Abstract
Background
Different methods have been described for laparoscopic hiatoplasty and hiatus hernia (HH) repair. All techniques are not standardized and the choice to reinforce or not the hiatus with a mesh is left to the operating surgeon’s preference. Hiatal surface area (HSA) has been described as an attempt at standardization; in case the area is > 4 cm2, a mesh is used to reinforce the repair.
Objective
The aim of this study was to describe a new patient-tailored algorithm (PTA), compare its performance in predicting crura mesh buttressing to HSA, and analyze outcomes.
Methods
Retrospective, single-center, descriptive study (September 2018–September 2021). Adult patients (≥ 18 years old) who underwent laparoscopic HH repair. Outcomes and quality of life measured with the disease-specific gastroesophageal reflux disease health-related quality of life (GERD-HRQL) and reflux symptom index (RSI) were analyzed.
Results
Fifty patients that underwent laparoscopic hiatoplasty and Toupet fundoplication were included. The median age was 61 years (range 32–83) and the median BMI was 26.7 (range 17–36). According to the PTA, 27 patients (54%) underwent simple suture repair while crural mesh buttressing with Phasix-ST® was used in 23 (46%). According to the HSA, the median hiatus area was 4.7 cm2 while 26 patients had an HSA greater than 4 cm2. The overall concordance rate between PTA and HSA was 94% (47/50). The median hospital stay was 1.9 days (range 1–8) and the 90-day complication rate was 4%. The median follow-up was 18.6 months (range 1–35). Hernia recurrence was diagnosed in 6%. Postoperative dysphagia occurred in one patient (2%). The GERD-HRQL (p < 0.001) and RSI (p = 0.001) were significantly improved.
Conclusions
The application of PTA for cruroplasty standardization in the setting of HH repair seems effective. While concordance with HSA is high, the PTA seems easier and promptly available in the operative theater with a potential increase in procedure standardization, reproducibility, and teaching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hiatal hernia (HH) occurs when contents of the abdominal cavity protrude through the esophageal hiatus of the diaphragm. The stomach is generally the principal viscera which migrates in the posterior mediastinum and thorax. The first attempt to treat this condition was described in 1919 by Soresi et al. [1]. In his work, Soresi affirmed the importance of anatomic diaphragmatic hiatus repair without tension. This concept was lately supported by Harrington et al. in 1928 [2, 3] that emphasized closure of the hiatus as essential for symptoms’ relief. While open crural repair has been the gold standard treatment since the beginning of the twentieth century, it has been progressively overcome by laparoscopic repair first described by Dallemagne et al. in 1991 [4].
Reconstruction of the diaphragmatic crura mandates careful evaluation of this complex anatomical region. Different surgical techniques have been described ranging from simple suture, pledge reinforcement, or mesh buttressing. All these techniques are not standardized, left to operating surgeon preference and “feeling” of a weak crura. This introduces a great heterogeneity because of different surgeon capabilities and expertise. A preliminary attempt at standardization was proposed by Granderath et al. in 2007 that described the hiatal surface area (HSA) [5]. Specifically, the area of the esophageal hiatus, that is compared to the area of an inverted triangle and semicircle, is calculated with a trigonometric formula. In case the HSA is greater than 4 cm2, crural mesh buttressing is advocated. This methodology foresees a laborious mathematical calculation while its utilization in the operative room setting may be laborious and not promptly available. Therefore, we previously proposed an easier “patient-tailored” algorithm (PTA) based on four different measurable parameters during laparoscopic repair in an attempt to standardize the hiatus repair [6].
Hence, the aim of this study was to describe the PTA approach, compare its performance in predicting crural mesh buttressing to the HSA, and analyze postoperative outcomes in a consecutive series of patients operated for symptomatic HH.
Materials and methods
In a single-center, retrospective, descriptive study from September 2018 to September 2021, we included all patients who underwent elective laparoscopic crural repair for symptomatic HH. Indications for surgery were in agreement with the latest SAGES Guidelines [7, 8]. All procedures were performed by two surgeons (A. A. and D. B.). The institutional review board (IRB) approved the study (HR#164-2021) and all patients signed a written informed consent. Inclusion criteria were (a) adult patients (≥ 18 years old) referred to our institution and operated for symptomatic HH and (b) elective laparoscopic cruroplasty with Toupet fundoplication. Exclusion criteria were (a) previous resective gastric operations and (b) patients unfit for surgery.
Baseline demographics and patient characteristics such as age, sex, body mass index (BMI) (kg/m2), comorbidities, type of hiatal hernia, operative data, and short-term surgical outcomes (90-day morbidity and mortality) were collected. Preoperative evaluation included esophagogastroduodenoscopy, swallow study, high-resolution esophageal manometry, and 24-h pH-impedance monitoring. Perioperative complications were defined in accordance with the modified Clavien-Demartines-Dindo classification [9].
Postoperative follow-up was standardized and outpatient visits were scheduled at 1, 6, and 12 months after the operation, and then yearly. Endoscopic and radiological (upper gastrointestinal swallow study and computed tomography) findings were collected. Barium swallow study and/or upper gastrointestinal endoscopy were performed between 6 and 12 months after surgery and repeated every year or at any time the patient complained of symptoms. The overall alimentary satisfaction was reported on a 1–10 visual analog scale. Patients with a score > 8 were considered the most satisfied with the procedure. Two validated self-administered questionnaires were used to assess patients’ symptoms and quality of life preoperatively and during follow-up. The disease-specific gastroesophageal reflux disease health-related quality of life (GERD-HRQL) is a 10-item disease-specific tool focused on heartburn, dysphagia, and gas bloat [10, 11]. Each symptom has an assigned score between 0 and 5. A final score is assigned based on the summary of individual scores, so a complete asymptomatic patient has a score of 0, while the most symptomatic patient has a score of 50. A score below 10 is considered normal. The reflux symptom index (RSI) is a nine-item, 45-point survey for respiratory symptoms assessment. RSI score lower than 13 is normal, RSI between 13 and 19 is considered abnormal but not pathologic, and RSI ≥ 19 is pathologic Improvement is defined as a decrease in RSI of > 5 points, with normalization being RSI < 13 and resolution defined as RSI > 13 and < 19 [12]. Hernia recurrence was defined as recurrent GERD symptoms with > 2 cm of gastric tissue above the diaphragmatic impression evidenced at follow-up upper endoscopy and/or swallow study [7, 8].
Outcomes
The primary outcome was to compare PTA performance with HSA in predicting crural mesh buttressing. Secondary outcomes were short-term (90-day) surgical complications, changes in esophageal symptoms, and patient-related quality of life assessed with GERD-HRQL and RSI.
Patient-tailored algorithm (PTA) and surgical technique
The PTA is based on four parameters [6]:
-
1.
Laparoscopic HH classification (Table 1). The type of hernia, based on the percentage of the herniated gastric body, is stratified into type I–II (1 point), type IIIa (2 points), and type IIIb–IV (3 points).
-
2.
Hiatus diastasis. After mediastinal esophageal dissection, the opening diameter of the hiatus is measured with a laparoscopic ruler just below the esophagus and classified as < 2 cm (1 point), ≥ 2 and < 4 cm (2 points), and ≥ 4 cm (3 points).
-
3.
Tropism. Pillars are categorized on the basis of the transverse length measured with a laparoscopic ruler. Pillars < 5 mm are defined hypoplasic (3 points) while pillars ≥ 5 mm are defined as normal (1 point).
-
4.
Presence of HH recurrence (2 points).
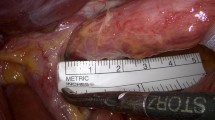
All patients underwent surgery according to a standardized procedure. Once the trocars are placed in the standard position for EGJ procedures, HH classification is assigned (Fig. 1). The gastrohepatic ligament is opened and the anterior aspect of the esophagogastric junction and the angle of His are exposed. Dissection is done circumferentially around the esophagus, thus creating a retroesophageal window to accommodate the fundoplication. The mediastinal dissection and esophageal downward mobilization for at least 3 cm are completed (Fig. 2). If present, the hernia sac and the lipoma are dissected. The wider opening diameter of the crura just below the esophagus (Fig. 3), the transverse diameter of the left/right pillar (Fig. 4), and the length of the right pillar are measured with a laparoscopic ruler (Fig. 5). PTA and HSA were calculated and compared in all patients. If PTA is ≤ 5, the crural reconstruction is performed with interrupted non-resorbable simple sutures (Prolene® 2.0-Ethicon). If PTA is > 5, a biosynthetic absorbable mesh (Phasix ST®-Poly-4-hydroxybutyrate (P4HB)-Bard) 10 × 7 cm U-shaped mesh was used for crural buttressing. The mesh is placed over the hiatoplasty and fixed with two resorbable sutures (Vycril® 2.0-Ethicon). Toupet fundoplication was fashioned according to the previously described “critical view” concept [13, 14]. Briefly, after fundic mobilization, in order to stabilize the wrap and reduce further manipulations of the gastric fundus, four “cardinal” non-absorbable stitches (Prolene® 2.0-Ethicon) are placed. Most cephalad include on each side the esophagus, the gastric fundus, and the crus. The other two sutures are placed 4 cm distally including the esophagus and the gastric wall. This clue, with respect of these key anatomical landmarks, allows us to assess the geometry of the fundoplication and ensures the lack of esophageal twisting (Supplementary Figure 1). A running barbed absorbable suture (3-0 polybutester/V-Loc, TM ®) is used to further secure the gastric wall to each side of the esophagus taking care not to include the anterior vagus nerve.
Statistical analysis
Continuous data are reported as median and interquartile range (IQR). Categorical data are reported using frequencies and proportions. Wilcoxon signed rank for paired data was performed as appropriate. Two-sided p values were computed. Statistical significance was considered when p value was equal or less than 0.05. All analyses and figures were carried out using R version 3.2.2 software [15].
Results
During the study period, 148 patients were operated for symptomatic HH through a laparoscopic approach according to the described PTA. Overall, 50 patients (33.7%) completed the intraoperative PTA vs. HSA assessment and were included in the final population to compare PTA vs. HSA performance in predicting crural mesh buttressing. Demographics of the patient population are shown in Table 2. The median age was 62 years (range 32–83) and the median BMI was 26.7 (range 16.9–36.7). Hypertension (52%), smoke (24%), and kyphosis (14%) were the most reported comorbidities. Heartburn (64%), regurgitation (26%), and chest pain (24%) were the most common typical esophageal symptoms while 52% of patients complained atypical symptoms. The median preoperative GERD-HRQL was 23 (range 0–44) while the median RSI was 13 (range 0–35). Esophagitis was diagnosed in 11 patients (22%; Los Angeles A 16%; Los Angeles B 4%; Los Angeles C 2%) while Barrett’s esophagus was present in 6 patients (12%). Ineffective esophageal motility was documented in 9 patients (18%). Overall, 86% of patients were taking chronic PPI therapy.
According to the intraoperative HH classification, type IIIA hernia was present in 46% of patients, type I–II in 32%, and type IIIB–IV in 22%. Overall, 16 patients (32%) were measured with hiatus diastasis < 2 cm, 32 patients (64%) had 2–4 cm of diastasis while 2 patients (4%) had > 4-cm diastasis. Pillars were hypoplastic (< 5 mm) in 10 cases (20%). Six patients (12%) underwent redo surgery for hernia and symptoms recurrence. The median operative time to complete all measurements and PTA was 4 minutes (range 2–5). According to the PTA, 27 patients (54%) underwent simple suture repair (score ≤ 5) while Phasix-ST® mesh was used in 23 (46%). The median HSA was 4.7 cm2 while 26 patients had a HSA greater than 4 cm2. Therefore, PTA and HSA gave the same indication in 47/50 patients with an overall 94% concordance rate (Table 3). Six patients were operated for symptomatic hernia recurrence; in these patients, PTA and HSA gave the same indication in 5/6 with an overall concordance rate of 83.3%.
In the majority of patients (66%), posterior cruroplasty was performed with 3 interrupted sutures while an additional anterior hiatoplasty was required in 4 patients (8%). The median operative time was 122 min (range 54–210). No intraoperative complications occurred and there were no conversions to open procedures. The mean postoperative hospital stay was 1.9 days (range 1–8). The 90-day postoperative complication rate was 4% (n = 2). The distribution of complications according to the modified Clavien classification was grade II (pleural effusion; n = 1) and grade IIIb (hydropneumothorax and pleural empyema; n = 1). There was no mortality (Clavien V).
The median follow-up was 18.6 months (range 1–35), with 41 patients (72%) having a minimum follow-up of 6 months. Hernia recurrence was diagnosed in three patients (6%) requiring redo surgery in one case. Overall, 92% of patients were off PPI with 8% taking daily or occasional PPI for residual symptoms. Postoperative occasional dysphagia was diagnosed in one patient (2%). Gas bloat syndrome was recorded in 18% of the patients (n = 9). At the last follow-up, the median GERD-HRQL (3, range 0–6; p < 0.001) and RSI (1.8, range 0–3; p = 0.001) were significantly improved compared to baseline.
Discussion
The application of a patient-tailored and standardized approach for hiatoplasty with PTA seems feasible, safe, and effective. Compared to HSA, it is easier and promptly available in operative room settings with a high concordance rate. PTA may be useful to increase procedure reproducibility, facilitate teaching, and standardize surgical outcomes.
Laparoscopic posterior cruroplasty represent a cardinal step for HH repair. Complete hernia sac excision, distal esophageal dissection, crural repair, and fashioning of a fundoplication are the mainstay of surgical repair. Adequate esophageal mobilization to obtain at least 3 cm of intra-abdominal esophagus without tension below the diaphragm allows to minimize axial tension. Similarly, adequate crural repair should be performed to minimize radial tension. This because both respiratory (breathing, sneezing, and coughing) and non-respiratory (vomiting, straining at stools, and laughing) movements exerts repetitive stress upon the crural repair, which if closed under tension may lead to repair disruption. The decision on how to approximate the crura is challenging because lack of standardization. Up to date, the decision on how repair the crura and if a mesh buttressing is needed is left to surgeons’ preference and “feeling” of a weak crura. This is source of heterogeneity among surgeons and poses an important problem in training surgeons in the first phase of the learning curve [16, 17]. The debate regarding the best way to close the crura is still ongoing [18–23]. A 2006 randomized controlled trial compared primary repair vs. primary repair reinforced with biological mesh in the setting of paraesophageal hernias. The authors found a statistically significant reduced 6-month recurrence rate for mesh repair (9% vs. 24%, p = 0.04) with no statistically significant differences in the long term (5 years). Previous systematic reviews and meta-analyses including both observational and randomized trials showed a statistically significant reduced recurrence rate for laparoscopic mesh-reinforced crura repair [22, 23]. By contrast, two recently published meta-analyses of randomized trials did not find any significant differences in term of hernia recurrence in the short- and medium-term follow-up comparing simple suture vs. mesh-augmented repair [24, 25]. However, some limitations and heterogeneity for primary outcomes limit the validity and robustness of such analyses. First, recurrence was not uniquely defined in all RCTs. Second, there is a relevant heterogeneity in the inclusion criteria between studies. Third, different surgeon experience, mesh type, location, shape, and crural fixation methods contributed to the heterogeneity. Finally, the mean follow-up is limited (up to 42 months). Therefore, because of the significant heterogeneity, a definitive evidence-based indication is far away to be drawn.
In an attempt to reduce heterogeneity related to technical variability and different surgeons’ expertise/preference, previous studies tried to propose a standardized methodology for crural repair. Fourtanier et al. described a disposable device (HiaTech®; Laboratoire Surgical IOC Saint Etienne, France) for hiatal orifice calibration and closure [26]. Granderath et al. proposed the use of the HSA to define the size of the hiatal defect [5, 17]. The rationale stands in the fact that as bigger is the hiatus area, greater is the difficulty of pillars approximation because tension with an increased risk for recurrence. Therefore, for large hernia (> 4 cm2), crural mesh buttressing was adopted to reinforce the hiatus and reduced radial tension. After intraoperative measurement of hiatus pillars length and diastasis with an endoscopic ruler, the HSA is calculated with a complex trigonometric formula. The angle of the crural commissure is calculated using an inverse trigonometric function.
This attempt at standardization has been reported to be safe and effective up to 28 months of follow-up [27, 28]. Notably, despite the HSA introducing an attempt at crural repair standardization based on laparoscopic measurement, it is a challenging trigonometric formula that is difficult to use in the operative room settings. Moreover, the HSA does not foresee the possibility of hernia recurrence in the calculation despite it having been indicated as an independent risk for relapse.
In an attempt to overcome the challenging use of HSA, the PTA has been previously introduced in our surgical practice [6]. Four parameters are considered based on previous studies reporting conceivable risk factors for recurrence (Table 1). First, large hernias and recurrence have been shown to be associated with an increased risk for relapse [29, 30]. Therefore, type IIIA and type IIIB-IV were given higher scores. Second, as reported by Granderath et al., the increasing diastasis of esophageal pillars poses the repair at higher radial tension with an increased risk for disruption [5, 17]. That’s the reason why diastasis between 2 and 4 cm was given 2 points while diastasis greater than 4 cm was given 3 points. Finally, pillar hypotropism has been shown to be an important component being associated with microscopic and ultrastructural modification of crural muscular fibers [31, 32]. Therefore, in the presence of pillar hypotropism, the patient was given 3 points while normal pillars gave 1 point. Toupet fundoplication was fashioned in all patients. In our center, we decided to perform Toupet fundoplication over Nissen fundoplication, regardless of esophageal motility pattern, in accordance with recently published studies that showed comparable results in terms of reflux control, quality of life, and a lower rate of dysphagia and gas bloat syndrome [33, 34]. The valve is fashioned following the “critical-view” concept by using four cardinal stitches to anchor the valve to the crura and esophagus. This visual cue allows us to respect the valve geometry thus avoiding esophageal twisting.
The laparoscopic HH repair according to the standardized PTA seems feasible and safe. Its utilization in the operative room setting is rapid and easily available as it does not require challenging trigonometric calculations. Notably, the operative time to complete the laparoscopic measurements and algorithm was 4 min. Therefore, the utilization of PTA in the operative room setting requires an additional reasonable operative time and does not affect the overall procedure time. Interestingly, the concordance rate between PTA and HSA is high with a tendency of being less prone to crural mesh buttressing. The postoperative overall morbidity and recurrence rates are 4% and 6%, respectively. These percentages are similar to previous studies reporting data for laparoscopic HH repair [35–38]. Furthermore, functional outcomes, postoperative dysphagia, patient satisfaction, and quality of life improvement seem comparable to previous reports [18, 39, 40]. Therefore, the PTA may represent a promising tool for technique standardization, reproducibility, and teaching to a young surgeon in the first phase of the learning curve with a low morbidity rate and satisfactory medium-term recurrence rate.
Limitations of the present study are related to the study design while possible preoperative selection bias could not be excluded. Furthermore, the limited patient cohort, follow-up, mesh/fundoplication type, and lack of comparison with non-standardized technique are supplementary limitations. Finally, as the study was conducted at a tertiary level, a referral center for surgical GERD treatment, results may not be generalizable. Therefore, data should be interpreted cautiously while future investigations are warranted.
Conclusions
The application of a “patient-tailored” and standardized approach for cruroplasty in the setting of laparoscopic HH repair seems effective. While concordance with HSA is high, the PTA seems easier to use and promptly available in the operative room settings with a potential increase in procedure standardization, reproducibility, and teaching.
References
Soresi AL (1919) Diaphragmatic hernia: its unsuspected frequency, its diagnosis technic for radical cure. Annals of Surgery. 69(3):254–270
Harrington S (1928) Diaphragmatic hernia. Arch Surg. 16:386–415
Stylopoulos N, Rattner DW (2005) The history of hiatal hernia surgery: from Bowditch to laparoscopy. Annals of Surgery. 241(1):185–193
Dallemagne B, Weerts JM, Jehaes C, Markiewicz S, Lombard R (1991) Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. 1(3):138–143
Granderath FA, Schweiger UM, Pointner R (2007) Laparoscopic antireflux surgery: tailoring the hiatal closure to the size of hiatal surface area. Surg Endosc 21(4):542–548
Aiolfi A, Cavalli M, Saino G, Sozzi A, Bonitta G, Micheletto G, et al. Laparoscopic posterior cruroplasty: a patient tailored approach. Hernia [Internet]. 2020 Apr 25 [cited 2022 Feb 4]; Available from: https://doi.org/10.1007/s10029-020-02188-5
Peters JH (2013) SAGES guidelines for the management of hiatal hernia. Surg Endosc. 27(12):4407–4408
for the SAGES Guidelines Committee, Kohn GP, Price RR, SR DM, Zehetner J, Muensterer OJ et al (2013) Guidelines for the management of hiatal hernia. Surg Endosc 27(12):4409–4428
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 240(2):205–213
Velanovich V (1998) Comparison of generic (SF-36) vs. disease-specific (GERD-HRQL) quality-of-life scales for gastroesophageal reflux disease. Journal of Gastrointestinal Surgery 2(2):141–145
Porta A, Aiolfi A, Musolino C, Antonini I, Zappa MA (2017) Prospective comparison and quality of life for single-incision and conventional laparoscopic sleeve gastrectomy in a series of morbidly obese patients. Obes Surg 27(3):681–687. https://doi.org/10.1007/s11695-016-2338-2
Belafsky PC, Postma GN, Koufman JA (2002) Validity and reliability of the Reflux Symptom Index (RSI). Journal of Voice 16(2):274–277
Bona D, Aiolfi A, Asti E, Bonavina L (2020 Jun) Laparoscopic Toupet fundoplication for gastroesophageal reflux disease and hiatus hernia: proposal for standardization using the “critical view” concept. Updates Surg 72(2):555–558
Aiolfi A, Cavalli M, Sozzi A, Lombardo F, Lanzaro A, Panizzo V, et al. Medium-term safety and efficacy profile of paraesophageal hernia repair with Phasix-ST® mesh: a single-institution experience. Hernia [Internet]. 2021 30 [cited 2022 Feb 4]; Available from: https://doi.org/10.1007/s10029-021-02528-z
Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reardon PR (2006) A modest proposal. Surg Endosc 20(6):995–995
Granderath FA (2007) Measurement of the esophageal hiatus by calculation of the hiatal surface area (HSA). Why, when and how? Surg Endosc 21(12):2224–2225
Siboni S, Asti E, Milito P, Bonitta G, Sironi A, Aiolfi A et al (2019) Impact of laparoscopic repair of large hiatus hernia on quality of life: observational cohort study. Dig Surg 36(5):402–408
Wade A, Dugan A, Plymale MA, Hoskins J, Zachem A, Roth JS (2016) Hiatal hernia cruroplasty with a running barbed suture compared to interrupted suture repair. Am Surg 82(9):e271–e274
Powell BS, Wandrey D, Voeller GR (2013) A technique for placement of a bioabsorbable prosthesis with fibrin glue fixation for reinforcement of the crural closure during hiatal hernia repair. Hernia. 17(1):81–84
Granderath FA (2003) Laparoscopic refundoplication with prosthetic hiatal closure for recurrent hiatal hernia after primary failed antireflux surgery. Arch Surg 1;138(8):902
Memon MA, Memon B, Yunus RM, Khan S (2016 Feb) Suture cruroplasty versus prosthetic hiatal herniorrhaphy for large hiatal hernia: a meta-analysis and systematic review of randomized controlled trials. Annals of Surgery 263(2):258–266
Memon MA, Siddaiah-Subramanya M, Yunus RM, Memon B, Khan S (2019) Suture cruroplasty versus mesh hiatal herniorrhaphy for large hiatal hernias (HHs): an updated meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 29(4):221–232
Petric J, Bright T, Liu DS, Wee Yun M, Watson DI (2022) Sutured versus mesh-augmented hiatus hernia repair: a systematic review and meta-analysis of randomized controlled trials. Annals of Surgery 275(1):e45–e51
Angeramo CA, Schlottmann F (2022) Laparoscopic paraesophageal hernia repair: to mesh or not to mesh. Systematic review and meta-analysis. Annals of Surgery 275(1):67–72
Fourtanier G (2007) A new method to calibrate the hiatus. Surg Endosc 21(9):1674–1675
Grubnik VV, Malynovskyy AV (2013) Laparoscopic repair of hiatal hernias: new classification supported by long-term results. Surg Endosc 27(11):4337–4346
Koch OO, Asche KU, Berger J, Weber E, Granderath FA, Pointner R (2011) Influence of the size of the hiatus on the rate of reherniation after laparoscopic fundoplication and refundopilication with mesh hiatoplasty. Surg Endosc 25(4):1024–1030
Endzinas Z, Jonciauskiene J, Mickevicius A, Kiudelis M (2007) Hiatal hernia recurrence after laparoscopic fundoplication. Medicina (Kaunas) 43(1):27–31
Little AG, Ferguson MK, Skinner DB (1986) Reoperation for failed antireflux operations. J Thorac Cardiovasc Surg 91(4):511–517
Fei L, del Genio G, Brusciano L, Esposito V, Cuttitta D, Pizza F et al (2007) Crura ultrastructural alterations in patients with hiatal hernia: a pilot study. Surg Endosc 21(6):907–911
Fei L, del Genio G, Rossetti G, Sampaolo S, Moccia F, Trapani V et al (2009) Hiatal hernia recurrence: surgical complication or disease? Electron Microscope Findings of the Diaphragmatic Pillars. J Gastrointest Surg 13(3):459–464
Håkanson BS, Lundell L, Bylund A, Thorell A (2019) Comparison of laparoscopic 270° posterior partial fundoplication vs total fundoplication for the treatment of gastroesophageal reflux disease: a randomized clinical trial. JAMA Surg 154(6):479
Broeders JAJL, Mauritz FA, Ahmed Ali U, Draaisma WA, Ruurda JP, Gooszen HG et al (2010) Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. British Journal of Surgery 97(9):1318–1330
Panici Tonucci T, Asti E, Sironi A, Ferrari D, Bonavina L (2020) Safety and Efficacy of Crura Augmentation with Phasix ST Mesh for Large Hiatal Hernia: 3-Year Single-Center Experience. J Laparoendosc Adv Surg Tech A 30(4):369–372. https://doi.org/10.1089/lap.2019.0726 Epub 2020 Jan 7
Niebisch S, Fleming FJ, Galey KM, Wilshire CL, Jones CE, Litle VR, Watson TJ, Peters JH (2012) Perioperative risk of laparoscopic fundoplication: safer than previously reported-analysis of the American College of Surgeons National Surgical Quality Improvement Program 2005 to 2009. J Am Coll Surg 215(1):61–68; discussion 68-9. https://doi.org/10.1016/j.jamcollsurg.2012.03.022
Bizekis C, Kent M, Luketich J (2006) Complications after surgery for gastroesophageal reflux disease. Thorac Surg Clin 16(1):99–108. https://doi.org/10.1016/j.thorsurg.2006.01.010
Asti E, Asti E, Lovece A, Bonavina L, Milito P, Sironi A, Bonitta G, Siboni S (2016) Laparoscopic management of large hiatus hernia: five-year cohort study and comparison of mesh-augmented versus standard crura repair. Surg Endosc 30(12):5404
Rodríguez-Luna MR, Pizzicannella M, Fiorillo C, Almuttawa A, Lapergola A, Mutter D, Marrescaux J, Dallemagne B, Perretta S (2021) Impact of surgical repair on type IV paraesophageal hernias (PEHs). Surg Endosc. https://doi.org/10.1007/s00464-021-08828-w
Patel NM, Puri A, Sounderajah V, Ferri L, Griffiths E, Low D, Maynard N, Mueller C, Pera M, van Berge Henegouwen MI, Watson DI, Zaninotto G, Hanna GB, Markar SR (2021) Para-Oesophageal hernia Symptom Tool (POST) Collaborative. Quality of life and symptom assessment in paraesophageal hernias: a systematic literature review of reporting standards. Dis Esophagus 12;34(7):doaa134
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in the studies involving human participates were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical standards
Appropriate Institutional Board Review approval was obtained for the study.
Human and animal rights
The procedures performed in this study are approved procedures for daily practice, and this descriptive retrospective study therefore does not include experiments on humans.
Informed consent
Patients were requested to sign the written informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Aiolfi, A., Sozzi, A., Cavalli, M. et al. Patient-tailored algorithm for laparoscopic cruroplasty standardization: comparison with hiatal surface area and medium-term outcomes. Langenbecks Arch Surg 407, 2537–2545 (2022). https://doi.org/10.1007/s00423-022-02556-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02556-y