Abstract
Mucolipidosis type IV (MLIV) is a rare, neurogenetic disorder characterized by developmental abnormalities of the brain, and impaired neurological, ophthalmological, and gastric function. Considered a lysosomal disease, MLIV is characterized by the accumulation of large vacuoles in various cell types. Recent evidence indicates that MLIV is caused by mutations in MCOLN1, the gene that encodes mucolipin-1 (ML1), a 65-kDa protein showing sequence homology and topological similarities with polycystin-2 and other transient receptor potential (TRP) channels. In this report, our observations on the channel properties of ML1, and molecular pathophysiology of MLIV are reviewed and expanded. Our studies have shown that ML1 is a multiple sub-conductance, non-selective cation channel. MLIV-causing mutations result in functional differences in the channel protein. In particular, the V446L and ΔF408 mutations retain channel function but have interesting functional differences with regards to pH dependence and Ca2+ transport. While the wild-type protein is inhibited by Ca2+ transport, mutant ML1 is not. Atomic force microscopy imaging of ML1 channels shows that changes in pH modify the aggregation and size of the ML1 channels, which has an impact on vesicular fusogenesis. The new evidence provides support for a novel role of ML1 cation channels in vesicular acidification and normal endosomal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucolipidosis type IV (MLIV) is a rare genetic disorder originally described by Berman et al. [8]. Clinically, MLIV is diagnosed by psychomotor retardation, and visual impairment [2]. MLIV patients also have abnormalities in white matter [14], indicative of a developmental brain disorder. At the cellular level, MLIV patients show abnormal vacuoles that stain with lysosomal markers in a number of cell types [15]. Vacuole accumulation is most prominent in secretory epithelial cells such as corneal epithelial [43], pancreas acinar [20], and stomach parietal cells [1]. Interestingly, parietal cells also manifest the only known specific biochemical abnormality of MLIV: achlorhydria associated with elevated gastrin secretion [40]. MLIV is caused by mutations in the recently discovered MCOLN1 gene that encodes a novel protein, mucolipin-1 (ML1) [5, 7, 44]. At least two other genes have been identified as homologous [13]. MCOLN1 mutations have been identified predominantly in Ashkenazi Jewish (AJ), but also in non-Jewish patients [3]. Two founder MCOLN1 mutations have been identified and account for 95% of mutant alleles in Ashkenazi MLIV families [5, 7, 44], in correlation with two haplotypes in the chromosomal region [42]. The most common AJ mutations result in a null-expression [44], suggesting that “lack of function” in ML1 causes the disease. Other mutations that only cause a minor change in the predicted sequence of ML1 result in varied phenotypes. These mutations range from the F408 deletion (ΔF408), an in-frame amino acid deletion identified in a patient with an unusually mild case of the disease, to more severe phenotypes [2]. Two patients have been reported with an intermediate form of MLIV, based on consecutive amino acid substitutions, namely V446L, and L447P, in the expected transmembrane segment 5 (TM5) of the protein (see below) [2]. These MLIV-causing mutations are expected to have ML1 activity, as recently reported [37].
Wild-type (WT) ML1 is a 580-amino acid protein, with an expected molecular mass of approximately 65 kDa. ML1 (TrpML1 following comprehensive nomenclature [32]) presents a transient receptor potential (Trp) channel-homologous region, particularly within amino acids 331–521, and an internal Ca2+ and Na+ channel pore region between amino acids 496 and 521. The topology of ML1 is expected to have the six putative TM domains prototypical of Trp channels [44]. The Trp-similar region spans TM3–6, with a putative pore-forming (P) loop between TM5 and 6. ML1 also contains two Pro-rich regions (aa 28–36 and 197–205), and a lipase serine active site domain (aa 104–114). A di-leucine motif (L-L-X-X) motif present at the carboxy-terminal end of ML1 may contribute to the expected late endosomal/lysosomal targeting. This is consistent with the late endosomal/lysosomal nature of MLIV, and the vacuolar manifestation in cells from MLIV patients. Further, ML1 shows sequence homology with the Caenorhabditis elegans ortholog gene, mutations in which also cause endocytic abnormalities [13, 21]. The human MCOLN1 gene rescues this defect [21]. ML1 also has strong topological homology with the polycystin-2 channel [44]. Recently, we have reported that ML1 is a non-selective cation channel [37]. Another report has shown that expression of the full-length MCOL1 cDNA in Xenopus oocytes is associated with the presence of large-conductance channels with permeability to Na+, K+, and Ca 2+ [24]. That study did not provide evidence, however, as to whether the channels observed are either endogenous, or, alternatively, complexes of ML1 with endogenous channels proteins, which may explain discrepancies with data obtained with the isolated protein [37]. In this report we review some of our original data, and provide new evidence that MLIV causing mutations in ML1 have distinct differences with respect to channel gating.
Materials and methods
In vitro translation of mucolipin-1 and proteoliposome preparations
WT-ML1 and two mutants, V446L-ML1 and ΔF408-ML1 were translated in vitro using a kit (Promega, Madison, Wisc., USA) as recently described [37]. Briefly, the human MCOLN1 cDNAs were sub-cloned in the pSV-SPORT1 expression vector to generate specific constructs. The cDNAs within the vector were transcribed in vitro and translated with the TnT-T7-coupled reticulocyte system (Promega). ML1-containing proteoliposomes were prepared with a lipid mixture of phosphatidylethanolamine (75%) and phosphatidylserine (25%) (Avanti Polar Lipids, Birmingham, Ala., USA). The phospholipid mixture was sonicated with a solution containing (in mM) 150 NaCl, 0.1 EDTA, 20 HEPES, pH 7.2, and 25 Na+-cholate. In vitro translated ML1 was added to the Na+-cholate solution (0.55 mM) and the phospholipid mixture (10 mg/ml). The mixture was dialyzed for 3 days in (in mM) 150 NaCl, 0.1 EDTA, 20 HEPES, pH 7.2, with three buffer changes to eliminate residual detergent and obtain the proteoliposomes used in the functional assays. Samples were separated by 4–12% SDS-PAGE and stained with Simply Blue Safe Stain (Invitrogen, Carlsbad, Calif., USA).
Solutions and changes in pH
The lipid bilayer solution was prepared as follows. A buffer solution was prepared with 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS) and 10 mM 2-(N-morpholino)ethanesulfonic acid (MES), and adjusted with KOH to pH 7.4. This solution had a final [K+] of 15 mM at room temperature. The solution also contained 10–15 μM CaCl2. To create a KCl chemical gradient KCl was added to the cis side of the chamber, to a final concentration of 150 mM. Whenever indicated, CaCl2 (500 mM) was added to the trans compartment to a final concentration of 90 mM.
Electrical recordings and data analysis
Ion channels were reconstituted as previously described [16, 17]. Briefly, lipid bilayers were formed with a mixture of synthetic phospholipids. The phospholipid composition of the lipid bilayers was seven parts of 1-palmitoyl-2-oleyl-sn-glycero-3-phosphatidylethanolamine and three parts of 1-palmitoyl-2-oleyl-sn-glycero-3-phosphatidylcholine (7:3, v/v, Avanti Polar Lipids) in n-decane (Sigma, St. Louis, Mo., USA) to final concentrations of 14 and 6 mM, respectively [16, 17]. All phospholipids used were 1-palmitoyl-2-oleoyl-based, the polar head group being choline (POPC) and ethanolamine (POPE). The lipid solution (~20–25 mg/ml) was used to “paint”, with a thin glass rod, the opening (250 μm diameter) of the polystyrene cuvette (CP13-150). The cuvette was inserted into a polyvinylchloride holder. Both sides of the lipid bilayer were bathed with an MOPS/MES-KOH-buffered solution as described above. Experiments were initiated by bathing the trans side of the bilayer in this solution after further addition of KCl (final 150 mM) to the cis side of the chamber. Holding potentials (Vh) were applied, and electrical signals recorded using a patch-clamp amplifier as previously reported [16]. The patch-clamp amplifier contained a 10-GΩ head-stage for lipid bilayers (PC-510A Warner Instruments, Hamden, Conn., USA). Output (voltage) signals were low-pass filtered at 1 kHz (−3 dB) with an eight-pole Bessel type filter (Frequency Devices, Haverhill, Mass., USA). Whenever indicated, electrical recordings of single-channel activity (current tracings), were filtered further (see Results) for display purposes only. PClamp v. 5.5.1 (Axon Instruments, Foster City, Calif., USA) was used for data analysis, and Sigmaplot v. 2.0 (Jandel Scientific, Corte Madera, Calif., USA) for statistical analysis and graphics. Open probability (Po) was obtained from exponential fitting of dwell-time histograms, and fitted to a Boltzmann equation [16, 17]. Significance, accepted at P<0.05, was obtained by Student’s t-test for paired samples. Data are expressed as mean±SEM for n experiments analyzed.
Atomic force microscopy
ML1 protein complexes were imaged with a Model 3000 atomic force microscope (AFM) attached to a Nanoscope IIIa controller (Digital Instruments, St. Barbara, Calif., USA) as recently reported [37]. In vitro-translated ML1-containing proteoliposomes used for the lipid-bilayer reconstitution studies were used for AFM imaging studies. Briefly, liposomes containing in vitro-translated ML1 were “baked” onto freshly cleaved mica and scanned with oxide-sharpened silicon nitride tips (DNP-S, Digital Instruments) under “tapping mode” conditions. Proteoliposomes were flattened in saline solution, containing (in mM) 0.2 CaCl2, 0.2 MgATP, 0.2 mercaptoethnol and 2 TRIS-HCl, pH 7.15–7.25. Samples were incubated for either ~15 min at 37°C or 30 min at room temperature, with similar results. Small volumes (~2 μl) of HCl (1 M) were added directly to the AFM scanning chamber to change pH. Data were processed with Image SXM v. 1.62 (Steve Barrett, Public Domain, 1999)
Results
Cation channel activity of human ML1
To determine whether human WT-ML1 is implicated in novel channel activity, we recently expressed ML1 [37] in an MCOL1−/− cell background. Endosomes were harvested from both negative control and cells over-expressing WT-ML1, and negative controls for comparison. Endosomal vesicles containing WT-ML1 showed spontaneous cation channel activity after reconstitution in the presence of 150 mM K+ on the cis side, and 15 mM K+ on the trans side of the reconstitution chamber. Cation-selective channels with a 130- to 150-pS single-channel conductance were observed, which were absent in endosomes from MCOLN1−/− cells. Large-conductance channels often “disorganized” into smaller channel levels, consistent with multi-channel complexes. The most frequent sub-conductance state was a 35-pS channel level. To confirm WT-ML1 cation channel activity, the expression vector containing the entire sequence for the human WT-ML1 was transcribed in vitro, translated, and dialyzed into liposomes (see Materials and methods and [37] for details). WT-ML1-containing liposomes were reconstituted in a lipid bilayer system. Spontaneous, cation-selective single-channel currents were observed, with the most frequent single-channel conductance of 46.3±9.44 pS (n=3). The conductance varied among preparations, suggesting channel complexes composed of various channel numbers. Single-channel recordings showed multiple substate levels. Mean versus variance analysis unmasked a main conductance of 35.8 pS and smaller single-channel conductances of 5.52 and 1.02 pS [37]. The Po of the ML1 channel in the most frequent open channel level was strongly voltage dependent, and decreased at negative potentials. This was characterized by fitting the Po versus holding potential data with the Boltzmann equation [37].
Channel activity of mutant ML1 and effect of pH
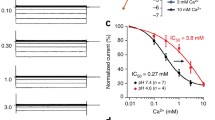
Channel activity was also found in endosomes expressing ΔF408- but not D362Y-ML1 [37]. While ΔF408-ML1 produces the mildest known case of the disease, D362Y-ML1 has been observed in two intermediate cases of the disease [2]. At least three conductance states, the smallest of which was 5 pS (only disclosed by mean vs. variance analysis), were unmasked. The multichannel behavior of ML1 was more clearly observed by channel inhibition elicited by decreasing cis pH from 7.4 to 6.4, which inhibited the WT-ML1 mean currents by 61.4% (26.6±4.42 vs. 10.2±3.19 pA, n=6, P<0.01). The pH-inhibited endosomal channel activity re-activated upon addition of KOH [37]. Both V446L, and ΔF408 ML1 mutants displayed spontaneous cation channel activity in asymmetrical K+, and K+/Na+ gradients. All channels showed multiple sub-conductance states (Fig. 1a). A single-channel conductance of 27.0±1.21 pS (n=4) was observed for ΔF408-ML1 and 42.2 pS (n=1), for V446L-ML1. Mean versus variance analysis also disclosed smaller single-channel conductances of 1.58±0.08 (n=3), and 1.79±0.11 pS (n=3) for ΔF408-ML1 and V446L-ML1, respectively. Conductances of 6.03 (n=3) and 5.02 pS (n=3), for the ΔF408 and the V446L mutants, respectively, were also obtained by mean versus variance analysis. Thus, the small unitary conductance was similar in both WT- and mutant ML1. Interestingly, a shift in the Boltzmann response to voltage was observed for the most frequent conductance state of the ΔF408-ML1 channel (Fig. 1b). To assess whether ML1 is permeable for Ca2+, channel activity was first determined in the presence of a K+ chemical gradient. All three, WT-, ΔF408- and V446L-ML1, showed spontaneous channel activity (Fig. 1a). Next, an opposing Ca2+ gradient was established by addition of 90 mM CaCl2 to the trans compartment (Fig. 2a). WT-ML1 channel activity was greatly reduced after Ca2+ addition. No negative (Ca2+-carrying) currents were observed (Fig. 2a, top). In contrast, both mutant ML1 proteins enabled Ca2+ movement. This blocking effect of Ca2+ on WT-ML1 was reversible, however, upon addition of EGTA (10 mM), to the cis compartment (Fig. 2b). Re-activation of Ca2+-carrying currents by WT-ML1 was also elicited by applying a strong holding potential (data not shown). Changes in K+ currents, however, did not revert after EGTA addition suggesting the movement of both cations through WT-ML1. The data indicate that while WT-ML1 is inhibited by Ca2+, both ΔF408 and V446L mutant ML1 drive Ca2+ currents.
Functional characterization of in vitro translated mucolipin-1 (ML1). a Representative single-channel currents of wild-type (WT)-ML1, and mutants V446L, and ΔF408 ML1. In vitro transcribed/translated ML1 constructs were incorporated into liposomes, and studied by reconstitution in a lipid bilayer system [37], in the presence of a K+ chemical gradient, with 150 and 15 mM KCl in the cis and trans compartments, respectively. Data are representative of 32 experiments. Holding potentials are 60 and 40 mV, for WT and mutant proteins, respectively. b Boltzmann distribution of open probability (Po) of the main conductance state of the channel, as a function of the holding potential (Vh). Experimental data (filled circles) are mean±SEM, n=4–7, and 3 experiments, for WT-, and ΔF408-ML1, respectively. Solid lines indicate best fit of data to a Boltzmann equation
Effect of Ca2+ on ML1 channel currents. a Single-channel currents of WT- and mutant ML1 in the presence of asymmetrical K+ and Ca2+. Experiments were initiated in a K+ chemical gradient, as in Fig. 1. Once channel activity was detected, CaCl2 was added to the trans compartment to a final concentration of 90 mM. Currents at negative potentials (Ca2+ driven) were observed. While WT-ML1 showed no negative currents, both ΔF408 and V446L mutant ML1 displayed channel activity. b Interestingly, Ca2+ currents by WT-ML1 could be initiated after addition of EGTA (10 mM) to the cis (counterlateral) compartment. Data are representative of three experiments. c In the presence of 90 mM Ca2+, in the trans compartment, the counter-lateral K+-driven currents are also reduced in WT-ML1. Data are representative of three experiments
AFM of mucolipin-1 channel clusters
The dispersive nature of the unitary conductances and presence of multiple sub-conductance states in the ML1 channels is suggestive of complexing of single channel clusters. Considering that ML1 may be located in highly acidic vesicular organelles, it is speculated that changes in pH may play a role in ML1 channel assembly, as postulated for other channels [10, 11, 41]. As a matter of fact, both Ca2+ and pH may be essential in determining the fusogenic properties of vesicular organelles. To test whether pH affects membrane fusion and modulates channel clustering, WT-ML1 containing liposomes were flattened onto freshly cleaved mica and scanned by AFM in saline solution, as recently reported [37]. WT-ML1 single-channel proteins were found at the “edge” of flattened liposomes. Multiprotein channel complexes changed in size at low pH (Fig. 3), in agreement with our recent findings [37]. Thus, spontaneous aggregation of WTML1 complexes at normal pH (7.15) changes at low pH (6.5). Further, a decreased pH helped to spread and fuse the flattened liposomes (Fig. 3), in agreement with the contention that lowering pH may be an important contributor to membrane fusion, a phenomenon mimicked by addition of divalent cations (Chasan et al, unpublished observations). These new data suggest that channel function by ML1 may control intravesicular pH and Ca2+, both contributors of membrane fusogenic capabilities.
Atomic force imaging of ML1 complexes. In vitro translated WT-ML1 containing proteoliposomes were flattened in solution onto freshly cleaved mica and imaged, in tapping mode AFM. a WT-ML1 complexes aggregated at normal pH (top, consecutive arrows). Low pH reduced the size of the complexes (bottom). Data are representative of three experiments. b Averaged scan lines (n=3) shows that distance between flattened liposomes (thick lines) is dramatically shorter after lowering pH. Dashed lines show the height of the lipid layers
Discussion
Three mucolipin genes have been found in humans, and other vertebrates, and only one in invertebrates e.g., Drosophila melanogaster (CG8743), and C. elegans (CUP-5) [13, 21]. The role(s) of the gene products is yet largely unknown, but information is mounting on similar roles in endocytotic vesicle trafficking. MLIV is a lipid storage disease, where impaired traffic of late endocytotic/lysosomal vesicle transport is observed [4, 6, 9, 13]. This is consistent with membrane abnormalities in MLIV patients, which is most pronounced in certain secretory epithelial cells [26]. In MLIV patient fibroblasts, high uptake and slow lipid degradation [20], and/or lipid sorting abnormalities [9] have also been reported. Thus, ML1 function is probably associated with vesicle trafficking. ML1 function may help stabilize intraorganelle homeostasis, which in its absence deteriorates, changing the recycling ability of the endosomal machinery. Loss-of-function mutations of the MCOLN1 gene homolog in the C. elegans (CUP-5), for example, lead to endocytotic defects, the formation of large lysosomal vacuoles, and increased apoptosis [13, 21]. The recently described mouse mucolipin-3 (ML3) localizes to cytoplasmic compartments of hair cells and stereocilia [12]. ML3 expression may play a critical role in vesicular structures and melanosome transport [23]. Although ML3 function is still unknown, skin pigmentation abnormalities, are associated with defects in vesicle function, and dysfunctional trafficking of late-stage melanosomes [25, 29, 47, 48]. In mice, ML3 deficiency causes deafness, a phenomenon that has been hypothesized to ML-related Trp-type channel activity [12].
The data in this report suggest that while WT-ML1 and the two mutants tested act as cation channels, the two MLIV-causing mutations that retain ML1 channel function have lost their ability to be inhibited by Ca2+. This is reminiscent of our recent findings indicating that both ΔF408- and V446L-MLI channels, have lost their ability to be regulated by lowering pH. Considering the importance of intravesicular pH in endosome/lysosomal function, it is expected that mechanisms linked to its regulation may be essential for the ability of vesicular organelles to fuse (Fig. 4). The atomic force imaging of WT-ML1-containing liposomes, the ability of which to spread and fuse was highly accelerated by changes in pH strongly supports this contention. Thus, low pH not only modified the state of aggregation of the unitary ML1 channels, and their intrinsic channel activity [37], but also the membrane properties of the liposomes. This may explain, in part, the pH effect on ML1, which is clearly different from that observed in the Trp-related channel PC2 [16]. The effect of lowering pH may reside in changes in the multiple sub-conductance states of the channels, which probably correlate functionally with each monomeric unit of the channel complex. This, however, will require further investigation. Interestingly, recent findings from our laboratory indicate that mutant ML1 channel complexes do not change in size at low pH [37]. In this context, the data suggest that the dysfunctional channel activity by mutant ML1 is most consistent with a “gain of function” rather than lack of function. Nonetheless, the major MLIV-causing mutations are expected to produce no protein, suggesting no contribution of the ML1 channel to cell function in the disease. The data provide support, however, for the idea that ML1 indeed is a functional Trp channel family member [31, 32]. Trp channels are quintessentially related to Ca2+ entry steps, and capacitative responses [22, 35, 36] in a number of sensory transduction events [18, 30, 46]. It is expected that ML1 channel function plays an important role in the ability of endosomal vesicles to fuse.
Hypothetical model of ML1 control of endosome/lysosomal function. a Hypothetical topology of WT-ML1 showing the six transmembrane domains (TM), and the pore region expected between TM5 and 6, with a P-loop. b Sequence comparison of human mucolipins 1 and 3, and human polycystin-2, in the distal region encompassing TMs 3–6. Both MLIV-causing mutations, tested in this report are indicated, which are adjacent to TMs 4 and 5, respectively. Interestingly, D326Y, tested in endosomal vesicles (see [37]), is without channel activity. c Hypothetical model of WT-ML1’s role in vesicular function. Cation channel function of normal ML1 (arrow) controls vesicular membrane potential ΔΨ and probably intravesicular Ca2+ content by switching off at low pH, which provides the driving force for H+ accumulation driven by other transporters. The inability of mutated ML1 to “shut-off” at low pH is in agreement with dissipation of the vesicular H+ gradient. ML1 function modifies vesicle fusogenesis by controlling intravesicular pH and Ca2+ [19]
Presently, no information is available as to the physiological location of WT-ML1. Nonetheless, the fact that MLIV, a lysosomal disease, is caused by mutations in a channel regulated by pH [37] and Ca2+ (this report), strongly suggests an important role in vesicular homeostasis. Most intracellular organelles along the endocytotic and secretory pathways, as well as lysosomes, have characteristic acidic intravesicular pH suited to their biological function. The establishment of a pH gradient within the endosomal compartment is key to a normal endocytic trafficking [27]. How this phenomenon is achieved is still unknown. However, vesicular acidification is a concerted process, where V-type ATPases elicit an uphill H+ electrochemical gradient that is accompanied by Cl− counterion movement via ClC-type channels and consequent intravesicular acidification (Fig. 4c). This H+ gradient is maintained and controlled by cation transport enabled by various proteins, including the Na+,K+-ATPase, and cation-selective channels. To date, little is known about the molecular identity of electrodiffusional cation-selective pathways in the endosome/lysosomal machinery. The expected presence of a functional ML1 in endosomes, combined with its ability to be regulated by Ca2+ and pH, provide the first indication for a functional mechanism implicated in this aspect of vesicular regulation. WT, but not mutant, ML1 was inhibited by low pH and high Ca2+. This suggests a sensitive regulatory mechanism for vesicular function, which is in agreement with previous evidence indicating that MLIV displays abnormal lysosomal pH [4]. A dysregulated (or absent) ML1 channel would likely cause this abnormality. Further studies will be required to assess whether compensatory mechanisms are at work, which complement yet unknown transport mechanisms in the absence of a functional ML1. Endosomal vesicles and Golgi complexes are permeable to counterions such as Cl− and K+, which can also affect vesicular pH by altering the vesicular membrane potential [39, 45]. ML1 cation channel function, may thus be considered relevant for the normal process of vesicular acidification [19, 38]. A feedback mechanism mediated by ML1 could help control intravesicular pH [19], and, most importantly, intravesicular resting potential, which is a main contributor to the driving force for lowering pH in intracellular vesicles [27, 28, 38]. Cation transport signaling events trigger vesicle trafficking and fusion [33], and Ca2+ release-coupled K+ transport across Ca2+ storage organelles such as the endoplasmic reticulum [33, 34]. Thus, ML1 may help regulate vesicular membrane potential, the process of acidification associated with normal vesicular function, and/or Ca2+ transport, into intracellular organelles.
References
Agnew BJ, Duman JG, Watson CL, Coling DE, Forte JG (1999) Cytological transformations associated with parietal cell stimulation: critical steps in the activation cascade. J Cell Sci 112:2639–2646
Altarescu G, Sun M, Moore D, Smith J, Wiggs E, Solomon B, Patronas N, Frei K, Gupta S, Kaneski C, Quarrell O, Slaugenhaupt S, Goldin E, Schiffmann R (2002) The neurogenetics of mucolipidosis type IV. Neurology 59:306–313
Bach G (2001) Mucolipidosis type IV. Minireview Mol Genet Metab 73:197–203
Bach G, Chen CS, Pagano RE (1999) Elevated lysosomal pH in Mucolipidosis type IV cells. Clin Chim Acta 280:173–179
Bargal R, Avidan N, Ben-Asher E, Olender Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D, Bach G (2000) Identification of the gene causing mucolipidosis type IV. Nat Genet 26:118–123
Bargal R, Bach G (1997) Mucolipidosis type IV: abnormal transport of lipids to lysosomes. J Inherit Metab Dis 20:625–632
Bassi MT, Manzoni M, Monti E, Pizzo MT, Ballabio A, Borsani G (2000) Cloning of the gene encoding a novel integral membrane protein, mucolipidin, and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet 67:1110–1120
Berman ER, Livni N, Shapira E, Merin S, Levij IS (1974) Congenital corneal clouding with abnormal systemic storage bodies: a new variant of mucolipidosis. J Pediatr 84:519–526
Chen CS, Bach G, Pagano RE (1998) Abnormal transport along the lysosomal pathway in mucolipidosis type IV disease. Proc Natl Acad Sci USA 95:6373–6378
Clay JR, Kuzirian A (2002) Trafficking of axonal K+ channels: potential role of Hsc70. J Neurosci Res 67:745–752
Clay JR, Kuzirian AM (2000) Localization of voltage-gated K+ channels in squid giant axons. J Neurobiol 45:172–184
Di Palma F, Belyantseva I, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K (2002) Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci USA 99:14994–14999
Fares H, Greenwald I (2001) Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nat Genet 28:64–68
Frei KP, Patronas NJ, Crutchfield KE, Altarescu G, Schiffmann R (1998) Mucolipidosis type IV: characteristic MRI findings. Neurology 51:565–569
Goldin E, Blanchette-Mackie EJ, Dwyer NK, Pentchev PG, Brady RO (1995) Cultured skin fibroblasts derived from patients with mucolipidosis 4 are autofluorescent. Pediatr Res 37:687–692
González-Perrett S, Batelli M, Kim K, Essafi M, Timpanaro G, Montalbetti N, Reisin IL, Arnaout MA, Cantiello HF (2002) Voltage dependence and pH regulation of human polycystin-2 mediated cation channel activity. J Biol Chem 277:24959–24966
González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF (2001) Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98:1182–1187
Goodman MB, Schwarz EM (2003) Transducing touch in Caenorhabditis elegans. Annu Rev Physiol 65:429–452
Grabe M, Oster G (2001) Regulation of organelle acidity. J Gen Physiol 117:329–343
Hammel I, Alroy J (1995) The effect of lysosomal storage diseases on secretory cells: an ultrastructural study of pancreas as an example. J Submicrosc Cytol Pathol 27:143–160
Hersh B, Hartwieg E, Horvitz H (2002) The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc Natl Acad Sci USA 99:4355–4360
Jayadev S, Petranka JG, Cheran SK, Biermann JA, Barrett J, Murphy E (1999) Reduced capacitative calcium entry correlates with vesicle accumulation and apoptosis. J Biol Chem 274:8261–8268
Kachar B, Battaglia A, Fex J (1997) Compartmentalized vesicular traffic around the hair cell cuticular plate. Hear Res 107:102–112
LaPlante JM, Falardeau J, Sun M, Kanazirska M, Brown EM, Slaugenhaupt SA, Vassilev PM (2002) Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett 532:183–187
Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer JA III, Pavan WJ (2002) Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci USA 99:4471–4476
Lubensky IA, Schiffmann R, Goldin E, Tsokos M (1999) Lysosomal inclusions in gastric parietal cells in mucolipidosis type IV: a novel cause of achlorhydria and hypergastrinemia. Am J Surg Pathol 23:1527–1531
Marshansky V, Ausiello DA, Brown D (2002) Physiological importance of endosomal acidification: potential role in proximal tubolopathies. Curr Opin Nephrol Hypertens 11:527–537
Marshansky V, Vinay P (1996) Proton gradient formation in early endosomes from proximal tubules. Biochem Biophys Acta 1284:171–180
Matesic LE, Yip R, Reuss AE, Swing DA, O’Sullivan TN, Fletcher CF, Copeland NG, Jenkins NA (2001) Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc Natl Acad Sci USA 98:10238–10243
Montell C (2001) Physiology, phylogeny, functions of the TRP superfamily of cation channels. Sci STKE 90:RE1
Montell C, Birnbaumer L, Flockerzi V (2002) The TRP channels, a remarkably functional family. Cell 108:595–598
Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX (2002) A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 9:229–231
Nguyen T, Chin WC, Verdugo P (1998) Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+. Nature 395:908–991
O’Rourke F, Soons K, Flaumenhauft R, Watras J, Baio-Larue C, Matthews E, Feinstein MB (1994) Ca2+ release by inositol 1,4,5-trisphosphate is blocked by the K+-channel blockers apamin and tetrapentylammonium ion, a monoclonal antibody to a 63 kDa membrane protein: reversal of blockade by K+ ionophores nigericin and valinomycin and purification of the 63 kDa antibody-binding protein. Biochem J 300:673–683
Putney JW Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS (2001) Mechanisms of capacitative calcium entry. J Cell Sci 114:2223–2229
Putney JW Jr, McKay RR (1999) Capacitative calcium entry channels. Bioessays 21:38–46
Raychowdhury MK, González-Perrett S, Montalbetti N, Timpanaro GA, Chasan B, Goldmann WH, Stahl S, Cooney A, Goldin E, Cantiello HF (2004) Molecular pathophysiology of mucolipidosis type IV. pH dysregulation of the human mucolipin-1 cation channel. Hum Mol Genet 13:617–627
Rybak SL, Lanni F, Murphy RF (2000) Theoretical considerations on the role of membrane potential in the regulation of endosomal pH. Biophys J 73:674–687
Schapiro F, Grinstein S (2000) Determinants of the pH of the Golgi complex. J Biol Chem 275:21025–21032
Schiffmann R, Dwyer NK, Lubensky IA, Tsokos M, Sutliff VE, Latimer JS, Frei KP, Brady RO, Barton NW, Blanchette-Mackie EJ, Goldin E (1998) Constitutive achlorhydria in mucolipidosis type IV. Proc Natl Acad Sci USA 95:1207–1212
Shuai JW, Jung P (2003) Optimal ion channel clustering for intracellular calcium signaling. Proc Natl Acad Sci USA 100:506–510
Slaugenhaupt SA, Acierno JSJ, Helbing LA, Bove C, Goldin E, Bach G, Schiffmann R, Gusella JF (1999) Mapping of the mucolipidosis type IV gene to chromosome 19p and definition of founder haplotypes. Am J Hum Genet 65:773–778
Smith JA, Chan CC, Goldin E, Schiffmann R (2002) Noninvasive diagnosis and ophthalmic features of mucolipidosis type IV. Ophthalmology 109:588–594
Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JSJ, Bove C, Kaneski CR, Nagle J, Bromley MC, Colman M, Schiffmann R, Slaugenhaupt SA (2000) Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet 9:2471–2478
Van Dyke RW, Belcher JD (1994) Acidification of three types of liver endocytic vesicles: similarities and differences. Am J Physiol 266:C81–C94
Voets T, Nilius B (2003) TRPs make sense. J Membr Biol 192:1–8
Wilson SM, Yip R, Swing DA, O’Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA (2000) A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA 97:7933–7938
Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA (2002) Identification of an organelle receptor for myosin-Va. Nat Cell Biol 4:271–278
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cantiello, H.F., Montalbetti, N., Goldmann, W.H. et al. Cation channel activity of mucolipin-1: the effect of calcium. Pflugers Arch - Eur J Physiol 451, 304–312 (2005). https://doi.org/10.1007/s00424-005-1448-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-005-1448-9