Abstract
Purpose
A defunctioning stoma is essential in reducing symptomatic leakage after colorectal surgery, particularly after lower anterior resection. Subsequent stoma closure is associated with morbidity and rarely mortality. This study aimed to identify the risk factors associated with post-operative complications related to stoma closure.
Methods
This retrospective cohort included patients who have undergone elective stoma closure between 2015 and 2017. Patient demographics, pre-morbidities, use of systemic therapy, stoma characteristics, and post-operative complications were retrieved from electronic records. Univariate and multivariate analysis was carried out to identify risk factors of stoma closure related morbidity.
Results
Ninety patients were included with a median age of 65 years, of which 58 (64.4%) of them were male. Sixty-nine (76.7%) patients had loop colostomy, while the rest had loop ileostomy. Fifty-four (60%) patients received neoadjuvant or adjuvant therapy. The median time interval from stoma creation to closure was 15 months. Nineteen (21.1%) patients had post-operative complications. The two most commonly observed post-operative complications were wound complications (16.7%) and intra-abdominal collections (6.7%). Fifteen (16.7%) patients developed an incisional hernia. The median follow-up time was 29 months. There was no 30-day mortality in this cohort. In multivariate analysis, adjuvant chemotherapy was associated with a higher risk of wound complications (p = 0.027). Higher risk of incisional hernia was seen in patients with history of hypertension (p = 0.046), use of adjuvant chemotherapy (p = 0.042) and stoma-related complications before closure (p = 0.002). Male patients might be associated with a higher risk of incisional hernia.
Conclusion
Adjuvant chemotherapy is associated with a higher risk of post-operative complications, particularly with wound complications. Male patients, hypertension, adjuvant chemotherapy, and stoma-related complications are associated with a higher risk of incisional hernia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A defunctioning stoma is an essential procedure in lower rectal cancer surgery. It can reduce symptomatic anastomotic leakage [1]. Stoma closure is often performed for functional, cosmetic, and social concerns, not to mention stoma-related complications [2]. However, the procedure is not without morbidities and complications. The overall complication rate ranged from 18.2 to 45.9% [3,4,5]. A small cohort has reported a reoperation risk of 3.37%. [5]. Diabetes was found to be an independent risk factor [6, 7]. The type of defunctioning stoma may affect the outcome. Some studies have suggested that, when compared with loop ileostomy, loop colostomy closure was associated with a higher risk of complication [8] and wound infection [9, 10]. The optimal timing (early versus delayed) of closure is controversial and is not determined yet [11,12,13,14,15,16,17]. Potential adverse effect of concurrent chemotherapy on closure surgery is also a concern, though the evidence was not strong [13]. This study aims to identify the risk factors affecting the morbidity of stoma closure after colorectal cancer surgery in our local population.
Materials and methods
This is a single-center retrospective cohort. Adult patients (> 18 years old) who underwent loop stoma closure during the period between 1st January 2015 and 31st December 2017, regardless of being ileostomy or colostomy, were included. Patients were excluded if the stoma was created for benign diseases (such as diverticulitis) or in emergency settings (such as diverting stoma for malignant obstruction). Patient demographics, tumor characteristics, surgical procedural details, stoma characteristics, use of neoadjuvant or adjuvant therapy, and preoperative condition were collected from the electronic patient record system. The data collection and analysis were performed in December 2019.
“Wound complication” was defined as pus discharge from the wound, prolonged wound dressing, or need of delayed/secondary suture. “Collection” was defined as any sizable collection which could be appreciated in computed tomography (CT) images. “Anastomotic leakage” was defined as free gas or disruption of anastomosis in CT images. “Anastomotic stricture” was defined as stenosis in which a normal 13.2-mm diameter colonoscope could not pass through or obstruction at anastomotic site evidenced by CT images. “Ileus” was defined as post-operative small bowel ileus requiring Ryle’s tube decompression. “Reoperation” was defined as any abdominal operation under general anesthesia, within 30 days after closure of stoma. “Incisional hernia” was defined as clinically detectable cough impulse over the closure site upon follow-up.
We have a standardized post-operative surveillance protocol for rectal cancer patients. After the index cancer surgery, we arrange clinical follow-up every 3 months up to 2 years. Afterwards, the follow-up interval changes to every 6 months until 5 years. The carcinoembryonic antigen (CEA) level is monitored. The pre-requisites of arranging closure of stoma are as follows. Firstly, patients have had their adjuvant chemotherapy completed. Secondly, they have had a contrast study done showing the absence of leakage or stricture at the anastomosis. Lastly, patients have a good general condition for operation under general anesthesia.
Usually, a surgical trainee, who is under the supervision of a specialist surgeon, performs the closure of stoma. An oval incision is made around the stoma followed by adhesiolysis and mobilization of the bowel. The stoma is either trimmed at the mucocutaneous junction or segmentally resected, subject to the surgeon’s preference. End to end anastomosis is performed in hand-sewn manner. A corrugated drain is routinely placed at the subcutaneous level before skin closure.
The statistical analysis was performed with SPSS 20.0. Categorical data are described using frequency, medians, and percentages. Continuous data are presented in medians and the range unless otherwise indicated. Logistic regression was used for univariate and multivariate analysis, p < 0.05 was considered as statistically significant. Univariate analysis of each potential risk factor was conducted. Significant factors in univariate analysis and important background demographics were included in the multivariate analysis.
Results
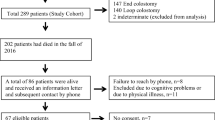
Ninety patients were included in the analysis (Fig. 1). Around one-fourth of the patients had loop ileostomy (n = 21), while the rest had loop colostomy (n = 69). Patient baseline demographics and premorbid conditions are presented in Table 1. Tumor characteristics and the type of index cancer surgery are shown in Table 2. The majority of patients had primary tumor located at the rectum (83%). There was an unusual case that required a covering ileostomy after a left hemicolectomy. That patient had a descending colon tumor with a colonic stent inserted as a bridge to elective surgery and was found to have edematous bowel-ends intra-operatively.
A total of 54 patients have received either neoadjuvant and/or adjuvant treatment (Table 3). Eleven of them had both neoadjuvant and adjuvant treatment. Out of the 28 patients who have received neoadjuvant therapy, 25 of them received neoadjuvant chemo-radiotherapy. A patient had neoadjuvant chemotherapy only for the treatment of liver metastasis. Two patients had neoadjuvant radiotherapy only because they were deemed unfit for chemotherapy. Thirty-seven patients received adjuvant treatment. The type of adjuvant treatment, the duration of treatment, and time interval from completion of adjuvant therapy to the closure of stoma are shown in Table 3.
Twenty-seven patients had stoma-related complications before the closure surgery (Table 4), including stoma prolapse (18.9%), parastomal hernia (10%), high output stoma (2.2%), and stoma stenosis (2.2%). Three patients had a parastomal hernia in addition to a prolapsed stoma. The median time interval from index cancer surgery to stoma closure was 15 months. The median operation time was 105 min and the median hospital stay was 8.5 days (Table 4).
Early post-operative complications occurred in nineteen (21.1%) patients (Table 5). Some of them suffered from more than one complication. Wound complications were common which occurred in 16.7% of our patients. Six patients developed intra-abdominal collections that required radiological drainage. There were two anastomotic leakages. One of them required reoperation and the other was treated with wound drainage and total parenteral nutrition. There was one anastomotic stricture that required endoscopic dilatation. Post-operative ileus and enterocutaneous fistula developed in two and one patients, respectively. The severity grading of these 19 patients according to Clavien-Dindo classification were listed in Table 5.
Three patients required early reoperations. Besides the anastomotic leakage mentioned above, another patient was reoperated for adhesive intestinal obstruction. The third patient had his corrugated drain slipped into the peritoneal cavity, which required wound exploration and drain retrieval on post-operative day 3. There were no mortality cases within 30 days of operation in this cohort.
The median follow-up time was 29 months. Three patients suffered from delayed rectal anastomotic leakage. These patients have had a contrast study confirming an intact anastomosis before closure. The leakages occurred 3 months, 8 months, and 2 years after surgery, respectively. They were likely related to previous pelvic irradiation rather than the closure surgery. In this cohort, fifteen patients (16.7%) developed an incisional hernia, of which two patients subsequently opted to undergo hernia repair. The incisional hernia was detected at 4 to 27 months after stoma closure surgery, with a median time interval of 10.5 months.
Univariate and multivariate analysis was performed to identify risk factors for different complications (Tables 6 and 7). Patient pre-morbidities were not associated with early post-operative complications. In the multivariate analysis, male sex (p = 0.044), history of hypertension (p = 0.046), use of adjuvant chemotherapy (p = 0.042), and stoma-related complications (p = 0.002) were risk factors of incisional hernia. Type of stoma (ileostomy versus colostomy) had no significant impact on overall post-operative complications (p = 0.078).
Patients receiving adjuvant chemotherapy before stoma closure had a statistically significant higher risk of wound complications (p = 0.027). We therefore performed a subgroup analysis by logistic regression on whether the duration of chemotherapy increases such risk. The result showed that the duration of chemotherapy (p = 0.934), the number of chemotherapy cycles (p = 0.601), and the time interval to stoma closure (p = 0.326) did not have a significant association with wound infections.
Discussion
Our study has confirmed that closure of loop stoma is not without risk. In our cohort, 21.1% of our patients had post-operative complications, which was comparable to previously reported studies [2,3,4,5]. We have identified that the use of adjuvant chemotherapy was an independent risk factor for wound complications. The use of adjuvant chemotherapy gave rise to a higher risk of incisional hernia as well. There are a few postulations on why adjuvant chemotherapy increases the risk of both short-term and long-term morbidity. First of all, the use of adjuvant chemotherapy may reduce patients’ general physique and healing power. Toxicity from chemotherapeutic agents can induce an immunocompromised state. Furthermore, the delay in the interval time to stoma closure after completion of adjuvant therapies may have a detrimental effect on the incidence of complications. Few studies have reported on a longer interval to closure increases the risk of early complication [11, 12, 14]. However, some reports have suggested the other way round [13, 16]. In our analysis, we could not demonstrate any relationship between the interval time to closure and the complication rate. Whether there is an optimal timing for closure remains inconclusive.
The occurrence of incisional hernia after stoma closure is not uncommon. With a median follow-up time of 29 months, the occurrence of incisional hernia was 16.7% in our cohort. Hypertension was found to be a risk factor for incisional hernia. This echoed the results of a few previous cohorts [18,19,20]. The underlying mechanism is not clear. Possible mechanisms include induction of endothelial dysfunction, aggravation of inflammation-induced hypoxia, inappropriate activation of inflammatory cytokines, and relation to metabolic syndrome. [21, 22]
Previous studies have learned that male patients were associated with a higher risk of developing incisional hernias. This can be due to a higher body mass index and higher intra-abdominal fat ratio in male [18]. In our study, male sex was not a significant factor in univariate analysis but was found to be a significant one in multivariate analysis. It is worth noting that the inclusion of sex in the multivariate analysis negatively affects the significance of adjuvant chemotherapy. This can be explained by the skewed distribution of our sample. Seventy-one percent of patients who had adjuvant chemotherapy were male. Future study with a normally distributed population is needed to clarify the effect of these two factors on the occurrence of incisional hernia.
In this study, we found that having stoma-related complications before the closure of stoma is associated with the development of incisional hernias. We have not come across any report on this association in the literature. It is not difficult to explain this phenomenon. First of all, the majority of stoma-related complications in our study were stoma prolapse and parastomal hernia, which share common predisposing factors with incisional hernia. These factors include weak fascial strength, large fascial defect, and obesity. Secondly, any of these complications increases technical difficulty during the procedure and the risk of early post-operative complications. Last but not least, stoma-related complications, especially high output stoma, may worsen patients’ nutritional status and hence reduce the ability of wound healing.
It is still controversial which type of loop stoma, an ileostomy or a colostomy, is a better option. Thus far, the results in the literature were conflicting and unconvincing. A colostomy is associated with a higher risk of complication rate, wound infection, and hernia [8,9,10, 23, 24]. On the other hand, an ileostomy was associated with a higher risk of dehydration, intestinal obstruction, and stoma prolapse [9, 25,26,27]. However, these associations were not observed in our cohort. Our ratio between ileostomy and colostomy was almost 1:3. Our surgeons have a preference for choosing loop colostomy over ileostomy. Such preference was influenced by the long waiting time for stoma closure in our public healthcare setting. The rationale behind this is that patients find it more convenient to handle colostomy content over ileostomy content. This ratio difference limits the ability to detect a statistically significant difference between these two groups.
Regarding the optimal time interval to closure, controversy still exists. A small retrospective cohort has advocated performing closure of loop ileostomy after at least 8.5 weeks to reduce morbidity [16]. Yet, some other studies have revealed an increased risk of complications if the time interval to ileostomy closure is beyond 6 months [11, 13, 15, 17]. In our study, the time interval to stoma closure had no significant effect on any of the post-operative complications. However, nearly all of our patients have to wait beyond 6 months due to the long waiting time in our public healthcare setting, prolonged adjuvant treatment, or anastomosis complication. Practice change and further study are needed to delineate whether earlier surgery within 6 months could lead to better results.
This study is limited by its retrospective nature. Quality of life (QOL) assessment after stoma closure was lacking. Functional disabilities such as gastrointestinal discomfort, alteration of bowel habit, or impaired psychological and social functioning after stoma closure have been reported [28, 29]. Future studies with QOL assessment, utilizing objective scoring systems such as SF-36, GLQI, and EuroQOL can give us better insight into this aspect.
This study was also limited by its small sample size and short follow-up interval. As the incidence of each complication was low, the statistical power of this cohort may not be adequate to detect a statistically significant result. Patients in this cohort have been followed up for 2–4 years. The incidence of incisional hernia was 16.7%, which is similar to the figure in the literature [7, 16]. The median time to clinical detection (without using imaging) of incisional hernia ranged from 25.2 to 32 months [7, 30]. Our median follow-up time was just 29 months. We expect a higher incidence of incisional hernia when the median follow-up time extends to over 3 years.
Conclusion
Closure of loop stoma is associated with both short-term and long-term morbidities. The use of adjuvant chemotherapy is associated with a higher risk of wound complications. Male sex, hypertension, use of adjuvant chemotherapy, and stoma-related complications before closure are associated with a higher risk of incisional hernia. With these findings in mind, we can provide in-depth patient education and counseling before stoma reversal.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Matthiessen P, Hallbook O, Rutegard J, Simert G, Sjodahl R (2007) Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer. Ann Surg 2007(246):207–214
Phatak UR, Kao LS, You YN, Rodriguez-Bigas MA, Skibber JM, Feig BW, Nguyen S, Chang GJ (2014) The impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol 21(2):507–512
Poskus E, Kildusis E, Smolskas E, Ambrazevicius M, Strupas K (2014) Complications after loop ileostomy closure: a retrospective analysis of 132 patients. Viszeralmedizin 30:276–280
Pokorny H, Herkner H, Jakesz R, Herbst F (2005) Mortality and complications after stoma closure. Arch Surg 140(10):956–960
Mengual-Ballester M, García-Marín JA, Pellicer-Franco E, Guillén-Paredes MP, García-García ML, Cases-Baldó MJ, Aguayo-Albasini JL (2012) Protective ileostomy: complications and mortality associated with its closure. Rev Esp Enferm Dig 104(7):350–354
Goret NE, Goret CC, Cetin K, Agachan AF (2019) Evaluation of risk factors for complications after colostomy closure. Ann Ital Chir 90:324–329
Sharp SP, Francis JK, Valerian BT, Canete JJ, Chismark AD, Lee EC (2015) Incidence of Ostomy site incisional hernias after stoma closure. Am Surg 81(12):1244–1248
Rullier E, Toux NL, Laurent C, Garrelon J-L, Parneix M, Saric J (2001) Loop ileostomy versus loop colostomy for defunctioning low anastomoses during rectal cancer surgery. World J Surg 25:274–278
Klink CD, Lioupis K, Binnebosel M, Kaemmer D, Kozubek I, Grommes J, Neumann UP, Jansen M, Willis S (2011) Diversion stoma after colorectal surgery: loop colostomy or ileostomy? Int J Color Dis 26(4):431–436
Williams NS, Nasmyth DG, Jones D, Smith AH (1986) De-functioning stomas: a prospective controlled trial comparing loop ileostomy with loop transverse colostomy. Br J Surg 73(7):566–570
Waterland P, Goonetilleke K, Naumann DN, Sutcliff M, Soliman F (2015) Defunctioning ileostomy reversal rates and reasons for delayed reversal: does delay impact on complications of ileostomy reversal? A study of 170 defunctioning ileostomies. J Clin Med Res 7(9):685–689
Baik H, Bae KB (2020) Low albumin level and longer interval to closure increase the early complications after ileostomy closure. Asian J Surg. https://doi.org/10.1016/j.asjsur.2020.09.007
Yin T-C, Tsai H-L, Yang P-F, Su W-C, Ma C-J, Huang C-W, Huang M-Y, Huang C-M, Wang J-Y (2017) Early closure of defunctioning stoma increases complications related to stoma closure after concurrent chemoradiotherapy and low anterior resection in patients with rectal cancer. World J Surg Oncol 15:80
Krebs B, Ivanecz A, Potrc S, Horvat M (2019) Factors affecting the morbidity and mortality of diverting stoma closure: retrospective cohort analysis of twelve-year period. Radiol Oncol 53(3):331–336. https://doi.org/10.2478/raon-2019-0037
Perez IR, Leon M, Pastor D, Dominguez JD, Cantero R (2014) Increased postoperative complications after protective ileostomy closure delay: an institutional study. World J Gastrointest Surg 6(9):169–174
Perez RO, Habr-Gama A, Seid VE, Proscurshim I, Sousa AH, Kiss DR, Linhares M, Sapucahy M, Gama-Rodrigues J (2006) Loop ileostomy morbidity: timing of closure matters. Dis Colon Rectum 49:1539–1545
Figueiredo MN, Mège D, Maggiori L, Ferron M, Panis Y (2015) When is the best time for temporary stoma closure in laparoscopic sphincter-saving surgery for rectal cancer? A study of 259 consecutive patients. Tech Coloproctol 19:469–474
Lorenz A, Kogler P, Kafka-Ritsch R, Ofner D, Perathoner A (2019) Incisional hernia at the site of stoma reversal - incidence and risk factors in a retrospective observational analysis. Int J Color Dis 34:1179–1187
Kaneko T, Funahashi K, Ushigome M, Kagami S, Goto M, Koda T, Nagashima Y, Shiokawa H, Koike J (2019) Incidence and risk factors for incisional hernia after closure of temporary ileostomy for colorectal malignancy. Hernia 23:734–738
Brook AJ, Mansfifeld SD, Daniels IR, Smart NJ (2018) Incisional hernia following closure of loop ileostomy: the main predictor is the patient, not the surgeon. Surgeon 16(1):20–26
Arima J, Huang C, Rosner B, Akaishi S, Ogawa R (2015) Hypertension: a systemic key to understanding local keloid severity. Wound Repair Regen 23:213–221
Huang C, Ogawa R (2014) The link between hypertension and pathological scarring: does hypertension cause or promote keloid and hypertrophic scar pathogenesis? Wound Repair Regen 22:462–466
Edwards DP, Leppington-Clarke A, Sexton R, Heald RJ, Moran BJ (2002) Stoma-related complications are more frequent after transverse colostomy than loop ileostomy: a prospective randomized clinical trial. Br J Surg 88(3):360–363
Tilney HS, Sains PS, Lovegrove RE, Reese GE, Heriot AG, Tekkis PP (2007) Comparison of outcomes following ileostomy versus colostomy for defunctioning colorectal anastomoses. World J Surg 31:1143–1152
Rondelli F, Reboldi P, Rulli A, Barberini F, Guerrisi A, Izzo L, Bolognese A, Covarelli P, Boselli C, Becattini C, Noya G (2009) Loop ileostomy versus loop colostomy for fecal diversion after colorectal or coloanal anastomosis: a meta-analysis. Int J Color Dis 24:479–488
Law WL, Chu KW, Choi HK (2002) Randomized clinical trial comparing loop ileostomy and loop transverse colostomy for faecal diversion following total mesorectal excision. Br J Surg 89(6):704–708
Guenaga KF, Lustosa SAS, Saad SS, Saconato H, Matos D (2008) Ileostomy or colostomy for temporary decompression of colorectal anastomosis. Systematic review and metaanalysis. Acta Cir Bras 23(3):294–303
Herrle F, Sandra Petrescu F, Weiss C, Post S, Runkel N, Kienle P (2016) Quality of life and timing of stoma closure in patients with rectal cancer undergoing low anterior resection with diverting stoma: a multicenter longitudinal observational study. Dis Colon Rectum 59(4):281–290
Reinwalds M, Blixter A, Carlsson E (2017) A descriptive, qualitative study to assess patient experiences following stoma reversal after rectal cancer surgery. Ostomy Wound Manage 63(12):29–37
De Robles MS, Bakhtiar A, Young CJ (2019) Obesity is a significant risk factor for ileostomy site incisional hernia following reversal. ANZ J Surg 89(4):399–402. https://doi.org/10.1111/ans.14983
Author information
Authors and Affiliations
Contributions
Study conception and design: Dr. C. Y J. Fok. Acquisition of data: Dr. C. Y J. Fok, Dr. T. L. D. Fung. Analysis and interpretation of data: Dr. C. Y J. Fok. Drafting of manuscript: Dr. C. Y J. Fok, Dr. T. L. D. Fung. Critical revision of manuscript: Dr. T. L. D. Fung, Dr. K. H. Kwok.
Corresponding author
Ethics declarations
Conflict of interest
Dr. C.Y.J. Fok, Dr. T.L.D. Fung and Dr. K.H. Kwok have no conflicts of interest or financial ties to disclose.
Ethics approval
This is a retrospective cohort, intended as an audit for service improvement, without a study protocol or affecting clinical management. Ethical board is not required.
Consent to participate
This study is conducted by retrospective review of patient data. Consent to participate is not applicable.
Consent for publication
This study is conducted by retrospective review of patient data. Consent to participate is not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fok, C.Y.J., Fung, T.L.D. & Kwok, K.H. Predictors of morbidity related to stoma closure after colorectal cancer surgery. Langenbecks Arch Surg 406, 349–356 (2021). https://doi.org/10.1007/s00423-020-02054-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-020-02054-z