structure impaired the voltage-dependent slow gate [30]. Although these studies suggest that the carboxy terminus exerts an important role in channel slow gating activity, future study is required to establish the molecular mechanism underlying this function.
Several recent studies have also suggested that the CBS domains may serve to regulate channel gating/function through nucleotide binding [32, 36]. Mutations predicted to impair ATP binding via the CBS domains resulted in alterations in the voltage-dependent gating of ClC-1 [36], such that open probability of the common gate would be enhanced at resting membrane potential. These authors suggest that nucleotide depletion and enhanced opening of ClC-1 channels may contribute to stabilizing the membrane potential of skeletal muscle cells during ischemia and metabolic stress. ATP depletion also results in significant activation of ClC-2 channel function [37], suggesting that both ClC-1 and ClC-2 may contribute to a cellular stress response. This idea was reinforced in a recent paper documenting a functional interaction between ClC-2 and Hsp90 [38]. Interestingly, a disease-causing mutation in the carboxy terminus of ClC-2 (G715E) leads to impaired ATP binding [32] and exhibits altered nucleotide regulation of voltage-dependent gating [39]. These findings stress the importance of developing an improved understanding of the molecular consequences of nucleotide interaction with the carboxy terminus of these proteins.
In summary, the common gating function of ClC channels appears to involve multiple domains within these dimeric proteins and likely requires their coordinated interaction. Several key questions will likely drive future research in this area. Namely, we need to determine how the cytosolic domains interact with each other and the functional consequences of this interaction on voltage-dependent slow gating. Furthermore, we need to identify the molecular basis for interaction of the cytosolic domains with the membrane domain and assess how such interaction engages the slow gate.
Heterodimerization may impact on ClC channel/transporter trafficking
Members of the ClC-3 cluster of ClC proteins exhibit an overlapping subcellular distribution. It is generally accepted that ClC-3A, ClC-4, and ClC-5 localize to endosomal compartments, where they facilitate endosomal acidification [40–43] through an unresolved mechanism. As previously mentioned, it has been determined that at least ClC-4, ClC-5, and possibly ClC-3 function as Cl-/H+ antiporters, yet the exact role for this activity in endosomal function has yet to be determined [2, 3]. Two splice variant forms of human ClC-3 (ClC-3A and a longer ClC-3B) have recently been described [44]. ClC-3B localizes to the Golgi compartments [45] and interestingly, co-expression of ClC-3B with ClC-3A leads to the partial re-direction of ClC-3A to the Golgi, possibly because association with ClC-3B confers a PDZ motif and interaction with the Golgi scaffolding protein, GOPC (see following section for further detail). These findings argue that the association of distinct family members may have important consequences with respect to organellar targeting. We have determined that ClC-4 and ClC-5 are co-expressed in recycling endosomes and can be co-immunoprecipitated from native tissues, i.e., renal proximal tubule epithelia [43]. It will be important to test the hypothesis that their interaction may promote co-localization in recycling endosomes, as the carboxy terminal PPXY motif of ClC-5, predicted to be important for its endocytosis, is not conserved in ClC-4. In this case, heterodimerization may confer a dominant endosomal trafficking motif to the complex. Alternatively, there are multiple endosomal trafficking motifs in both proteins that may promote endocytosis of ClC-4 independent of ClC-5, despite the lack of the PY motif. Therefore, future studies are required to determine the functional consequences of the interaction between endosomal ClC transporters and the relative role of distinct endosomal targeting motifs.
Interactions with cytoskeletal/scaffolding and cytosolic proteins contribute to ClC channel/transporter function and trafficking
It is well known that channel/transporter proteins can form functional interactions with cytosolic and/or other membrane proteins and that these interactions can have a vital impact on their physiological activity. In the following paragraphs, we will discuss putative interactions between ClC and cytosolic proteins, including kinases, phosphatases, ubiquitin ligases, and cytoskeletal proteins.
Cytoskeletal/scaffolding proteins
Several ClC family members have been found to associate with cytoskeletal elements. Investigation of the functional consequences of these interactions has provided considerable insight into the functional modulation of these channels. For example, disruption of the actin cytoskeleton following treatment with cytochalasin D or latrunkulin results in significant activation of ClC-2 currents [46], supporting a role for actin remodeling in modulating the functional properties of the ClC-2 channel. Biochemical studies further revealed that the N-terminus of ClC-2 (residues 31–74) can associate directly with the actin cytoskeleton via electrostatic interactions [46]. This finding is significant, in light of the evidence implicating a functional role for ClC-2 in cell-cycle-dependent events, which are usually accompanied by major cytoskeletal rearrangement and actin remodeling [47–49]. Interestingly, the endosome-associated ClC-5 was also reported to interact directly with a cytoskeletal protein, cofilin, via its carboxy terminal tail [50]. Cofilin, which is an actin-binding protein, promotes disassembly of actin filaments, and has been implicated in actin remodeling [51]. Phosphorylation of cofilin leads to actin stabilization and a decrease in albumin trafficking, a function thought to be modulated by ClC-5 [50]. These findings suggest that the function and/or trafficking of ClC-5 may be mediated through associations with the actin cytoskeleton.
ClC-2 is also thought to interact directly or indirectly with the microtubule-associated dynein motor complex [52]. This association was initially defined in biochemical studies employing purified ClC-2 protein as bait for interacting proteins in detergent-solubilized rodent brain lysate. Dynein was identified in the column eluant by mass spectrometry. Both ClC-2 and dynein are likely to interact in vivo as they could be co-immunoprecipitated from hippocampal membranes. The functional significance of this interaction was demonstrated by the finding that the subcellular distribution of endogenous ClC-2 in a fibroblastic cell line was dependent on the integrity of the dynein motor complex [52]. This finding provides the first direct evidence to suggest that the functional expression of ClC-2 at the cell surface is regulated through endosomal trafficking.
Extensive investigations have proven that a protein’s subcellular localization is highly dependent on “localization motifs”, which dictate interactions with specific organelle-resident proteins. This has also been shown for members of the ClC family. ClC-3A localizes to synaptic vesicles and late endosomal compartments and plays a vital role in acidification of these compartments, as revealed in studies of vesicles obtained from ClC-3 knockout mice [40]. ClC-3B, on the other hand, localizes to the Golgi compartment [45]. This protein has an extended C-terminal cytosolic domain that contains a unique PDZ (PSD95/Dlg/ZO-1) domain-binding motif, which is implicated in ClC-3B’s interaction with the second PDZ-binding domain motif of several scaffolding proteins including EBP50 and the Golgi-associated protein, GOPC (Golgi-associated PDZ and coiled-coil motif-containing protein) [44]. The interaction of ClC-3B with GOPC via its carboxy terminus possibly accounts for its Golgi localization. ClC-3A lacks this PDZ domain-binding motif and this may contribute to its distinct localization in the late endosomal compartment [45].
Interactions with kinases, phosphatases and ubiquitin ligases alter ClC channel/transporter activity and trafficking
Electrophysiological studies suggest that kinase- and phosphatase-mediated reactions significantly modulate the functional properties of several members of the ClC family, including ClC-1, ClC-2, and ClC-3. In general, although there are some exceptions [53], agonists that enhance cellular kinase activity suppress chloride flux through these proteins, whereas those that promote phosphatase activity increase ClC function at the cell surface [37, 54–56]. Several kinases have been implicated in the regulation of ClC-2, including PKA, PKC, the M-phase-specific p34cdc2/cyclin B kinase and serum and glucocorticoid inducible kinase isoform 3 [48, 54–57]. Type 1 protein phosphatases have been implicated in the functional regulation not only of mammalian ClC-2 (i.e. PP1, PP2A phosphatases), but also the related Caenorhabditis elegans CLH-3 (i.e. the C. elegans-specific CeGLC 7α, 7β phosphatases) [37, 54]. As these phosphatases have been implicated in controlling C. elegans mitotic and meiotic cell cycle events [58–60], it has been suggested that CLH-3 may be functionally important in cell-cycle-dependent events. As CLH-3 exhibits biophysical and regulatory properties that are similar to ClC-2 [37, 47, 61] an analogous functional role may be ascribed to ClC-2 in mammals. Indeed, a single C-terminal serine residue in rabbit ClC-2 is directly phosphorylated by the M-phase specific p34cdc2/cyclin B kinase [48, 49].
Recently, a detailed study by Denton et al. provided evidence for a direct protein interaction between CLH-3 and GCK-3 (germinal center kinase-3) [61]. Yeast two-hybrid studies first identified GCK-3 as a binding partner for the C-terminus of the CLH-3 isoform, CLH-3b. Interestingly, heterologous expression of GCK-3 elicited a dramatic inhibition of recombinant CLH-3b in HEK293 cells. Conversely, disruption of endogenous GCK-3 gene expression in worm oocytes by siRNA led to constitutive activation of CLH-3 currents, supporting the biological significance of this interaction [61]. Denton and colleagues suggested that there may be a key role for this kinase in the cell-cycle-dependent modulation of CLH-3b. As the mammalian homolog of GCK-3, PASK (proline alanine-rich STE-20 related kinase) is a cytoskeletal-associated kinase that is highly expressed in neuronal and epithelial cells [62], it is possible that ClC-2 may be modulated in a similar manner, though future studies are required to validate this claim.
Several investigations have pointed to an additional role for kinase activity in modulating the cell surface stability of ClC-2. First, phosphoinositide (PI) 3-kinase, which has been implicated in the endocytic trafficking of other membrane proteins, such as the transferrin receptor [63] has been proposed to regulate ClC-2 function [64]. Furukawa and colleagues provided evidence to suggest that phosphorylation of the ClC-2 on its carboxy terminus by p34cdc2/cyclin B kinase leads to enhanced targeting to the proteasomal degradation pathway [48, 49]. These findings suggest that the cell surface expression of ClC-2 channels, i.e., channel number, may be regulated in a phosphorylation-dependent manner and future studies are required to identify the protein-binding partners which mediate regulated endosomal trafficking. We have already alluded to the role of dynein in the trafficking of ClC-2. The function of dynein is modified by phosphorylation [65] and this regulation may potentially account for phosphorylation-dependent changes in the cell surface expression of ClC-2.
A distinct type of post-translational modification, ubiquitination, may also modify trafficking of the endosomal ClC protein, ClC-5 [66, 67]. ClC-5 possesses a proline-rich motif in an intervening region between the two CBS domains of its carboxy terminus, similar, but not identical to the PPXY motif utilized by ENaC [66]. In vitro binding studies led to the hypothesis that this region of the ClC-5 protein binds the E3 ubiquitin ligase Nedd 4.2, or the Nedd 4-like protein WWP2, possibly leading to its ubiquitination, entry into the endosomal pathway and/or degradation. Consistent with this hypothesis, mutation of this proline-rich motif results in an increase in the relative cell surface expression of ClC-5 in in-vivo studies [66, 67]. It has been proposed that Nedd 4.2 also modulates the cell surface expression and function of ClC-K, through direct action on Barttin, a β subunit which possesses a PPXY motif [11, 68]. However, the role of ubiquitination in ClC-5 trafficking and degradation remains vague and future research is required to determine the role of this modification in intracellular trafficking in the context of multiple additional trafficking motifs. For example, alternative internalization motifs may be utilized in the case of ClC-3 and ClC-4, as neither of these endosome-localized transporters possesses a PPXY motif.
Conclusions
In this review, we have highlighted the importance of intramolecular and intermolecular interactions in mediating the intrinsic channel/transporter function of ClC proteins. However, an understanding of the molecular mechanisms linking domain–domain interactions to protein function remains to be elucidated, awaiting the generation of further structural models. Finally, we discussed the role of intermolecular interactions in the context of post-translational modification of ClC proteins and subcellular trafficking. Even though this field is in its infancy, there are tantalizing suggestions of the impact of these interactions on the function of ClC channels/transporters at the cell surface and in different subcellular compartments.
References
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82:503–568
Scheel O, Zdebik AA, Lourdel S, Jentsch TJ (2005) Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436:424–427
Picollo A, Pusch M (2005) Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436:420–423
Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, Jentsch TJ (1992) The skeletal muscle chloride channel in dominant and recessive human myotonia. Science 257:797–800
Haug K, Warnstedt M, Alekov AK, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H, Heils A (2003) Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet 33:527–532
Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP (1997) Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet 17:171–178
Lloyd SE, Pearce SH, Fisher SE, Steinmeyer K, Schwappach B, Scheinman SJ, Harding B, Bolino A, Devoto M, Goodyer P, Rigden SP, Wrong O, Jentsch TJ, Craig IW, Thakker RV (1996) A common molecular basis for three inherited kidney stone diseases. Nature 379:445–449
Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215
Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poet M, Steinfeld R, Schweizer M, Kornak U, Jentsch TJ (2005) Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J 24:1079–1091
Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ (2000) ClC-5 Cl–channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature 408:369–373
Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl- channel beta-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414:558–561
Middleton RE, Pheasant DJ, Miller C (1994) Purification, reconstitution, and subunit composition of a voltage-gated chloride channel from Torpedo electroplax. Biochemistry 33:13189–13198
Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R (2002) X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415:287–294
Dutzler R, Campbell EB, MacKinnon R (2003) Gating the selectivity filter in ClC chloride channels. Science 300:108–112
Accardi A, Miller C (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature 427:803–807
Estevez R, Schroeder BC, Accardi A, Jentsch TJ, Pusch M (2003) Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron 38:47–59
Engh AM, Maduke M (2005) Cysteine accessibility in ClC-0 supports conservation of the ClC intracellular vestibule. J Gen Physiol 125:601–617
Maduke M, Williams C, Miller C (1998) Formation of CLC-0 chloride channels from separated transmembrane and cytoplasmic domains. Biochemistry 37:1315–1321
Hebeisen S, Fahlke C (2005) Carboxy-terminal truncations modify the outer pore vestibule of muscle chloride channels. Biophys J 89:1710–1720
Pusch M, Ludewig U, Jentsch TJ (1997) Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. J Gen Physiol 109:105–116
Saviane C, Conti F, Pusch M (1999) The muscle chloride channel ClC-1 has a double-barreled appearance that is differentially affected in dominant and recessive myotonia. J Gen Physiol 113:457–468
Wu F, Roche P, Christie PT, Loh NY, Reed AA, Esnouf RM, Thakker RV (2003) Modeling study of human renal chloride channel (hCLC-5) mutations suggests a structural–functional relationship. Kidney Int 63:1426–1432
Lin YW, Lin CW, Chen TY (1999) Elimination of the slow gating of ClC-0 chloride channel by a point mutation. J Gen Physiol 114:1–12
Lorenz C, Pusch M, Jentsch TJ (1996) Heteromultimeric CLC chloride channels with novel properties. Proc Natl Acad Sci USA 93:13362–13366
Weinreich F, Jentsch TJ (2001) Pores formed by single subunits in mixed dimers of different CLC chloride channels. J Biol Chem 276:2347–2353
Jordt SE, Jentsch TJ (1997) Molecular dissection of gating in the ClC-2 chloride channel. EMBO J 16:1582–1592
Pusch M, Jordt SE, Stein V, Jentsch TJ (1999) Chloride dependence of hyperpolarization-activated chloride channel gates. J Physiol 515(Pt 2):341–353
Cid LP, Niemeyer MI, Ramirez A, Sepulveda FV (2000) Splice variants of a ClC-2 chloride channel with differing functional characteristics. Am J Physiol Cell Physiol 279:C1198–C1210
Varela D, Niemeyer MI, Cid LP, Sepulveda FV (2002) Effect of an N-terminus deletion on voltage-dependent gating of the ClC-2 chloride channel. J Physiol 544:363–372
Estevez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ (2004) Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol 557:363–378
Hebeisen S, Biela A, Giese B, Muller-Newen G, Hidalgo P, Fahlke C (2004) The role of the carboxyl terminus in ClC chloride channel function. J Biol Chem 279:13140–13147
Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG (2004) CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113:274–284
Sintchak MD, Fleming MA, Futer O, Raybuck SA, Chambers SP, Caron PR, Murcko MA, Wilson KP (1996) Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell 85:921–930
Schwappach B, Stobrawa S, Hechenberger M, Steinmeyer K, Jentsch TJ (1998) Golgi localization and functionally important domains in the NH2 and COOH terminus of the yeast CLC putative chloride channel Gef1p. J Biol Chem 273:15110–15118
Carr G, Simmons N, Sayer J (2003) A role for CBS domain 2 in trafficking of chloride channel CLC-5. Biochem Biophys Res Commun 310:600–605
Bennetts B, Rychkov GY, Ng HL, Morton CJ, Stapleton D, Parker MW, Cromer BA (2005) Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J Biol Chem Jul 20; [Epub ahead of print] doi:10.1074/jbc.M502890200
Rutledge E, Denton J, Strange K (2002) Cell cycle- and swelling-induced activation of a Caenorhabditis elegans ClC channel is mediated by CeGLC-7alpha/beta phosphatases. J Cell Biol 158:435–444
Hinzpeter A, Lipecka J, Brouillard F, Baudouin-Legros M, Dadlez M, Edelman A, Fritsch J (2005) Association between HSP90 and the ClC-2 chloride channel upregulates channel function. Am J Physiol Cell Physiol July 27; [Epub ahead of print] doi:1152/ajpcell.00209.2005
Niemeyer MI, Yusef YR, Cornejo I, Flores CA, Sepulveda FV, Cid LP (2004) Functional evaluation of human ClC-2 chloride channel mutations associated with idiopathic generalized epilepsies. Physiol Genomics 19:74–83
Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ (2001) Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29:185–196
Hara-Chikuma M, Yang B, Sonawane ND, Sasaki S, Uchida S, Verkman AS (2005) ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. J Biol Chem 280:1241–1247
Hara-Chikuma M, Wang Y, Guggino SE, Guggino WB, Verkman AS (2005) Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem Biophys Res Commun 329:941–946
Mohammad-Panah R, Harrison R, Dhani S, Ackerley C, Huan LJ, Wang Y, Bear CE (2003) The chloride channel ClC-4 contributes to endosomal acidification and trafficking. J Biol Chem 278:29267–29277
Ogura T, Furukawa T, Toyozaki T, Yamada K, Zheng YJ, Katayama Y, Nakaya H, Inagaki N (2002) ClC-3B, a novel ClC-3 splicing variant that interacts with EBP50 and facilitates expression of CFTR-regulated ORCC. FASEB J 16:863–865
Gentzsch M, Cui L, Mengos A, Chang XB, Chen JH, Riordan JR (2003) The PDZ-binding chloride channel ClC-3B localizes to the Golgi and associates with cystic fibrosis transmembrane conductance regulator-interacting PDZ proteins. J Biol Chem 278:6440–6449
Ahmed N, Ramjeesingh M, Wong S, Varga A, Garami E, Bear CE (2000) Chloride channel activity of ClC-2 is modified by the actin cytoskeleton. Biochem J 352(Pt 3):789–794
Rutledge E, Bianchi L, Christensen M, Boehmer C, Morrison R, Broslat A, Beld AM, George AL, Greenstein D, Strange K (2001) CLH-3, a ClC-2 anion channel ortholog activated during meiotic maturation in C elegans oocytes. Curr Biol 11:161–170
Furukawa T, Ogura T, Zheng YJ, Tsuchiya H, Nakaya H, Katayama Y, Inagaki N (2002) Phosphorylation and functional regulation of ClC-2 chloride channels expressed in Xenopus oocytes by M cyclin-dependent protein kinase. J Physiol 540:883–893
Zheng YJ, Furukawa T, Ogura T, Tajimi K, Inagaki N (2002) M phase-specific expression and phosphorylation-dependent ubiquitination of the ClC-2 channel. J Biol Chem 277:32268–32273
Hryciw DH, Wang Y, Devuyst O, Pollock CA, Poronnik P, Guggino WB (2003) Cofilin interacts with ClC-5 and regulates albumin uptake in proximal tubule cell lines. J Biol Chem 278:40169–40176
Bamburg JR, Wiggan OP (2002) ADF/cofilin and actin dynamics in disease. Trends Cell Biol 12:598–605
Dhani SU, Mohammad-Panah R, Ahmed N, Ackerley C, Ramjeesingh M, Bear CE (2003) Evidence for a functional interaction between the ClC-2 chloride channel and the retrograde motor dynein complex. J Biol Chem 278:16262–16270
Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ (2004) Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol 556:353–368
Fritsch J, Edelman A (1996) Modulation of the hyperpolarization-activated Cl- current in human intestinal T84 epithelial cells by phosphorylation. J Physiol 490:115–128
Cuppoletti J, Tewari KP, Sherry AM, Ferrante CJ, Malinowska DH (2004) Sites of protein kinase A activation of the human ClC-2 Cl(-) channel. J Biol Chem 279:21849–21856
Kajita H, Whitwell C, Brown PD (2000) Properties of the inward-rectifying Cl- channel in rat choroid plexus: regulation by intracellular messengers and inhibition by divalent cations. Pflugers Arch 440:933–940
Palmada M, Dieter M, Boehmer C, Waldegger S, Lang F (2004) Serum and glucocorticoid inducible kinases functionally regulate ClC-2 channels. Biochem Biophys Res Commun 321:1001–1006
Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, Lin R, Smith MM, Allis CD (2000) Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102:279–291
Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M (2002) The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr Biol 12:798–812
Rogers E, Bishop JD, Waddle JA, Schumacher JM, Lin R (2002) The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J Cell Biol 157:219–229
Denton J, Nehrke K, Yin X, Morrison R, Strange K (2005) GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol 125:113–125
Ushiro H, Tsutsumi T, Suzuki K, Kayahara T, Nakano K (1998) Molecular cloning and characterization of a novel Ste20-related protein kinase enriched in neurons and transporting epithelia. Arch Biochem Biophys 355:233–240
Martys JL, Wjasow C, Gangi DM, Kielian MC, McGraw TE, Backer JM (1996) Wortmannin-sensitive trafficking pathways in Chinese hamster ovary cells Differential effects on endocytosis and lysosomal sorting. J Biol Chem 271:10953–10962
Bali M, Lipecka J, Edelman A, Fritsch J (2001) Regulation of ClC-2 chloride channels in T84 cells by TGF-alpha. Am J Physiol Cell Physiol 280:C1588–C1598
King SM (2000) The dynein microtubule motor. Biochim Biophys Acta 1496:60–75
Schwake M, Friedrich T, Jentsch TJ (2001) An internalization signal in ClC-5, an endosomal Cl-channel mutated in dent’s disease. J Biol Chem 276:12049–12054
Hryciw DH, Ekberg J, Lee A, Lensink IL, Kumar S, Guggino WB, Cook DI, Pollock CA, Poronnik P (2004) Nedd4-2 functionally interacts with ClC-5: involvement in constitutive albumin endocytosis in proximal tubule cells. J Biol Chem 279:54996–55007
Embark HM, Bohmer C, Palmada M, Rajamanickam J, Wyatt AW, Wallisch S, Capasso G, Waldegger P, Seyberth HW, Waldegger S, Lang F (2004) Regulation of CLC-Ka/barttin by the ubiquitin ligase Nedd4-2 and the serum- and glucocorticoid-dependent kinases. Kidney Int 66:1918–1925
Acknowledgements
The authors would like to acknowledge the contributions of Winnie Luong, Dr. Raha Mohammad-Panah, Dr. Canhui Li, and Dr. Mohabir Ramjeesingh to the ideas and several of the experiments discussed in this review. Our research was supported by the Heart and Stroke Foundation of Canada and the Canadian Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhani, S.U., Bear, C.E. Role of intramolecular and intermolecular interactions in ClC channel and transporter function. Pflugers Arch - Eur J Physiol 451, 708–715 (2006). https://doi.org/10.1007/s00424-005-1513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-005-1513-4