Abstract
Background
The interest in correlation between hospital and surgeon practice volume and postoperative outcomes has grown considerably over the last decades; it has been suggested that surgery is likely to be associated with higher cure rates, lower morbidity and more favourable results in cost-effectiveness when performed in a high-volume setting. The aim of this paper is to undertake an evidence-based literature review of the relationship between surgical volume and clinical outcomes in parathyroidectomy for primary hyperparathyroidism. We used accepted quality markers to identify the relationship between volume and outcome with a view to defining a reproducible minimal surgical volume-related standard of care in parathyroid surgery.
Methods
A peer review literature analysis of volume and outcomes in parathyroid surgery was carried out and assessed from an evidence-based perspective. Results were discussed at the 2019 Conference of the European Society of Endocrine Surgeons devoted to “Volumes, Outcomes and Quality Standards in Endocrine Surgery”.
Results
Literature reports no prospective randomised studies; thus, a low level of evidence may be achieved.
Conclusions
Parathyroid surgery is at increased risk of failures, morbidity and need for reoperations and cost when performed in low-volume settings; thus, it should be concentrated in dedicated settings, with adequate annual volume and expertise. Acceptable results may be achieved moving parathyroid surgery cases away from low-volume settings (< 15 parathyroidectomies/year). Challenging procedures (primary hyperparathyroidism without unequivocal preoperative localization, hereditary variants, paediatric patients, reoperations) should be confined to high-volume settings (> 40 parathyroidectomies/year).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The academic interest in the correlation between hospital and surgeon practice volume and postoperative outcomes has grown considerably over the last 2 decades. Cardiopulmonary, oesophago-gastric, colonic and pancreatic surgery have been clearly demonstrated to have better outcomes when performed in a high-volume setting [1,2,3] and the volume-outcome relationship in endocrine surgery has now also been extensively studied. In parathyroid surgery, most studies suggest that parathyroidectomy is likely to be associated with higher cure rates and lower morbidity when performed in a high-volume setting [4,5,6,7] but there are also isolated reports of satisfactory results in lower volume settings [8], especially when dealing with a single preoperatively localised parathyroid adenomas. It remains the case, however, that national and international guidelines consistently recommend that parathyroidectomies should be performed by surgeons with an appropriate case-load, training and experience in the management of primary hyperparathyroidism (pHPT) but rarely if ever defining the minimal annual case-load, training or experience advocated or indeed the evidence on which this opinion is formulated.
The relative importance of hospital or institutional case-load rather than individual surgeon is debated in some fields of surgery. In complex surgical procedures, hospital volume is reported to reduce morbidity, shorten the length of stay and reduce the overall cost of care [9]. However, in other surgical specialties such as endocrine surgery, the hospital volume appears to be less important, and it is the workload, expertise and experience of the individual surgeon that appears more relevant [10]. Unfortunately, most of the literature on the relationship between volume and outcome whether surgeon specific or institutional is scientifically weak. Furthermore, national guidance often focuses on the structure and process of service provision rather than the clinical outcomes.
The aim of this study was to perform an evidence-based literature review of surgical volumes and the related outcomes in pHPT surgery. The ultimate goal was to use quality markers that correlate the relationship between surgical volume and outcomes in parathyroid surgery. With this data, we aimed to define a minimal surgical volume-related standard of care that could reliably ensure optimal outcomes in parathyroid surgery.
Materials and methods
A Medline search of articles pertinent to volume and outcomes in parathyroid surgery was performed using PubMed (National Library of Medicine) to December 2018. Keywords and medical subject headings (MeSH) were used and combined by Boolean operators, specifically (“parathyroidectomy” or “parathyroid surgery”) AND (“volume” or “experience” or “quality”). A manual search of references in the eligible papers was also performed to identify additional relevant articles. The search was limited to papers based on human studies and written in English.
Levels of evidence and possible grades of recommendation were considered according to the criteria stated by a modified Sackett’s classification [11] and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system [12]. According to Sackett’s classification, the strength of a recommendation has been graded “A” when supported by studies with a level of evidence I (meta-analysis or large randomised trials with clear cut-off results and low risk for error); “B” when supported by level II studies (small randomised trials and moderate to high risk for error); and “C” when supported by level III (non-randomised, prospective with contemporaneous control trials), level IV (non-randomised trials with historical controls, retrospective analysis) or level V studies (case series without controls, expert opinion). In the GRADE system, the strength of recommendations has been defined as “strong” or “weak”; the quality of evidence has been indicated by cross-filled circles: “⊕OOO” denotes very low-quality evidence (any estimate of effect is very uncertain); “⊕⊕OO”, low quality (further research is very likely to have an important impact on confidence in the estimate of effect and likely to change the estimate); “⊕⊕⊕O”, moderate quality (further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate); and “⊕⊕⊕⊕”, high quality (further research is very unlikely to change the confidence in the estimate of effect).
A review of the literature led to the production of a draft document that was presented and discussed at the 8th Conference of the European Society of Endocrine Surgeons (ESES) devoted to “Volumes, Outcomes and Quality Standards in Endocrine Surgery” held in Granada (Spain), May 16–18, 2019. This is a revised paper that incorporates the outcomes of the discussions.
Results
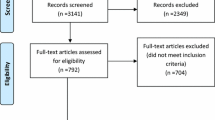
The initial literature search yielded 821 articles that were selected after screening of the title, abstracts and full text. After exclusion of articles containing insufficient details and inclusion of further articles following cross referencing, 18 studies met the criteria for inclusion into the present analysis (Table 1) [4, 5, 7, 8, 13,14,15,16,17,18,19,20,21,22,23,24,25,26].
There were no prospective randomised studies. Most studies were based on retrospective cohorts or prospectively collected data from large administrative databases. All papers showed an evidence grade between III and V according to Sackett’s classification, with a subsequent limited strength of recommendations. Many papers were biased by the inclusion of other neck endocrine operations (thyroidectomy) and by the presence of self-reported outcomes without external review; the largest studies were based on data from national or regional databases, potentially biased in turn by incomplete information and suboptimal coding. Meta-analysis was not possible because the reported data, outcomes and categorisation of surgical volume were extremely heterogeneous and not comparable.
Quality benchmarks in parathyroid surgery: correlation between case-load and outcomes
Quality outcomes in parathyroid surgery have been interpreted from either a clinical or an economic point of view or both. The main clinical outcome identified in the literature was the cure rate [4, 5, 8, 13, 14, 16, 18, 20, 21, 25]; the rate of persistent/recurrent pHPT [19]; the need for reoperative surgery after unsuccessful initial surgery [4, 5, 14, 18, 23]; the morbidity rate, including general (cardiovascular, endocrine, gastrointestinal, haematological, vascular, neurological, urological, respiratory, infections and wound) or surgical endocrine-specific complications (laryngeal nerve injury, voice disturbance, neck hematoma, definitive postoperative hypoparathyroidism) [4, 5, 13,14,15,16,17, 19, 20, 22,23,24,25,26]; and the mortality rate [14, 22, 23]. Economic items included operative time, length of hospital postoperative stay, costs and hospital charges [4, 16, 17, 22, 24, 26].
Nearly all the available literature suggested a self-evident correlation between higher surgical volume (i.e. the number of annually performed parathyroidectomies) and a better clinical and economic outcome. Specifically, parathyroid surgery performed in a high-volume setting achieved a significantly increased cure rate (90–99% compared with 70–97% in lower volume setting) [13, 14, 25] and a lower persistent pHPT rate (4.2–6% vs 9.6–15%) [13, 19]. Higher volume parathyroid surgery correlated with both a lower need for reoperation after unsuccessful initial surgery (1.4% vs 6.5%) [23] and a lower rate of avoidable reoperations because of persistent pHPT (13–22% vs 78–89%) [4, 5].
When parathyroid surgery was performed in a low-volume setting, morbidity was 1.8 to 7 times higher [22, 24, 26] and the surgery-specific endocrine morbidity rate was significantly higher (1.91–4.4% vs 0.99–3.5%) [14, 23]. The adverse outcome rates were also higher in parathyroid reoperations (3.76% vs 1.48%) [14], and in the paediatric population (14% vs 10% for general complication and 11% vs 8% for surgical endocrine-specific complications) [16]. Specifically, high-volume surgeon parathyroidectomies were complicated less frequently by vocal cord paralysis (absolute difference 1.5% vs 6%) [5, 7], by less postoperative hypoparathyroidism (4% vs 14%) [13, 15] and neck hematoma compared with low-volume setting [15, 20]. Low-volume parathyroid practice appears to present an increased mortality (6.7-fold increased risk, or 1% vs 0.04%) compared with a high-volume practice [14, 22].
Several papers reported that a low-volume setting correlates with as much as 6 times longer length of stay [15, 18, 22], a 7-fold increased risk of hospitalisation greater than 2 days [24, 26], a longer postoperative stay in adults (mean 2.1 vs 1.8 days) [16] and children (2 vs 1.5 days) [17]. Furthermore, high-volume surgeons were more likely to perform parathyroid surgery as outpatient [15], with a reported absolute difference of over 25.5% [7]. As a consequence, hospital charges [22] and average cost for a low-volume parathyroid surgeon was significantly higher than those managed by high-volume surgeons (65–300% in adults [15, 24, 26] and 9–36% in children [16, 17]). Some papers also reported that operative time was reduced for high-volume surgeons, especially when performing a bilateral neck exploration [21].
Defining high- and low-volume surgery
At least three different methods have been applied in literature to identify thresholds for low and high-volume surgeons/centres. Arbitrary cut-off categorisation was used in almost all papers except for two in which a subdivision in quantiles (quartiles or percentiles) was applied [24, 26]. Only one paper used a mathematic formula correlating outcomes and volume [23]. In some papers, the case-load was dichotomized between high and low-volume [5, 16, 18], and in others, a variety of intermediate categories were assessed. Some have opted not to fix specific thresholds [4, 21]. Table 1 summarises the characteristics of papers analysed, the suggested cut-off, the analysed outcomes and the main findings.
The threshold used to define high-volume parathyroid surgeon/centre varies from 10 to 100 annual cases and low-volume surgeons/centres have been defined as anything from 1 to 50. Irrespective of this definition range, the majority of studies confirmed the need to concentrate parathyroid surgery into a high-volume setting whenever feasible or at least to move patients away from the very lowest volume case-load setting in order to improve the cure rate, reduce the reoperation rate, morbidity and overall cost of care. Malmaeus [13] suggested that special focused training in parathyroid surgery is needed to optimise the cure rate and minimise the complications. This view has been reiterated by Yeh et al. [19] who also advocated the designation of regional high-volume centres. Neychev [25] did not fix any specific threshold, but suggested that improvement is achievable by identifying qualified endocrine surgeons.
Sosa et al. [14] categorised surgeons into four volume groups (cut-offs 10, 20 and 50 cases/year) and showed that the lowest volume surgeons had a significantly higher complication rate compared with the highest volume ones in primary operations. In reoperative parathyroidectomy, the difference in complications was significant comparing lowest volume to low-medium volume (10–20 procedures/year) surgeons. Stavrakis [15] categorised surgeons in six groups: A (surgeons performing 1 to 3 procedures/year), B (4 to 8), C (9 to 19), D (20 to 50), E (51 to 99) and F (100 or more procedures). A statistically significant difference was observed in the rate of postoperative haemorrhage when comparing A with F groups. Therefore, the authors assumed that surgeons performing 3 or less parathyroidectomies/year should be considered very low-volume surgeons and that a cut-off of 4 cases/year could be considered a minimum safety value to assure an acceptable safety level. The authors also concluded that only surgeons performing over 100 parathyroidectomies/year should be considered high-volume surgeons, which, however, would represent just 1% of all surgeons performing endocrine operations. The overall implication remains that outcomes could be improved immediately by moving patients away from lowest volume centres (< 4 cases/year). Al Qurayshi [24, 26] categorised surgeons into low (< 25 percentile, 1–2 parathyroidectomies/year), intermediate (25–75 percentile, 3–19 parathyroidectomies a year) and high-volume (> 75 percentile, 20 or more parathyroidectomies a year) and confirmed that procedures performed by low-volume surgeons were associated with increased risks of postoperative complications and longer hospital stay.
Other studies used arbitrary categorisations. Meltzer [7] showed an improvement in results (less complications, more outpatient procedures) for surgeons performing more than 40 procedures/year compared with those performing 20 or less parathyroidectomies/year. Noureldine [22] categorised three groups: low (< 2 cases/year), intermediate (2–13 cases/year) and high-volume surgeons (≥ 14 procedures/year), finding advantages in terms of morbidity, mortality, length of stay and hospital charges for higher surgeon volume.
Chen [18] used a cut-off of 50 parathyroidectomies/year, and found a lower failure rate after parathyroidectomies performed in high-volume hospitals. These results appear in contrast with Chow [8] that showed good results in terms of cure rate in a low-volume hospital (≤ 10 parathyroidectomies/year), even if a not negligible morbidity rate (1.9% transient and 1% permanent vocal fold palsy) was reported. Mitchell [5] categorised hospitals into low-volume (< 20 cases/year) and high-volume (20 or more cases), reporting a dramatic drop of avoidable reoperations in the latter. Yeh [19] also applied a categorisation to subdivide hospitals in low (< 3 cases/year), medium (3–6) and high-volume (7 or more cases/year), showing a progressive, non-linear decrease in persistent disease, reaching a cure rate of 96%. Abdulla [23] suggested that an average number of 10 parathyroidectomies/year seemed to be sufficient to achieve an acceptable reoperation rate of 1%.
Surgeon volume vs institutional volume
Some studies focused on the individual surgeon case-load, seeking the definition of “high-volume surgeon” [7, 14,15,16,17, 20,21,22, 24,25,26] whilst others focused on hospital case-load, looking for “high-volume centres” [4, 5, 8, 13, 18, 19, 23]. Nearly all studies suggest that the specific annual case-load of an individual surgeon is considerably more relevant than hospital volume [4, 5, 15, 18, 25] in determining a favourable outcome. Stavrakis reported that, even if a high degree correlation (r = 0.55) between surgeon and hospital volume was present, the hospital volume activity was the dominating determinant of length of stay and costs, whilst it had no effect on complication rate which was predictable only by the individual surgeon case-load [15].
Parathyroid volume vs combined neck endocrine volume
Whilst thyroid and parathyroid surgery share some anatomical and surgical features, the literature suggests that the operative volume is organ-specific, so that operative volume in thyroid surgery may not improve the outcomes in parathyroid surgery [5, 15]. Useful data in this regard has been collected from patient cohorts referred to a tertiary care centre for reoperative surgery where the trend appears to be that centres performing high thyroid but low parathyroid surgery numbers had significantly higher risk for reoperative parathyroid surgery [5]. In this study, reoperations were deemed “avoidable” in a much higher numbers when the first operation was performed in a low parathyroidectomy volume centre (78% vs 22%), even in case of concomitant high-volume thyroid volume. Thus, the magnitude of the surgeon volume effect appears to be parathyroid specific [15], although most thyroid high-volume centres tend to be equally high-volume for parathyroid surgery [14].
Localised vs unlocalised disease
Very few studies have analysed the role of case-load and experience according to the preoperative localization in pHPT surgery. Some papers suggested that satisfactory results may be achieved even by low-volume surgeons when preoperative ultrasonography or scintiMIBI scanning identify an unequivocal solitary parathyroid adenoma, reporting a cure rate of 98.1% [8], presumably also due to patient selection. As previously stated, however, the rate of avoidable failures after primary neck exploration has been found to be significantly higher in low-volume compared with high-volume hospitals (100% vs 17%, respectively) even when accurate preoperative localization was achieved [5]. Parathyroid disease with equivocal or negative preoperative localization comes with a significantly higher risks of failure (OR 1.96 and 2.5 respectively) but of note is the fact that the lowest surgical success rate (79.5%) was found among scan-negative/equivocal patients treated at low-volume hospitals. Indeed, low/medium hospital case-load and negative/equivocal scintiMIBI scan results appear to be a troublesome combination of independent risk factors for persistent disease [19].
Hereditary pHPT and reoperations for persistent/recurrent pHPT
No paper has formally compared the results of parathyroid surgery in hereditary pHPT and the case-load of the surgeon or institution. This may be because of the rarity of the diseases or because complex surgery tends to gravitate towards specialist centres already. However, it is also true that some hereditary pHPT patients are simply not being diagnosed and the results are to be found embedded within the reoperative parathyroidectomy data.
Discussion
Primary hyperparathyroidism is the third commonest endocrine disease and parathyroidectomy has become a relatively common operation. A cure rate of over 95% at initial surgery is reported by expert parathyroid surgeons who are, however, responsible for just only 4% of procedures [15, 27]. Most parathyroidectomies are performed in a setting where publication of results is less likely. Failure to cure pHPT may lead to the need for reoperations that come with a lower cure rate, more morbidity and increased cost. For these reasons, in recent years, an increasing number of studies have addressed the topic of the relationship between hospital and/or surgeon case-load and outcomes in parathyroid surgery.
Whilst understandably no prospective randomised studies have been performed, the existing literature reports a strong relationship between higher case-load and better clinical and economic outcomes in parathyroid surgery [14, 15, 24, 26]. Higher annual case-loads are consistently reported to achieved significantly increased cure rates [13, 14, 25], lower rates of persistent pHPT, less frequent need for reoperations [13, 19], fewer avoidable reoperations [4, 5] and up to sevenfold lower morbidity [22, 24, 26] than in a low-volume setting. Vocal cord paralysis, postoperative hypoparathyroidism and neck hematoma [13, 15, 20] are all less common in high-volume practices and this effect appears to be amplified in the case of reoperations and in the paediatric population [16]. Whilst difficult to demonstrate a causal link, the significantly increased mortality risk (6- to 25-fold) in low-volume settings also cannot be ignored [14, 22].
The available literature may always be accused of being suboptimal but overwhelmingly supports the conclusion that parathyroid surgery in a high-volume setting may be more cost-effective in part due to the shorter length of stay or increased rate of outpatient surgery [7, 15,16,17,18, 22, 24, 26] even if comparison of absolute costs and hospital charges varies between national health care systems and may not comparable.
It is accepted that the published literature presents limitations and requires careful interpretation and that the huge data from anonymised administrative databases and national registries may have limited in accuracy due to possible coding errors, under-reporting of complications that arise after discharge and the unreliability of long-term outcomes. However, the error is probably universal and comparative data from the same sources and associated conclusions are likely to be valid.
Whilst there is a fair but scientifically weak conclusion that parathyroidectomy is best performed in high-volume settings, the definitions of “low” and “high-volume” surgeons/hospitals remain unclear, varying between 1–50 and 20–100 annual parathyroidectomies, respectively. The literature is too heterogeneous to fix a definitive threshold, so organisations often use linguistics rather than number thresholds: the American Association of Endocrine Surgeons guidelines state that parathyroidectomy should be conducted by surgeons with adequate training and experience [28]. A recent literature systematic review [29] proposed to fix a threshold volume of 15–20 parathyroidectomy/year to satisfy the minimal criteria of safety and to fix a threshold of 30–40 procedures to define “high-volume”. Since in some settings these minimal thresholds cannot be achieved, a compromise has been proposed to include the surgeon’s thyroidectomy practice to reach the minimal volume even though the literature does not support this approach [5] since a high thyroidectomy case-load in the context of few parathyroidectomies comes with a significantly higher risk for persistent pHPT. Parathyroid surgery appears to require specific expertise, knowledge of embryology, surgical anatomy, pathophysiology that all underpin the figure of the “experienced parathyroid surgeon” previously recommended by Doppman [30].
Another controversial area is the definition of the relationship between experience, expertise and surgeon annual case-load. Even if expert and experienced surgeons usually perform parathyroidectomy at high-volume some have extensive experience but lesser practice volumes at any given time. Sosa and colleagues [14] analysed the impact of surgeon experience to the annual case-load by assessing the number of years in practice after completing an advanced training in endocrine surgery. They found that in high-volume surgeons, less major morbidity occurred after primary operations and reoperations, with a further decrease of major complications and in-hospital death rate directly correlated to the length of previous experience. Thus, it could be argued that better results might be obtained by dedicated and specialised surgeons, with adequate annual case-load, previous experience, targeted training and specific knowledge of parathyroid surgery-related issues.
General surgical literature has placed great importance on hospital in addition to surgeon volume in optimising outcomes and reducing costs, but this seems to refer to high-risk surgical procedures that require prolonged postoperative and multidisciplinary management [9, 10]. In endocrine surgery, the impact of the individual surgeon seems more relevant [4, 5, 15, 18, 25]. Indeed, Stavrakis and colleagues reported that hospital volume has no effect on complication rates, but may influence length of stay and costs, whilst it is surgeon volume that has the main influence on clinical outcomes and in particular is a predictor of complications [15]. The surgeon volume effect appears to be non-linear, with a sevenfold difference between the extremes of the volume spectrum, whilst the broad “middle ground” of surgeons performing 4 to 99 endocrine operations yearly achieved similar complication rates [15]. As suggested above, satisfactory results were again reported also by experienced surgeons operating at low-volume centres [25]. However, it should be underlined that in most cases, surgeon and hospital volumes coexist since high-volume surgeons usually operate at high-volume centres [5, 15].
Parathyroid surgery in most cases is performed after multiple localization studies with the site of the abnormal gland already established prior to surgery. It has been reported that satisfactory results may be obtained in this setting also by low-volume surgeons [8] with appropriate patient selection, although intervention on only localised disease would deprive patients that might benefit from surgery. Indeed, the fact that low-volume surgeons gravitate towards localised disease whilst high-volume centres operate on all comers achieving similar cure rates of around 97% [31] is an indirect marker of the value of expertise. Localised disease is not, however, a guarantee of cure in all hands since even when the disease has been positively identified before surgery, low-volume surgeons still have worst outcomes [5, 15]. This finding has been supported by population-based data from the USA where the poorest outcomes and lowest cure rates were found among un-localised disease patients treated at low-volume hospitals [19].
Similar conclusions may be reached when considering the role of case-load and experience in parathyroid surgery at the more challenging end of the spectrum. Parathyroidectomy in the context of hereditary pHPT requires an understanding of the genotypic and phenotypic background of the disease and a thorough knowledge of the guidance on this topic; challenging bilateral neck explorations (and sometimes multiglandular parathyroidectomy) with search for ectopically located disease may be required [32]. These requirements automatically place the patients in a specialist context but little data is available on the differing outcomes encountered depending on the surgical volume of the surgeon involved. Moreover, many hereditary pHPT patients are in the paediatric age group where there is evidence that high-volume practice is associated with lower complication rates, hospital lengths of stay and cost of care (Sosa 2008). The recommendation based on expert opinion is that such surgery is best performed in the appropriate environment which invariable means in the context of high-volume surgery [17].
A final special mention has to be made of reoperative parathyroid surgery which is associated with a lower cure rate and consistently higher morbidity [33]. Most inexperienced surgeons would not be keen to undertake reoperative surgery and the American Association of Endocrine Surgeons guidelines for the management of pHPT [28] and the collaborative UK/US guidelines on reoperative parathyroid surgery [34] both recommend that parathyroid reoperations should be performed by high-volume parathyroid surgeons. Most literature focuses on the reason why the first parathyroidectomy failed [1] rather than on the failure to cure at subsequent operations. It is certainly the case that most previously unidentified parathyroid adenomas are located in the conventional neck surgical field [35] so repeat surgery would seem logically and self-evidently best performed by a surgeon with greater experience [36]. However, the evidence-based to confirm this recommendation is based almost entirely on common sense and expert opinion rather than scientific proof.
Conclusions
Parathyroid surgery appears to be associated with an increased risk of failure to cure, need for reoperation, morbidity, possibly mortality and increased costs when performed in low-volume setting. As a consequence, there is a desire to move away from the lowest volume parathyroid surgeons towards more experienced surgeons in a high-volume setting. Expert and experienced surgeons currently represent a minority and are not ubiquitously available; so any centralization has to be accommodated to local circumstances since it may not always practical, feasible nor politically implementable. However, patients armed with the existing evidence may ask why they should be exposed to worse outcomes and higher risk and lead this drive. There will remain a role for local centres with adequate minimal case-load and with acceptable results for patients that prefer local surgery or who are unable or unwilling to travel to dedicated centres.
Summary and recommendations
In conclusion, after presenting the results of this review in an evidence-based perspective at the 8th European Society of Endocrine Surgeons Conference (May 16–18, 2019, Granada, Spain), a set of conclusions were agreed upon, followed by the recommendation grade and evidence level.
-Is it possible to identify quality benchmarks in parathyroid surgery?
Quality in pPTH surgery can be measured according to the clinical and economic outcomes. The cure rate, rate of persistent pHPT and need for reoperations, general and surgical endocrine-specific morbidity and eventually mortality should be measured by each parathyroid surgeon to assess the level of performance (recommendation level: C, evidence level: III; GRADE: strong; ⊕⊕⊕O).
-Is there any correlation between case-load and outcomes?
Case-load and outcomes correlate well in parathyroid surgery. High-volume settings unequivocally achieve better clinical and economic results. The role of expertise, experience and specific competence should be taken in account together with the annual case-load (recommendation level: C, evidence level: III; GRADE: strong; ⊕⊕OO).
-Is the impact on outcomes of individual surgeon and hospital case-load different?
Specific experience and the case-load of the surgeon appears to be more relevant than hospital volume in achieving good outcomes in parathyroid surgery (recommendation level: C, evidence level: III; GRADE: strong; ⊕⊕OO).
-Can outcomes in parathyroid surgery be influenced by thyroid surgery case-load?
Parathyroid surgery needs specific and adequate knowledge and competence; thyroid surgery case-load may affect the outcomes of parathyroid surgery but probably to a limited extent and should not be considered when defining a requisite surgical case-load for parathyroid surgery (recommendation level: C, evidence level: III; GRADE: weak; ⊕OOO).
-How can high and low-volume parathyroid surgeons be defined?
The definition of high and low-volume parathyroid surgeon remains arbitrary. Consensus gravitates towards a threshold of 40 and 15 annual procedures as cut-off for the former and the latter, respectively (recommendation level: C, evidence level: III; GRADE: weak; ⊕OOO).
-Should parathyroid surgery be concentrated in high-volume settings?
Parathyroid surgery is at increased risk of morbidity, failures and need for reoperations when performed in low-volume settings; thus, it should be concentrated in dedicated settings, with adequate annual volume and expertise. Whenever possible, local centres with adequate minimal case-load and expertise might be designated at least at regional level. Acceptable results may be achieved simply moving parathyroid surgery cases away from low-volume settings (recommendation level: C, evidence level: III; GRADE: strong; ⊕⊕OO).
Challenging procedures (pHPT without unequivocal preoperative localization, hereditary pHPT, paediatric patients, reoperations) should be confined to high-volume setting (recommendation level: C; evidence level: III; GRADE: strong; ⊕⊕OO).
References
Zarebczan B, Chen (2011) Influence of surgical volume on operative failures for hyperparathyroidism. Adv Surg 45:237–248
Schlottmann F, Strassle PD, Charles AG, Patti MG (2018) Esophageal cancer surgery: spontaneous centralization in the US contributed to reduce mortality without causing health disparities. Ann Surg Oncol 25:1580–1587
Krautz C, Nimptsch U, Weber GF, Mansky T, Grützmann R (2018) Effect of hospital volume on in-hospital morbidity and mortality following pancreatic surgery in Germany. Ann Surg 267(3):411–417
Chen H, Zeiger MA, Gordon TA, Udelsman R (1996) Parathyroidectomy in Maryland: effects of an endocrine center. Surgery 120(6):948–953
Mitchell J, Milas M, Barbosa G, Sutton J, Berber E, Siperstein A (2008) Avoidable reoperations for thyroid and parathyroid surgery: effect of hospital volume. Surgery 144(6):899–906
Bergenfelz AOJ, Wallin G, Jansson S, Eriksson H, Mårtensson H, Christiansen P, Reihnér (2011) Results of surgery for sporadic primary hyperparathyroidism in patients with preoperatively negative sestamibi scintigraphy and ultrasound. Langenbeck’s Arch Surg 396:83–90
Meltzer C, Klau M, Gurushanthaiah D, Tsai J, Meng D, Radler L, Sundang A (2017) Surgeon volume in parathyroid surgery-surgical efficiency, outcomes, and utilization. JAMA Otolaryngol Head Neck Surg 143(8):843–847
Chow TL, Choy CY, Lam SH (2015) Focused parathyroidectomy without intra-operative parathyroid hormone monitoring for primary hyperparathyroidism: results in a low-volume hospital. J Laryngol Otol 129:788–794
Birkmeyer JD, Skinner JS, Wennberg DE (2002) Will volume-based referral strategies reduce costs or just save lives? Health Aff 21(5):234–241
Chowdhury MM, Dagash H, Pierro A (2007) A systematic review of the impact of volume of surgery and specialization on patient outcome. Br J Surg 94:145–161
Sackett DL (1989) Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 95(suppl):2–4
Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, for the GRADE Working Group (2008) Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Malmaeus J, Granberg PO, Halvorsen J, Akerström G, Johansson H (1988) Parathyroid surgery in Scandinavia. Acta Chir Scand 154(7–8):409–413
Sosa JA, Powe NR, Levine MA, Udelsman R, Zeiger MA (1998) Profile of a clinical practice: thresholds for surgery and surgical outcomes for patients with primary hyperparathyroidism: a national survey of endocrine surgeons. J Clin Endocrinol Metab 83:2658–2665
Stavrakis AI, Ituarte PH, Ko CY, Yeh MW (2007) Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery 142:887–899
Sosa JA, Tuggle CT, Wang TS, Thomas DC, Boudourakis L, Rivkees S, Roman SA (2008) Clinical and economic outcomes of thyroid and parathyroid surgery in children. J Clin Endocrinol Metab 93(8):3058–3065
Tuggle CT, Roman SA, Wang TS, Boudourakis L, Thomas DC, Udelsman R, Sosa JA (2008) Pediatric endocrine surgery: who is operating on our children? Surgery 144(6):869–877
Chen H, Wang TS, Yen TW, Doffek K, Krzywda E, Schaefer S, Sippel RS, Wilson SD (2010) Ann Surg 252(4):691–695
Yeh MW, Wiseman JE, Chu SD, Ituarte PH, Liu IL, Young KL, Kang SJ, Harari A, Haigh PI (2011) Population-level predictors of persistent hyperparathyroidism. Surgery 150(6):1113–1119
Dehal A, Abbas A, Al-Tememi M, Hussain F, Johna S (2014) Impact of surgeon volume on incidence of neck hematoma after thyroid and parathyroid surgery: ten years’ analysis of nationwide in-patient sample database. Am Surg 80(10):948–952
Karakas E, Schneider R, Rothmund M, Bartsch DK, Schlosser K (2014) Initial surgery for benign primary hyperparathyroidism: an analysis of 1,300 patients in a teaching hospital. World J Surg 38(8):2011–2018
Noureldine SI, Abbas A, Tufano RP, Srivastav S, Slakey DP, Friedlander P, Kandil E (2014) The impact of surgical volume on racial disparity in thyroid and parathyroid surgery. Ann Surg Oncol 21(8):2733–2739
Abdulla AG, Ituarte PH, Wiggins R, Teisberg EO, Harari A, Yeh MW (2012) Endocrine surgery as a model for value-based health care delivery. Surg Neurol Int 3:163. https://doi.org/10.4103/2152-7806.1051
Al-Qurayshi Z, Randolph GW, Srivastav S, Kandil E (2016) Outcomes in endocrine cancer surgery are affected by racial, economic, and healthcare system demographics. Laryngoscope 126:775–781
Neychev VK, Ghanem M, Blackwood SL, Aragon Han P, Fazeli R, Schneider E, Najafian A, Bloch DC, Bard MC, Klarsfeld JH, Zeiger MA, Lipton RJ (2016) Parathyroid surgery can be safely performed in a community hospital by experienced parathyroid surgeons: a retrospective cohort study. Int J Surg 27:72–76
Al-Qurayshi Z, Hauch A, Srivastav S, Kandil E (2017) Ethnic and economic disparities effect on management of hyperparathyroidism. Am J Surg 213:1134–1142
Saunders BD, Wainess RM, Dimick JB, Doherty GM, Upchurch GR, Gauger PG (2003) Who performs endocrine operations in the United States? Surgery 134(6):924–931
Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, Doherty GM, Herrera MF, Pasieka JL, Perrier ND, Silverberg SJ, Solorzano CC, Sturgeon C, Tublin ME, Udelsman R, Carty SE (2016) The American Association of Endocrine Surgeons Guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 151(10):959–968
Melfa G, Porrello C, Cocorullo G, Raspanti C, Rotolo G, Attard A, Gullo R, Bonventre S, Gulotta G, Scerrino G (2018) Surgeon volume and hospital volume in endocrine neck surgery: how many procedures are needed for reaching a safety level and acceptable costs? A systematic narrative review. G Chir 39(1):5–11
Doppman JL (1986) Reoperative parathyroid surgery: localization procedures. In: Rothmund M, Wells Jr. SA (eds) Parathyroid surgery. Prog Surg. Basel, Karger, vol 18, pp 117–132. https://doi.org/10.1159/000412364
Jinih M, O’Connell E, O’Leary DP, Liew A, Redmond HP (2017) Focused versus bilateral parathyroid exploration for primary hyperparathyroidism: a systematic review and meta-analysis. Ann Surg Oncol 24:1924–1934
Iacobone M, Carnaille B, Palazzo FF, Vriens M (2015) Hereditary hyperparathyroidism - a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbeck’s Arch Surg 400(8):867–886
Chadwick D. 2017 BAETS 5th National Audit Report. https://www.baets.org.uk/wp-content/uploads/BAETS-Audit-National-Report-2017.pdf & ISBN 978-0-9929942-0-4
Stack BC Jr, Tolley NS, Bartel TB, Bilezikian JP, Bodenner D, Camacho P, JPDT C, Dralle H, Jackson JE, Morris JC 3rd, Orloff LA, Palazzo F, Ridge JA, Scott-Coombes D, Steward DL, Terris DJ, Thompson G, Randolph GW (2018) AHNS Series: do you know your guidelines? Optimizing outcomes in reoperative parathyroid surgery: definitive multidisciplinary joint consensus guidelines of the American Head and Neck Society and the British Association of Endocrine and Thyroid Surgeons. Head Neck 40(8):1617–1629
McIntyre CJ, Allen J, Constantinides VA, Jackson JE, Tolley NS, Palazzo FF (2015) Patterns of disease in patients at a tertiary referral centre requiring reoperative parathyroidectomy. Ann R Coll Surg Engl 97(8):598–602
Mihai R, Barczynski M, Iacobone M, Sitges-Serra A (2009) Surgical strategy for sporadic primary hyperparathyroidism an evidence-based approach to surgical strategy, patient selection, surgical access, and reoperations. Langenbeck’s Arch Surg 394(5):785–798
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iacobone, M., Scerrino, G. & Palazzo, F.F. Parathyroid surgery: an evidence-based volume—outcomes analysis. Langenbecks Arch Surg 404, 919–927 (2019). https://doi.org/10.1007/s00423-019-01823-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-019-01823-9