Abstract
Background
The importance of preoperative chemotherapy in a multimodality management of patients with colorectal liver metastases (CRLM) has been demonstrated. We analyse the carcinoembryonic antigen (CEA) changes following neoadjuvant chemotherapy in patients with CRLM who underwent liver resection.
Methods
The final cohort included 107 eligible patients. Increased CEA levels following neoadjuvant chemotherapy were defined as the increase of baseline CEA level at diagnosis of CRLM compared with the CEA level after completion of neoadjuvant chemotherapy. Disease-free survival (DFS), post-recurrence survival (PRS) and overall survival (OS) were calculated using both Kaplan-Meier and multivariate Cox-regression methods.
Results
CEA increase was associated with decreased PRS and OS (HR 2.69; 95 % CI, 1.28–5.63; p = 0.009, and HR 2.50; 95 % CI, 1.12–5.56; p = 0.025, respectively) in multivariate analysis, but there was no association between CEA changes and DFS. CEA increase was only associated with disease progression during preoperative chemotherapy (p = 0.014). Interestingly, this association was not absolute, as only 5 of the 11 patients with disease progression demonstrated CEA increase. Regarding the remaining 12 patients with CEA increase, according to RECIST criteria, eight patients demonstrated partial response and four patients stable disease.
Conclusion
In this study, we demonstrated the CEA increase following neoadjuvant chemotherapy as an adverse prognostic factor for PRS, and OS but not for DFS in patients undergoing liver resection for liver-only colorectal metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of colorectal liver metastases (CRLM) by surgical resection has become the standard of care over the last two decades. Surgical resection of CRLM has been shown to increase the patients’ survival, with a 5-year overall survival (OS) ranging between 35 and 58 % for resected cases and with approximately 16 % of these patients being disease-free 10 years after hepatectomy [1, 2]. Although the criteria for liver resection in these patients have expanded, only 10 % of patients who present with CRLM are candidates for hepatectomy [3].
The importance of preoperative chemotherapy as part of multimodal management of these patients has already been demonstrated [4]. Preoperative chemotherapy can be used in three ways: (1) neoadjuvant setting to downsize the liver metastases, (2) allow demonstration of tumour biology, (3) in some cases to convert patients with tumours considered irresectable into candidates for a liver resection, with the survival statistics of this group being similar to the initially resectable cases: ∼30 % of 5-year survival [5, 6]. The vast majority of patients with CRLM who undergo liver resection receive neoadjuvant chemotherapy.
Risk stratification to select patients for post-hepatectomy adjuvant follow-up and therapy is necessary. Traditional prognostic factors, e.g. primary tumour stage, interval from diagnosis of primary tumour to diagnosis of CRLM, size and number of hepatic metastasis and presence of extrahepatic disease, have been used either individually or combined (clinical prognostic scores) for decades for this risk stratification. This has been challenged in recent years [7–9]. The main reason is that these factors are based on data from the preneoadjuvant chemotherapy era. More data indicate that tumour biology is a superior prognostic factor when compared to the traditional measurements of tumour volume [10, 11]. The need for new reliable prognostic factors which more precisely model tumour response to neoadjuvant chemotherapy and thus tumour biology is imperative. Response to neoadjuvant chemotherapy has been shown to be a powerful prognostic factor for patients undergoing liver resection for CRLM. This response can be measured according to the size changes of the liver metastases, their metabolic response as this is demonstrated by sequenced PET scans, and possibly by changes in carcinoembryonic antigen (CEA) levels.
The purpose of our study was to ascertain whether the change in CEA alone following neoadjuvant chemotherapy is related to the disease-free survival (DFS), post-recurrence survival (PRS) and OS of patients with liver-only colorectal metastases undergoing hepatectomy after neoadjuvant chemotherapy.
Materials and methods
A prospectively collected surgical database from the Royal Marsden Hospital, London, was interrogated to identify the patients who underwent liver resection for liver-only colorectal metastases following neoadjuvant chemotherapy between January 2005 and December 2012. All patients were operated on by one of two liver surgeons (SM and AZK). Patients who had an R2 resection and patients who died because of post-operative complications were excluded. Patients with normal CEA (≤3 mcg/L) both at diagnosis and after completion of neoadjuvant chemotherapy and patients with unavailable data regarding CEA level at the time of diagnosis and after completion of neoadjuvant chemotherapy were also excluded.
All patients had agreed multidisciplinary team meetings. The extent of liver disease was determined using Primovist-enhanced MRI. CT and FDG-PET of the chest, abdomen and pelvis were performed in all patients to exclude extrahepatic disease. MRI was also used to evaluate the response to chemotherapy. In patients with a primary tumour in situ, the timing for the resection of CRLM was determined by the multidisciplinary team. Determinants of the above decision were the localization of the primary tumour, OMS Performance Status and the extent of necessary hepatectomy. Ιntra-operative ultrasound was performed in all patients and the surgical plan modified accordingly.
For each patient, the institutional electronic records were checked and data was collected regarding the following: (a) standard demographics, (b) primary colorectal tumour, (c) CRLM characteristics, (d) preoperative chemotherapy, (e) response to preoperative chemotherapy, (f) liver resection and (g) DFS, PRS and the OS. DFS was calculated from the date of hepatectomy to the date of disease recurrence and was censored at the last follow-up or at the time of death if the patients remained tumour free at that time. PRS was calculated from the date of recurrence to the date of death. OS was calculated from the time of hepatectomy to the date of death and was censored at the last follow-up. Follow-up data for all patients were available.
The study was approved by the local ethics committee and Institutional Review Board.
Statistical analysis
Statistical analysis was performed with the Statistical Package of the Social Sciences (SPSS), version 17.0. The primary end points of the study were DFS, PRS and OS, respectively. CEA increase following neoadjuvant chemotherapy was determined as the increase of CEA level at the diagnosis of CRLM comparing with the CEA level after completion of neoadjuvant chemotherapy.
Chi-square test was used for calculating the association between patients’ and tumour’s categorical characteristics and CEA change. The impact of these features on DFS, PRS and OS was analysed using the Kaplan-Meier method. Survival outcomes between groups were compared with the log-rank test. A p value of less than 0.05 was considered statistically significant. The factors associated with DFS, PRS or the OS (p < 0.1) in univariate analysis were used for the performance of the multivariate Cox-regression analysis.
Results
One hundred and forty-nine patients were identified from the prospectively collected surgical database from the Royal Marsden Hospital, and 107 patients met criteria for inclusion in the study. Other patients were excluded for the following reasons: incomplete resection/R2 resection (n = 4), post-operative death (n = 2), missing data for the calculation of CEA change following neoadjuvant chemotherapy (n = 8) and normal CEA both at diagnosis of CRLM and after completion of neoadjuvant chemotherapy (n = 28).
Patient demographics, characteristics of CRLM at diagnosis, details of surgical resection and correlation of these characteristics with CEA change following neoadjuvant chemotherapy are shown in Table 1. In the majority of patients (69 %), neoadjuvant chemotherapy was based on oxaliplatin, with the remaining receiving an irinotecan-based regimen. Patients received a median of 5 cycles of neoadjuvant chemotherapy (range 1–24 cycles). In total, 38 patients (36 %) received a combination of doublet cytotoxic neoadjuvant chemotherapy with bevacizumab. Eleven patients (10 %) experienced disease progression during preoperative chemotherapy. CEA increased following neoadjuvant chemotherapy in 17 patients (16 %). As demonstrated in Table 1, CEA increase was only associated with disease progression during preoperative chemotherapy (p = 0.014). Interestingly, this association was not absolute, as only 5 of the 11 patients with disease progression demonstrated CEA increase. Regarding the remaining 12 patients with CEA increase, according to RECIST criteria [12], eight patients demonstrated partial response and four patients stable disease.
Median follow-up for the entire study population was 34 months (1 to 103 months) and for the survivors was 41 months (1 to 103 months). During follow-up, 79 patients (74 %) developed tumour recurrence, 73 of these (92 %) during the first 2 years after hepatectomy. Forty patients (38 %) died during follow-up. While CEA increase was not associated with a recurrence rate (82 vs. 72 %, p = 0.550), it was strongly associated with an increase in death rate (71 vs. 31 %, p = 0.002). For the entire study population, 3- and 5-year DFS rates were 26 and 23 % and OS rates were 71 and 55 %, respectively.
Univariate analysis (Table 2) demonstrated that more than three liver metastases at diagnosis (HR 1.80; 95 % CI, 1.06–3.04; p = 0.028), bilobar distribution (HR 1.82; 95 % CI, 1.15–2.90; p = 0.011), preoperative administration of more than 6 cycles of chemotherapy (HR 2.44; 95 % CI, 1.48–4.03; p < 0.001) and disease progression during neoadjuvant chemotherapy according to RECIST criteria [12] (HR 3.22; 95 % CI, 1.67–6.23; p < 0.001) were associated with a decreased DFS. CEA increase was not associated with DFS (HR 1.62; 95 % CI, 0.90–2.90; p = 0.102) (Fig. 1) (Table 2). The only factor that remained statistically associated with DFS in multivariate analysis was disease progression during preoperative chemotherapy (HR 3.41; 95 % CI, 1.62–7.16; p = 0.001).
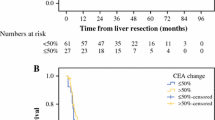
Regarding OS, univariate analysis (Table 2) revealed that bilobar distribution of lesions (HR 2.52; 95 % CI, 1.35–4.71; p = 0.004), preoperative administration of more than 6 cycles of chemotherapy (HR 2.12; 95 % CI, 1.06–4.26; p = 0.034), no adjuvant post-hepatectomy chemotherapy (HR 2.94; 95 % CI, 1.52–5.67; p = 0.001) and CEA increase following neoadjuvant chemotherapy (HR 2.77; 95 % CI, 1.40–5.49; p = 0.003) were associated with decreased OS (Fig. 2). Patients with CEA increase had a median OS of 29 months, and median OS was 81 months in patients with stable or decreased CEA. Three- and 5-year OS rates were 42 and 19 %, respectively, in patients with CEA increase and 78 and 63 %, respectively, in patients with stable or decreased CEA. In multivariate analysis, factors that remained statistically associated with OS included bilobar distribution of CRLM (HR 2.39; 95 % CI, 1.13–5.07; p = 0.023) and CEA increase (HR 2.50; 95 % CI, 1.12–5.56; p = 0.025) (Table 2).
A subgroup analysis of the 79 patients who experienced recurrence demonstrated that only CEA increase (HR 2.69; 95 % CI, 1.28–5.63; p = 0.009) was independently associated with reduced post-recurrence survival. Patients with CEA increase showed a median PRS of 23 months, while patients with stable or decreased CEA showed a median PRS of 40 months (Fig. 3).
Discussion
Ninety-seven percent of patients with colon cancer have high levels of CEA in the serum [13–15]. Hara et al. have reported that in patients undergoing liver resection with curative intent for CRLM, an elevated post op CEA level is associated with a probability of recurrence of 70–90 %, yet 10 % when CEA level was in the normal range [16]. Additionally, Araujo et al. have reported that 15 ng/mL could be considered as a high specificity and positive predictive value for recurrence when measured the first 6 months following liver resection in patients with CRLM [17].
In this study, we demonstrate that CEA increase during neoadjuvant chemotherapy is an independent prognostic factor associated with reduced PRS and OS, but not DFS in patients with liver-only colorectal metastases who subsequently undergo curative liver resection. The only factor associated with CEA increase was disease progression during neoadjuvant chemotherapy, according to RECIST criteria [12]. Notably, despite the strong association between CEA increase and disease progression, there was no complete match between these parameters. Although patients with a CEA increase had a median OS of 29 months, patients with a stable or decreased CEA had a median OS of 81 months.
The prognostic value of the disease response to neoadjuvant chemotherapy in patients with CRLM has been highlighted in recent years. Our team has recently reported the association between radiological disease progression and decreased DFS [10]. In this study, we have found similar findings, as the radiological disease progression is the only factor independently associated with DFS. Lau et al. have also reported an association of metabolic response, as determined by the proportional change in PET scan parameters following neoadjuvant chemotherapy, with both DFS and OS [11]. The third parameter that reflects the response of the tumour to neoadjuvant chemotherapy and which has been reported to be linked with OS but not with DFS is the CEA change following neoadjuvant chemotherapy [18]. Stremitzer et al. investigated a CEA change in conjunction with DFS and OS in a group of 88 patients with CRLM who underwent liver resection with curative intent and concluded that a CEA change of less than 50 % is associated with decreased OS but not DFS. To determine the optimal cutoff value of 50 %, they applied receiver operating curve (ROC) analysis [18]. In this study, we divided patients into two groups based on the CEA change following neoadjuvant chemotherapy: patients with an increase in CEA and patients with stable or a decrease in CEA after neoadjuvant chemotherapy. We believe that this categorization is more reliable and reproducible than the determination of an optimal cutoff level which would be applicable only regarding this population under study and therefore would not be reproducible. Our choice of categorization can be supported by results of two further studies that investigated the prognostic value of CEA change in patients with non-metastatic resectable rectal cancer [19, 20]. While both studies concluded that CEA change following neoadjuvant treatment is not associated with decreased OS, one study used 70 % as optimal cutoff value of CEA change and the other one 50 % [19, 20].
Our findings are supported by the report of Stremitzer et al. which demonstrated that increased CEA following neoadjuvant chemotherapy is associated with decreased OS. Increasingly, data indicate that tumour biology is a superior prognostic factor when compared to the traditional measurements of tumour volume [10, 11]. This finding is even more important when it comes to CRLM because of the high probability of micrometastatic disease not readily detectable by follow-up imaging studies. Assessment of response to neoadjuvant chemotherapy via an accessible way to define tumour biology may have strategic value. Currently, the only widely accepted method for the assessment of tumour response to neoadjuvant chemotherapy is the radiologic response, more specifically the RECIST criteria [12]. However, these criteria aim mainly at adopting a common parameter in regards to the response of solid tumours to chemotherapy so as for results from phase II and phase III studies to be comparable, and not to delineate risk stratification for patients in terms of post-resection adjuvant follow-up and therapy. Lau et al. have demonstrated that metabolic response to neoadjuvant chemotherapy is superior to radiologic response according to RECIST criteria as a prognostic factor in patients with CRLM undergoing liver resection [11]. Similarly, both reported results from Stremitzer’s work and from our study demonstrate that CEA change is a predictive factor superior to radiologic response regarding OS [18]. On the other hand, in an era characterised by the increasing use of biological agents such as bevacizumab or immunological therapies in the neoadjuvant setting for patients with CRLM, RECIST criteria appear to be inferior to morphological changes of liver metastases for the assessment of response to treatment [21]. More recent criteria that are used for targeted therapies include the Choi response criteria for gastrointestinal stromal tumours, modified RECIST criteria for hepatocellular carcinoma and immune-related response criteria for melanoma. The positron emission tomography response criteria in solid tumours and the Cheson criteria make use of positron emission tomography to provide functional information and help determine tumour viability [22].
All of the above data indicate the need for a marker superior to RECIST criteria for the evaluation of response to neoadjuvant treatment. For the assessment of metabolic response, a repeat PET scan following completion of neoadjuvant chemotherapy is required in our opinion. As this is not performed routinely in most cancer centres for economic and availability reasons, we believe that assessment of CEA change following neoadjuvant chemotherapy could be a reasonable method for additional assessment of response to neoadjuvant chemotherapy.
Τhe greatest restriction of our study is its retrospective nature, and as such, a selection bias is a possibility. In an effort to homogenise the study population, we excluded patients with extrahepatic disease and included only patients with potentially curative resections. Furthermore, all patients were operated on by one of two surgeons at the same institution and they all underwent similar preoperative staging, MDM assessment and same protocol decision tree.
Conclusion
We have shown that CEA increase following neoadjuvant chemotherapy is an independent prognostic factor associated with both PRS and OS in patients with liver-only colorectal metastases who undergo curative liver resection. We suggest validation of this finding in an independent cohort and consideration of risk stratification for post-hepatectomy adjuvant follow-up and therapy.
References
Pawlik TM, Schulick RD, Choti MA (2008) Expanding criteria for respectability of colorectal liver metastases. Oncologist 13(1):51–64
Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y (2007) Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25(29):4575–4580
Bismuh H, Adam R, Levi F et al (1996) Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 224(4):509–520
Kemeny N (2007) Presurgical chemotherapy in patients being considered for liver resection. Oncologist 12:825–839
Nordlinger B, Van Cutsem E, Rougier P et al (2007) Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European colorectal metastases treatment group. Eur J Cancer 43:2037–2045
Adam R, Delvart V, Pascal G et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657, discussion 57–8
Neal CP, Garcea G, Doucas H, Manson MM et al (2006) Molecular prognostic markers in resectable colorectal liver metastases: a systematic review. Eur J Cancer 42:1728–1743
Tanaka K, Shimada H, Fujii Y, Endo I et al (2004) Pre-hepatectomy prognostic staging to determine treatment strategy for colorectal cancer metastases to the liver. Arch Surg 389:371–379
Fong JG, Fortner R, Sun MF, Brennan MF et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–321
Neofytou K, Smyth EC, Giakoustidis A, Khan AZ et al (2014) Elevated platelet to lymphocyte ratio predicts poor prognosis after hepatectomy for liver-only colorectal metastases, and it is superior to neutrophil to lymphocyte ratio as an adverse prognostic factor. Med Oncol 31(10):239–248
Lau LF, Williams DS, Lee ST, Scott AM et al (2014) Metabolic response to preoperative chemotherapy predicts prognosis for patients undergoing surgical resection of colorectal cancer metastatic to the liver. Ann Surg Oncol 21(7):2420–2428
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National cancer institute of the United States. National cancer institute of Canada. J Natl Cancer Inst 92(3):205–216
Gold P, Freedman SO (1965) Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med 121:439–462
Gold P, Shuster J, Freedman SO (1978) Carcinoembryonic antigen (CEA) in clinical medicine: historical perspectives, pitfalls and projections. Cancer 42(3 Suppl):1399–1405
Thomson DM, Krupey J, Freedman SO, Gold PB (1969) The radioimmunoassay of circulating carcinoembryonic antigen of the human digestive system. Proc Natl Acad Sci U S A 64(1):161–167
Hara M, Sato M, Takahashi H, Takayama S et al (2013) Carcinoembryonic antigen elevation in post-hepatectomy patients with colorectal cancer liver metastasis indicates recurrence with high accuracy. Hepatogastroenterology 60(128):1935–1939
Araujo RL, Gönen M, Allen P, DeMatteo R et al (2015) Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol 13
Stremitzer S, Stift J, Graf A, Singh J et al (2014) CEA Change After Neoadjuvant Chemotherapy Including Bevacizumab and Clinical Outcome in Patients Undergoing Liver Resection for colorectal liver metastases. Ann Surg Oncol 17
Kim CW, Yu CS, Yang SS, Kim KH (2011) Clinical significance of pre- to post-chemoradiotherapy s-CEA reduction ratio in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol 18(12):3271–3277
Huang CS, Lin JK, Wang LW, Liang WY et al (2014) Assessment of the value of carcinoembryonic antigen reduction ratio as a prognosis factor in rectal cancer. Am J Surg 208(1):99–105
Boonsirikamchai P, Asran MA, Maru DM, Vauthey JN et al (2011) CT findings of response and recurrence, independent of change in tumor size, in colorectal liver metastasis treated with bevacizumab. AJR Am J Roentgenol 197(6):W1060–W1066
Tirkes T, Hollar MA, Tann M, Kohli MD et al (2013) Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics 33(5):1323–1341. doi:10.1148/rg.335125214
Authors’ contributions
Kyriakos Neofytou: conception and design of research, preparation of manuscript, statistical analysis
Alexandros Giakoustidis: conception and design of research, preparation of manuscript
Mafalda Costa Neves: preparation of manuscript
Dawn Morrison: preparation of manuscript
Dimitris Giakoustidis: critical review of manuscript
Aamir Z Khan: critical review of manuscript
Justin Stebbing: critical review of manuscript
Satvinder Mudan: conception and design of research, preparation of manuscript, critical review and approval of manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
Supported by the National Institute for Health Research Royal Marsden/Institute for Cancer Research Biomedical Research Centre
Conflicts of interest
All the authors declare no conflict of interest related to the present research.
Additional information
Mr. Neofytou and Mr. Giakoustidis contributed equally.
Synopsis
We analyse the CEA changes following neoadjuvant chemotherapy in patients with CRLM who underwent liver resection. CEA increase following neoadjuvant chemotherapy appears to be an independent prognostic factor associated with both PRS and OS in patients with liver-only colorectal metastases who undergo curative liver resection.
Rights and permissions
About this article
Cite this article
Neofytou, K., Giakoustidis, A., Neves, M.C. et al. Increased carcinoembryonic antigen (CEA) following neoadjuvant chemotherapy predicts poor prognosis in patients that undergo hepatectomy for liver-only colorectal metastases. Langenbecks Arch Surg 402, 599–605 (2017). https://doi.org/10.1007/s00423-016-1415-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-016-1415-2