Abstract
IL-1β reduces the activity and protein expression of Na+-K+-ATPase in rat kidney cells. The aim of the present study was to elucidate the signalling pathway involved, using the LLC-PK1 cell line. In these cells IL-1β caused a time and concentration-dependent decrease in the protein expression of the Na+-K+-ATPase. Inhibition of extracellular signal-regulated kinase (ERK), nuclear factor-κB (NF-κB) and cyclooxygenase (COX), but not p38 mitogen-activated kinase (MAPK), abolished the effect of the cytokine on the pump. The activation of NF-κB by IL-1β was maximal at 20 min and declined thereafter. Inhibition of the transcription factor by pyrrolidinediethyldithiocarbamate (PDTC) down-regulated the ATPase. The effects of IL-1β on the pump and NF-κB were prevented by the COX inhibitor indomethacin. Exogenous PGE2 reduced protein expression of the ATPase within 15 min, even in presence of an ERK inhibitor. It is concluded that IL-1β stimulates the mitogen and extracellular signal regulated protein kinase kinase/extracellular signal regulated protein kinase (MEK/ERK) pathway. This activates NF-κB, thus leading to increased COX-2 expression and PGE2 release. PGE2 in turn inhibits NF-κB and reduces the protein expression of Na+-K+-ATPase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal epithelia play a key role in the excretion of ions and molecules and in the maintenance of salt and water balance. The principal regulator of transepithelial ion transport is the Na+-K+-ATPase, a ubiquitously expressed pump responsible for the generation of the Na+ gradient that drives many cellular activities such as active transport processes and the production of action potentials.
The Na+-K+-ATPase has two subunits, α and β. The catalytic α-subunit (~113 kDa) contains the binding sites for Na+, K+, ATP and ouabain [19]. The β-subunit is needed for the activity of the complex and for the insertion of the pump in the plasma membrane. Many α- and β-subunit isoforms have been identified [8] and can assemble in different combinations. The α1β1 association is found nearly in every tissue and is considered to be the housekeeping isozyme. It is also the principle isozyme in the kidney [25].
Interleukin-1 beta (IL-1β) is a proinflammatory cytokine produced by macrophages. It alters renal electrolyte reabsorption and induces natriuresis [3, 4, 12, 15, 22, 30] and diuresis [16]. Recently, we have unravelled the mechanism underlying these effects of the cytokine by demonstrating a decrease in the activity and protein expression of Na+-K+-ATPase in both medullary and cortical kidney cells isolated from rats treated with IL-1β [16]. Inhibition of the pump is expected to decrease the Na+ gradient and Na+ reabsorption, thus leading to natriuresis, and to decrease the osmotic gradient, thus leading to diuresis. Although the effect of the cytokine on the pump has been well established, the signalling pathway involved is still ill-defined.
IL-1β is known to exert a diversity of biological effects through an activation of the mitogen-activated protein (MAP) kinase (MAPK) cascades, including the extracellular signal-regulated kinase (ERK), c Jun amino-terminal kinase (JNK) and the p-38 MAPK [23]. Once activated, MAPKs in turn phosphorylate a variety of intracellular substrates including certain transcription factors like nuclear factor-κ B (NF-κB) [14, 15, 23, 24].
NF-κB is a nuclear transcription factor that resides in its inactive state in the cytoplasm, complexed to an inhibitory protein IκB. Upon activation of the complex, ΙκB undergoes sequential phosphorylation on Ser-32 and Ser-36 by IκB kinase, resulting in its subsequent ubiquitination and degradation, thus releasing NFκB for translocation to the nucleus where it activates transcription of various genes [2].
Prostaglandins (PG) are also thought to mediate part of the response to IL-1β. By activating JNK or p38 MAPK, IL-1β induces cyclooxygenase-2 (COX-2) expression and PGE2 production [10, 14]. Although there are two COX isoforms (COX-1 and -2), only COX-2 is inducible by cytokines, growth factors and bacterial endotoxins [27, 29]. COX-1 is constitutively active and unaffected by IL-1 β or tumour necrosis factor-α (TNF-α) [9]. Activation of NF-κB is necessary for the induction of COX-2 expression in many, but not all cells. Although TNF-α activates NF-κB and induces COX-2 gene expression in rat vascular smooth muscle cells [6], the two effects are unrelated to each other, suggesting that the transcriptional regulation of COX-2 by NF-κB is dependent on the type of cell [6].
It is apparent that although many of the mediators of IL-1β signalling have been identified, they are not ubiquitous and their positions relative to each other in the signalling pathway are still ill-defined. The aim of this work was thus to determine the signal transduction pathway activated by IL-1β through which the cytokine exerts its effect on renal Na+-K+-ATPase. For this the porcine renal proximal tubule cell line (LLC-PK1) was used and the involvement of already established intermediates of IL-1β action like NF-κB, MAPK and PGE2 was studied. Knowing the mode of action of the cytokine would help in modulating the different undesirable effects and symptoms that accompany some inflammatory reactions and may help also in circumventing the natriuresis and diuresis that always accompany high levels of IL-1β.
Materials and methods
Culture media, fetal bovine serum (FBS), penicillin/streptomycin, trypsin-EDTA and Hanks balanced salt solution (HBSS) were purchased from Gibco (Paisley, UK). The MAPK inhibitors PD98059 and SB 202190 were from Calbiochem (San Diego, Calif., USA). Pyrrolidinediethyldithiocarbamate (PDTC), indomethacin, cycloheximide, actinomycin D and prostaglandin E2 (PGE2) were obtained from Sigma (St Louis, Mo., USA). Recombinant human interleukin-1β was from Endogen (Woburn, Mass., USA). Rabbit anti-Na+-K+-ATPase α1 polyclonal antibody and anti-rabbit IgG horseradish peroxidase (HPR)-conjugated antibody were from Upstate Biotechnology (Lake Placid, N.Y., USA, Cat. No. 06-520 and 12-348 respectively). Anti-IκBα monoclonal antibody was from Biosource International (Camarillo, Calif., USA), while goat anti-mouse IgG HPR-conjugated antibody and the enhanced chemiluminescence (ECL) kit were from Santa Cruz Biotechnology, (Santa Cruz, Calif., USA). Polyvinylidene difluoride (PVDF) membranes, protein assay reagent and rainbow marker were from Biorad (Hercules, Calif., USA). The LLC-PK1 cell line (porcine renal proximal tubule cells) was a generous gift from Dr. Rula Barhoumi-Mouneimbeh from Texas A&M University, USA.
Cell culture and treatments
Cells were cultured in DMEM-F12 medium supplemented with 10% heat-inactivated FBS, penicillin (100 µg/ml) and streptomycin (100 µg/ml) in a humidified incubator (95% air, 5% CO2) at 37°C in 100-mm culture dishes. The medium was replenished twice a week and at 80% confluence the cells were treated with IL-1β. Prior to treatment, cells were washed with HBSS and then incubated for different periods (30, 60, 90, 120, 150 or 180 min) in growth medium to which IL-1β was added at different concentrations (5, 7.5, 10, 15 or 20 ng/m), to determine the optimal concentration and incubation period. Once determined, they were employed in all experimental treatments. The specificity of the response was investigated by studying the effect of heat-inactivated IL-1β (heated for 3 min to 120°C) on the pump expression. For this LLC-PK1 cells were incubated with the heated cytokine at the optimal concentration and time (15 ng/ml for 2 h).
When the cells were to be treated with PGE2, the latter was added to the incubation medium at 1 nM for 15, 30 or 45 min. Inhibitors or their vehicle were added, when their effect was to be studied, 15 min prior to the addition of IL-1β or PGE2. The MAPK inhibitors, PD98059 and SB202190 were added at a final concentration of 50 µM; indomethacin (dissolved in DMSO) and PDTC were added at 100 µM. Actinomycin D and cycloheximide were added at 3 and 5 µg/ml respectively. When activation of NFκB was to be studied, cells were incubated for 20 min with IL-1β or the inhibitors.
At the end of the incubation period, the cells were harvested, lysed, homogenized in a Polytron homogenizer at 4°C for 4 min and assayed for protein using the Bio-Rad assay and samples were used for Western blot analysis.
Na+-K+-ATPase assay
Cell homogenates were diluted in TRIS buffer (in mM): 200 NaCl; 5 MgCl2; 2 EGTA; 5 KCL; 200 TRIS-HCl, pH 7.4, to a final protein concentration of 5.2 mg/ml and incubated with saponin (0.02%) for 30 min at room temperature. Aliquots (50 µl) were then incubated at 37°C for 10 min in the presence or absence of ouabain (4 mM final concentration). The reaction was initiated by addition of ATP to a final concentration of 1.25 mM and terminated after a 1-h incubation at 37°C by addition of trichloroacetic acid (200 µl, 11%). The samples were centrifuged at 3,000 g for 5 min and the amount of inorganic phosphate (Pi) liberated in the supernatant measured colourimetrically according to [28]. The activity of the enzyme was determined as the ouabain-inhibitable Pi liberated and the percentage recovery was calculated as follows:
Western blot analysis
Protein expression of Na+-K+-ATPase was determined by Western blot analysis. Membrane proteins (30 µg) were loaded equally and resolved on 8% polyacrylamide gels using the Mini-Protean 3 system (Bio-Rad). Samples were warmed to 37°C for 30–45 min before loading and then transferred to a PVDF membrane using a Bio-Rad Mini-Trans-blot cell. The PVDF membrane was washed, blocked and incubated with a rabbit polyclonal primary anti-Na+/K+-ATPase α-1 IgG and with a goat anti-rabbit HRP conjugated secondary antibody. Signals were detected using enhanced chemiluminescence with luminol reagent.
The state of activation of NF-kB was determined by monitoring changes in the protein expression of the inhibitory IκB protein by Western blot analysis, and was conducted as above except that the membrane was incubated with an IKB-α antibody added at 1 µg/ml and the secondary antibody used was an HRP-conjugated goat anti-rabbit IgG.
The expression of β actin was used to check for equal loading. Autoradiographs were quantified densitometrically using appropriate software (Gel-Pro Analyzer v. 2, Media Cybernetics, Silver Spring, Md., USA). Signal intensities were normalized with respect to that of β-actin and results reported as percentages of the control value.
Statistical analysis
The significance of differences between means was established by a one-way ANOVA followed by a Tukey-Kramer multiple comparisons test.
Results
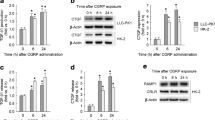
IL-1β caused a concentration and time-dependent decrease in the activity and protein expression of Na+-K+-ATPase in LLC-PK1 cells (Figs. 1, 2). The maximal effect was observed at 2 h and 10 ng/ml. Heat-inactivated IL-1β did not have any effect on the pump when added at the optimal concentration and for the optimal period.
a Effect of different concentrations of interleukin-1β (IL-1β) on protein expression of the α1-subunit of the Na+-K+-ATPase in LLC-PK1 cells. Cells were incubated for 2 h. Results are representative of an experiment repeated 3 times. b Effect of different concentrations of IL-1β on the activity and protein expression of the Na+-K+-ATPase. Means±SEM. Protein expression was quantified using Gel-Pro Analyzer (v. 2) software and is shown as a percentage of the signal obtained with the control. The letters identify the columns for purposes of comparison: P<0.05: c vs. e; P<0.01: a vs. b, b vs. e; P<0.001: a vs. c, a vs. d, a vs. e, a vs. f, a vs. g. Activity was assayed by measuring the ouabain-inhibitable release of inorganic phosphate: P<0.05: b′ vs. e′; P<0.01: a′ vs. b′; P<0.001: a′ vs. c′, a′ vs. d′, a′ vs. e′, a′ vs. f′, a′ vs. g′
a Time course effect of the effect of IL-1β on the protein expression of the α1-subunit of Na+-K+-ATPase. Cells were incubated for different periods with IL-1β (10 ng/ml). Results are representative of an experiment repeated 3 times. b Effect of duration of incubation with IL-1β on the activity and protein expression of Na+-K+-ATPase. Means±SEM; quantification details as in Fig. 1. P<0.05: b vs. c, b vs. d, b vs. g, c vs. e, d vs. e; P<0.01: e vs. g; P<0.001: a vs. b, a vs. c, a vs. d, a vs. e, a vs. f, a vs. g. Activity is reported as the percentage recovery of the enzyme activity. P<0.05: a′ vs. b′, b′ vs. e′; P<0.01: a′ vs. c′, a′ vs. d′, a′ vs. g′; P<0.001: a′ vs. e′, a′ vs. f′
Involvement of MAPK
To study the involvement of the MAPK cascades in the cytokine effect on the pump, cells were incubated in the simultaneous presence of IL-1β and PD98059 or SB202190, inhibitors of mitogen and extracellular signal regulated protein kinase kinase/extracellular signal regulated protein kinase (MEK/ERK) and p38 MAPK respectively (Fig. 3). The effect of the cytokine was still apparent in the presence of the p38 MAPK inhibitor, but not when MEK was inhibited.
a Effect of PD 98058 (50 µM) and SB 202190 (50 µM) on protein expression of the α1-subunit of the Na+-K+-ATPase. The inhibitors were added 15 min before the addition of IL-1β after which the cells were incubated for 120 min. Results are representative of an experiment repeated 3 times. b Quantification of the results, quantification details as in Fig. 1. P<0.001: a vs. b, a vs. f, b vs. c, b vs. d, b vs. e, c vs. f, d vs. f, e vs. f
Involvement of NF-κB
In this study, NFκB activation was studied by monitoring changes in the protein expression of IκBα, which is degraded upon activation of the transcription factor. IL-1β (10 ng/ml) activated NF-κB maximally by 20 min and the activation decreased thereafter (Fig. 4) until it returned to basal levels. Incubation of LLC-PK1 cells for 2 h with PDTC, an inhibitor of NF-κB, down-regulated the pump (Fig. 5) and in the simultaneous presence of PDTC and IL-1β, the protein expression of the ATPase was not different from that seen in the presence of PDTC or IL-1β alone.
a Time course effect of IL-1β (10 ng/ml) on the protein expression of the inhibitor of nuclear factor-κB (IκB) unit. Results are representative of an experiment repeated 3 times. b Changes in protein expression of IκB compared with control. Means±SEM, details as in Fig. 1. P<0.05: a vs. i; P<0.01: a vs. b, c vs. d, d vs. e, c vs. f, c vs. g, c vs. h; P<0.001: a vs. c
a Effect of pyrrolidinediethyldithiocarbamate (PDTC) (50 µM) on protein expression of the α1-subunit of the Na+-K+-ATPase in the presence or absence of IL-1β (10 ng/ml). Results are representative of an experiment repeated 3 times. b Quantification of the results. Means±SEM, details as in Fig. 1. P<0.01: a vs. b; P<0.001: a vs. c, a vs. d
Position of MEK/ERK relative to NF-κB in the signalling pathway
IL-1 β added in presence of PD98059, an inhibitor of the MEK/ERK pathway, did not cause any activation of NF-kB at 20 min (Fig. 6).
a Effect of mitogen-activated protein kinase (MAPK) inhibitor PD98059 (PD) on protein expression of the IκB subunit. PD (50 µM) was added to cells 15 min before the addition of IL-1β and cells were incubated thereafter for 20 min. Results are representative of an experiment repeated 3 times. b Quantification of the results; means±SEM; details as in Fig. 1. P<0.01: a vs. b, b vs. c, b vs. d
Effect of indomethacin
The IL-1β-induced down-regulation of the Na+-K+ pump and NF-κB activation were prevented by indomethacin, the classic, non-selective inhibitor of COX (COX-1 and -2) (Fig. 7).
a Effect of indomethacin (100 µM) on the protein expression of the α1-subunit of the Na+-K+-ATPase in the presence and absence of IL-1β (10 ng/ml). Results are representative of an experiment repeated 3 times. b Quantification of results; means±SEM; details as in Fig. 1. P<0.001: a vs. d; P<0.01: b vs. d, c vs. d. c Effect of IL-1β (10 ng/ml) on the expression of IκB subunit in presence or absence of indomethacin (100 µM). Results are representative of an experiment repeated 3 times. d Quantification of results; means±SEM; details as in Fig. 1. P<0.01: a vs. b, b vs. d; P<0.001: b vs. c
Effect of PGE2
Exogenous PGE2 added to the cells reduced protein expression of the pump within 15 min (Fig. 8) and inactivated NF-κB (Fig. 9c, d). These responses were not influenced by PD98050, a MEK/ERK inhibitor (Fig. 9). PDTC, an inhibitor of NF-κB, decreased the protein expression of Na+-K+-ATPase, and a further decrease was elicited by the simultaneous presence of PGE2 (Fig. 10).
a Time course of the effect of 1 nM prostaglandin E2 (PGE 2 ) on the protein expression of the α1-subunit of the Na+-K+-ATPase. Results are representative of an experiment repeated 3 times. b Quantification of results; means±SEM; details as in Fig. 1. P<0.001: a vs. b, a vs. c, a vs. d, a vs. e
a Effect of 1 nM PGE2 on the protein expression of the α1-subunit of the Na+-K+-ATPase in presence of the MEK/ERK inhibitor PD98059 (50 µM). Results are representative of an experiment repeated 3 times. b Quantification of results; means±SEM; details as in Fig. 1. P<0.001: a vs. c, a vs. d. b vs. c; P<0.01: b vs. d. c Effect of 1 nM PGE2 on the activation of NF-κB in the presence of PD98059 (50 µM). Results are representative of an experiment repeated 3 times. d Quantification of results; means±SEM; details as in Fig. 1. P<0.001: a vs. c, a vs. d, b vs. c, b vs. d
a Effect of PGE2 (1 nM) in presence and absence of PDTC (100 µM) on protein expression of the α1-subunit of Na+-K+-ATPase. b Quantification of results; means±SEM; details as in Fig. 1. P<0.001: a vs. b, a vs. c, a vs. d
Effect of PGE2 in presence of inhibitors of translation or transcription
To determine whether PGE2 affects transcription or translation of the pump, cells were incubated with PGE2 in presence or absence of actinomycin D or cycloheximide, inhibitors of transcription and translation respectively. Either inhibitor, added alone, decreased the expression of the pump and further inhibition was observed in the simultaneous presence of PGE2, which was similar to that obtained with PGE2 alone (Fig. 11).
a Western blot showing the effect of PGE2 in presence or absence of actinomycin or cycloheximide on the protein expression of the α1 subunit of the Na+-K+-ATPase. b Quantification of results; means±SEM; details as in Fig. 1. P<0.01: a vs. b, a vs. c, a vs. d, a vs. e, a vs. f, b vs. d, b vs. e, b vs. f; P<0.05: c vs. d, c vs. e, c vs. f
Discussion
IL-1β caused in LLC-PK1 cells a concentration and time-dependent decrease in the activity and protein expression of Na+-K+-ATPase (Figs. 1, 2). This finding is consistent with similar findings in kidney cortical cells isolated from IL-1β-treated rats [16] and with the inhibitory effect on ATPase activity and ouabain binding in MDCK cells [7]. The inhibition in activity correlated closely with the decrease in the pump expression and can thus be ascribed to a reduction in the number of enzyme molecules.
IL-1β is known to signal through activation of the MAPK cascades [17, 23] that have as their terminal effectors the ERKs, JNKs and p38 MAPKs. IL-1β modulates the three types of MAPK in mammalian cells [23]. The kinases are activated by distinct upstream MAPK kinases (MEK, SEK and MKK3) that recognize and phosphorylate threonine and tyrosine residues within a tripeptide motif (Thr-X-Tyr) on MAPK, a process that is required for their activation.
To determine whether the effect of IL-1β on the Na+-K+-ATPase is mediated through the MAPK cascades, cells were incubated in the simultaneous presence of IL-1β and PD98059 or SB202190, inhibitors of MEK/ERK and p38 MAPK respectively (Fig. 3). Inhibition of MEK, but not p38 MAPK, abrogated the effect of IL-1β on the pump, suggesting that ERK, but not p38 MAPK, is involved in regulating pump expression. In contrast, p38 MAPK rather than ERK mediates the effect of Il-1β on the pump in Caco-2 cells [1]. It can be concluded that different MAPKs are involved in the signalling pathways of the cytokine in different cells.
Activated MAPKs are known to phosphorylate a variety of intracellular substrates including certain transcription factors like NF-κB [11]. NF-κB resides in its inactive form in the cytosol, complexed with inhibitory proteins known as IκBs [21]. Phosphorylation of IκB by IκB kinase leads to its dissociation from the complex and its subsequent degradation by proteasomes. The liberated NF-κB then translocates into the nucleus, where it activates the transcription of various genes. Leung et al. [18] have reported that IL-1β induces, within 5 min, IκB phosphorylation, degradation and nuclear translocation in numerous cell types [26]. Our results showed also that activation of NF-κB by IL-1β was apparent as early as 10 min, reached its peak at 20 min and decreased in magnitude thereafter (Fig. 4).
Activated NF-κB is known to increase in turn the expression of IκB, and newly synthesized IκB enhances the dissociation of NF-κB from the DNA, because the affinity of NF-κB for IκB is higher than its affinity for the κB sites on DNA, initiating thus a negative feedback loop [21]. This may be one reason for the transient activation of NF-κB observed at 20 min.
To determine whether such activation is necessary for basal expression of the pump or for the effect of the cytokine, LLC-PK1 cells were incubated for 2 h with PDTC, an inhibitor of NF-κB, in presence or absence of IL-1β (Fig. 5). The inhibitor alone reduced the protein expression of the ATPase indicating that the transcription factor NF-κB is involved in its basal expression. When IL-1β was added simultaneously with PDTC, no further decrease in the pump expression was observed beyond that seen with PDTC or IL-1β alone. This suggests that if IL-1β acts through NF-κB, its effect should be inhibitory, and because the transcription factor was already inhibited no further effect was observed. Since the activation of the transcription factor preceded the down-regulatory effect of the cytokine on the pump, it can be postulated that transient NF-κB activation is needed for the cytokine effect on the ATPase.
IL-1 β added in presence of PD98059, an inhibitor of the MEK/ERK pathway, did not activate NF-kB at 20 min (Fig. 6) implying that MAPKs are involved in the activation process and are situated upstream from the transcription factor in the signalling pathway. This finding is in line with the well-established activation of NF-κB by the MAPK cascades [24]. On the other hand, IL-1β induces, through activation of some MAPK, COX-2 expression and PGE2 production [12, 13], which in turn inhibits NFκB translocation to the nucleus [20]. In the present study, the IL-1β-induced down-regulation of the Na+-K+ pump was prevented by indomethacin, a COX inhibitor (Fig. 7). Since IL-1β enhances PGE2 production by inducing gene expression of COX-2 with little effect on COX-1 [10], PGE2 may be a potential mediator of the cytokine action on the ATPase. In support of this hypothesis, exogenous PGE2 added to the cells also down-regulated the pump within 15 min (Fig. 8) and inactivated NF-κB (Fig. 9c, d). Both responses were unaffected by PD98050, an inhibitor of MEK/ERK, inferring that the MAPK is upstream from PGE2 (Fig. 9).
Although PGE2 inhibited NF-κB, the transcription factor may still not necessarily be involved in the pathway leading to down-regulation of Na+-K+-ATPase. To clarify this point, the effect of PGE2 on the pump was studied in presence of PDTC, an inhibitor of NF-κB. PDTC alone reduced the expression of the pump, and a further reduction was observed in the simultaneous presence of PGE2 (Fig. 10), which could be due to an additional inhibition of NF-κB by the prostaglandin over and above that due to PDTC, or to the activation of another pathway not involving NF-κB.
It could be concluded that NF-κB is involved in the basal expression of the Na+-K+-ATPase and that IL-1β exerts its effect on the pump by activating the MEK/ERK cascade, which in turn activates NF-κB leading to an increase in the gene expression of COX-2 and consequently PGE2 release. The increase in PGE2 in turn inhibits NF-κB, leading to a decrease in the synthesis of new pump molecules. The basal level of PGE2 is relatively low and does not inhibit the basal activity of NF-κB. The time frame for the whole pathway is consistent with the proposed sequence of events. IL-1 β needed 120 min to exert its effect on the pump, whereas, PGE2 needed only 15 min to exert the same effect.
Transitory activation of NF-κB is thus needed to increase the level of PGE2, which is the key and end player in the cytokine action on the Na+-K+-ATPase. Since its effect appeared as early as 15 min, and because the turnover rate of the Na+-K+-ATPase molecules could not be as short as 15 min, there may be a further mechanism by which PGE2 decreases protein expression of the pump, in addition to the inhibitory effect on basal expression mediated through inhibition of NF-κB. This mechanism may include recruitment of pump molecules to cytosolic compartments and their degradation, in an endocytotic process that could be similar to that described by Chibalin et al. [5] to explain the dopamine-induced decrease in Na+-K+-ATPase activity in proximal tubule cells. Although this hypothesis is speculative and remains to be tested, it is supported by the data obtained with inhibitors of transcription and translation. Since the simultaneous addition of PGE2 and the inhibitors produced a further decrease in the pump protein expression over that obtained with the inhibitors alone (Fig. 11), it can be inferred that PGE2 has a mechanism of action that does not involve transcription or translation. On the other hand because the addition of actinomycin D or cycloheximide to PGE2 did not exert any further down-regulatory effect beyond that exerted by PGE2 alone, it could be speculated that translation and transcription were already inhibited by PGE2.
In conclusion, IL-1β activates NF-κB transiently, leading to PGE2 production. PGE2, at high levels, acts along two lines: on one hand it inhibits NF-κB and contributes to the termination of its activation, initiated by IL-1β. On the other hand it promotes endocytosis and degradation of Na+-K+-ATPase molecules through an as yet unknown mechanism. It should be noted however that the signal transduction pathway activated by the cytokine is not the same in all types of cells. In Caco-2 cells [1], IL-1β increases PGE2 production and exerts a similar effect on the pump, but via different mediators. In these cells, AP-1 contributes to the basal expression of the pump in addition to other still unidentified transcription factors, while NF-κB is not involved. PGE2 acts independently of NF-κB and AP-1 and promotes endocytosis and degradation. Whether the unidentified transcription factors are inhibited in parallel is unknown.
The signal transduction pathway of IL-1β on the Na+-K+-ATPase in LLC-PK1 is shown in Fig. 12.
References
Al-Sadi R, Kreydiyyeh SI (2003) Mediators of interleukin-1 beta action on the Na+K+-ATPase in Caco-2 cells. Eur Cytokine Netw 14:83–90
Barnes PJ (1997). Nuclear factor-kappa B. Int J Biochem Cell Biol 29:867–870
Beasley D, Dinarello CA, Cannon JG (1988) Interleukin-1 induces natriuresis in conscious rats: role of renal prostaglandins. Kidney Int l33:1050–1065
Caverzasio J, Rizzoli R, Dayer JM, Bonjour JP (1987) Interleukin-1 decreases renal Na+ reabsorption: possible mechanism of endotoxin-induced natriuresis. Am J Physiol 252:F943–F946
Chibalin AV, Katz AI, Berggre PO, Bertorello AM (1998) Receptor-mediated inhibition of renal Na+-K+-ATPase is associated with endocytosis of its alpha and beta-subunits. Am J Physiol 273:C1458–C1465
Chen G, Wood EG, Wang SH, Warner TD (1999) Expression of cyclooxygenase-2 in rat vascular smooth muscle cells is unrelated to nuclear factor-κB activation. Life Sci 64:1231–1242
Cohen-Luria R, Moran A, Rimon G (1994) Cyclooxygenase inhibitors suppress inhibitory effect of PGE2 on Na-K-ATPase in MDCK cells. Am J Physiol 267:F94–F98
Crambert G, Hasler U, Beggah AT, Yu C, Modyanov N, Horisberger JD, Lelievre L, Geering K (2000) Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J Biol Chem 275:1976–1986
Diaz A, Chepenik KP, Korn JH, Reginato AM, Jimenez SA (1998) Differential regulation of cyclooxygenases 1 and 2 by interlukin-1 beta, tumor necrosis factor-alpha, and transforming growth factor-beta 1 in human lung fibroblasts. Exp Cell Res 241:222–229
Dinarello CA (2000) Proinflammatory cytokines. Chest 118:503–508
Eder J (1997) Tumour necrosis factor alpha and interleukin 1 signalling: do MAPKK kinases connect it all? Trends Pharmacol Sci 18:319–322
Guan Z, Buckman SY, Miller BW, Springer LD, Morrison AR (1998) Interleukin-1 beta-induced cyclooxygnease-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem 273:28670–28676
Husted RF, Zhang C, Stokes JB (1998) Concerted actions of IL-1β inhibit Na+ absorption and stimulate anion secretion by IMCD cells. Am J Physiol 275:F946–F954
Jiang B, Brecher P, Cohen RA (2001) Persistent activation of nuclear factor-κB by interleukin-1 beta and subsequent inducible NO synthase expression requires extracellular signal-regulated kinase. Arterioscler Thromb Casc Biol 21:1915–1920
Kohan DE, Merli CA, Simon EE (1989) Micropuncture localization of the natriuretic effect of interleukin-1. Am J Physiol 256:F810–F813
Kreydiyyeh S, Al-Sadi R (2002) Interluekin-1β increases urine flow rate and inhibits protein expression of Na+/K+ ATPase in the rat jejunum and kidney. J Interferon Cytokine Res 22:1041–1048
Laporte JD, Moore PE, Lahiri T, Schwartzman IN, Panettieri RA Jr, Shore SA (2000) P38 MAP kinase regulates IL-1 beta responses in cultured airway smooth muscle cells. Am. J Physiol 279:L932–L941
Leung K, Betts JC, Xu L, Nabel GJ (1994) The cytoplasmic domain of the interleukin-1 receptor is required for nuclear factor-kappa B signal transduction. J. Biol Chem 269:1579–1582
Lingrel JB, Kuntzweiler T (1994) Na+-K+-ATPase. J Biol Chem 209:19659–19662
Poligone B, Baldwin AS (2001) Positive an negative regulation of NF-kappa B by COX-2: roles of different prostaglandins. J Biol Chem 42:38658–38664
Rothwarf DM, Karin M (1999) The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE 26:RE1
Sakairi Y, Ando Y, Tabei K, Kusano E, Asano Y (1994) Interleukin-1 inhibits Na+ and water transport in rabbit cortical collecting duct. Am J Physiol 266:F674–F680
Saklatvala J, Dean J, Finch A (1999) Protein kinase cascades in intracellular signaling by interleukin-I and tumour necrosis factor. Biochem Soc Symp 64:63–77
Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S (1997) Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 198:35–49
Skou JC, Esmann M (1992). The Na,K-ATPase. J Bioenerg Biomembr 24:249–261
Stylianou E, O’Neill LA, Rawlinson L, Edbrooke MR, Woo P, Saklatvala J (1992) Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J. Biol Chem 267:15836–15841
Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182
Taussky HH, Shorr E (1953) Microcolorimetric method for determination of inorganic phosphorous. J Biol Chem 202:675–685
Vane JR, Bakhle YS, Botting RM (1998) Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120
Zeidel ML, Brady HR, Kohan DE (1991) Interleukin-1 inhibition of Na+-K+-ATPase in inner medullary collecting duct cells: role of PGE2. Am J Physiol 261:F1013–F1016
Acknowledgements
This work was supported from a grant from the University Research Board and the National Lebanese Council for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kreydiyyeh, S.I., Al-Sadi, R. The signal transduction pathway that mediates the effect of interleukin-1 beta on the Na+-K+-ATPase in LLC-PK1 cells. Pflugers Arch - Eur J Physiol 448, 231–238 (2004). https://doi.org/10.1007/s00424-004-1242-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-004-1242-0