Abstract
Purpose
The effectiveness of a transanal drainage tube for the prevention of anastomotic leakage (AL) is still uncertain. This study aimed to investigate the impact of anal decompression on AL after rectal cancer surgery.
Methods
We retrospectively reviewed 536 rectal cancer patients who underwent low anterior resection without diverting stoma, with (n = 154) or without (n = 382) placing of a transanal drainage tube, between January 2005 and December 2014. Risk factors for AL were analyzed, and propensity score matching analysis was used to compensate for the differences in baseline characteristics.
Results
AL occurred in 50 (9.3 %) of the patients. Male sex (odds ratio [OR] 3.097, p = 0.005), high ASA score (OR 3.505, p = 0.025), and neoadjuvant chemoradiation (OR 2.506, p = 0.018) were independent predictors of AL on multivariable analysis. After propensity score matching, transanal drainage tube tended to lessen rates of grade C AL with definite peritonitis (1.9 vs. 5.8 %, p = 0.077), although there was no difference in the incidence of AL in patients with or without transanal drainage tubes (5.8 vs. 9.1 %, p = 0.278).
Conclusions
Placement of a transanal drainage tube was not associated with a reduction in the total incidence of AL after low anterior resection for rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of total mesorectal excision (TME) [1], more rectal cancer patients can now be treated with sphincter-preserving surgery with improved oncologic outcome and quality of life [2, 3]. However, anastomotic leakage (AL), the rate of which has been reported to be 5–19 % in rectal cancer surgery [4], still remains an important concern for colorectal surgeons, because this complication often results in reoperation and increased morbidity and mortality [4].
Several previous studies have reported clinical, tumor-related, and surgery-related risk factors for AL, including male sex, obesity, high American Society of Anesthesiologists (ASA) score, advanced stage, lower tumor level, perioperative transfusion, and multiple firings of the linear stapler [4–6]. In addition, several surgical techniques and methods such as diverting stoma [7, 8], abdominal drains [9], reinforcing sutures [10, 11], and fibrin adhesives [12] have been used in attempts to reduce the incidence of AL. Some other studies report the use of transanal drainage tubes for the prevention of AL after rectal cancer surgery on the assumption that increased neorectal pressure during the early postoperative period may result in AL [13–18]. However, the results of these studies are inconsistent.
Therefore, we have designed this study to investigate the impact of anal decompression using a transanal drainage tube on AL after low anterior resection without fecal diversion.
Patients and methods
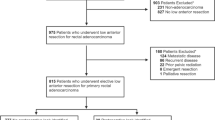
This study was approved by the institutional review board of our institution. We retrospectively reviewed data from the rectal cancer database at our institution collected prospectively between January 2005 and December 2014 and included those who underwent low anterior resection using a standard double-stapling anastomosis without diverting stoma. Low rectal cancer patients who underwent intersphincteric resection or diverting stoma were excluded, because transanal drainage tubes are not indicated in these cases.
The preoperative clinical evaluation included colonoscopy, abdominopelvic and chest computed tomography (CT), and pelvic magnetic resonance imaging or endorectal ultrasound for local staging. The distance of the tumor from the anal verge (AV) was assessed with a digital rectal examination and rigid rectoscopy. The location of the tumor was classified into three groups according to the distance from the AV: lower rectum (0–5 cm), middle rectum (6–10 cm), and upper rectum (11–15 cm).
For clinical T3-4 or N1-2 mid-to-low rectal cancers, neoadjuvant chemoradiation was delivered (45.0–50.4 Gy plus 5-fluorouracil/leucovorin) and radical surgery was performed 6–10 weeks after completion of neoadjuvant therapy. All of the operations were performed by a single surgeon (H.R.K.). All patients underwent standard TME and regional lymphadenectomy, followed by a double-stapling anastomosis. The application of a transanal drainage tube was routinely carried out in operations after January 2013. Following anastomosis formation, a 10-Fr rubber catheter with two or three side holes near the tip was gently inserted into the neorectum via the anus and positioned with the tip 5–10 cm proximal to the anastomosis. In most cases, the tube was removed on the third postoperative day, unless it was spontaneously removed.
Postoperative adjuvant treatment was determined by the attending physician based on the pathologic stage and the general condition of the patient. For patients with stage II rectal cancer with high-risk features for systemic recurrence, or stage III rectal cancer, 5-fluorouracil-based postoperative adjuvant treatment was recommended. For patients who received neoadjuvant chemoradiation, postoperative adjuvant chemotherapy was recommended for all patients irrespective of surgical pathology results. For mid-to-low rectal cancer patients with pathologic stage T3-4 or N1-2 who did not receive neoadjuvant therapy, postoperative radiotherapy was also administered.
All patients underwent mechanical bowel preparation with polyethylene glycol. Second-generation cephalosporins were routinely administered just before surgery. Urinary catheters were removed on postoperative day 2 or 3 at the surgeon’s discretion. Pain was managed with opioid-based patient-controlled analgesia and additional nonsteroidal anti-inflammatory drugs. Diet was resumed within 3 days of surgery unless obstructive symptoms such as vomiting occurred. Mainly one Jackson-Pratt drain was inserted around the anastomotic site during the operation and was removed within 5 days following surgery if AL was not definite.
AL was defined as a defect of the intestinal wall at the anastomotic site that results in a communication with the bowel lumen or a pelvic abscess close to the anastomosis [19]. All ALs were confirmed by using rigid sigmoidoscopy, CT, or operative findings. According to the proposal by the International Study Group of Rectal Cancer in 2010 [19], AL was classified into three grades: grade A, requiring no intervention; grade B, requiring active therapeutic intervention without re-laparotomy; and grade C, requiring re-laparotomy. Urgent re-laparotomy was performed if patients showed acute symptoms such as pain, fever, and leukocytosis, with pus or fecal discharge through the pelvic drain or AL confirmed on CT. In patients without acute symptoms, re-laparotomy was considered in cases with pelvic abscess around the anastomosis or rectovaginal fistula at the surgeon’s discretion.
Categorical variables were compared using the χ2 or Fisher’s exact test. Multivariable logistic regression analysis was used to determine independent risk factors for AL. Variables with p < 0.10 on univariable analysis were entered into a logistic regression model. To compensate for the differences in baseline characteristics and to prevent selection bias, we conducted propensity score matching analysis. Propensity scores were derived using binary logistic regression for each patient who had a transanal drainage tube and for each patient who did not, using covariates of age, sex, tumor location, BMI, ASA score, operation time, pathologic stage, tumor size, and preoperative chemoradiation. Subsequently, patients with transanal drainage tube were matched to patients without transanal drainage tube according to propensity scores. All results were considered significant at p < 0.05. Statistical analyses were carried out using SPSS software version 21.0 (IBM Inc., Armonk, NY, USA).
Results
The incidence of AL was 9.3 % (50/536). Most (39/50, 78.0 %) of them underwent re-laparotomy (grade C), while five patients required therapeutic intervention without re-laparotomy (grade B), and six cases were detected incidentally and required no therapeutic intervention (grade A). Among the 38 patients with grade C AL, 26 (68.4 %) had acute symptoms of peritonitis and required urgent surgical intervention, whereas the other 12 patients had rectovaginal fistula (1/12) or pelvic abscess around anastomotic site (11/12) but no definite evidence of peritonitis.
Table 1 shows the risk factors for AL. On univariable analysis, male sex (p = 0.003), long operation time (p = 0.046), and neoadjuvant chemoradiation (p = 0.041) were associated with AL. High ASA score (p = 0.063), large tumor (p = 0.095), and transanal drainage tube (p = 0.078) also tended to be associated with AL. On multivariable logistic regression analysis, male sex (odds ratio [OR] 3.097, 95 % confidence interval [CI] 1.400–6.851, p = 0.005), high ASA score (OR 3.505, 95 % CI 1.170–10.500, p = 0.025), and neoadjuvant chemoradiation (OR 2.506, 95 % CI 1.168–5.375, p = 0.018) were independent risk factors for the occurrence of AL.
Compared to patients without transanal drainage tube, those with transanal tube were younger (p < 0.001), had lower T stage (p < 0.001), smaller tumors (p = 0.042), and shorter operation times (p < 0.001) (Table 2). After propensity score matching, 308 patients were well balanced for variables considered in the propensity score method (Table 2).
In the entire cohort, the incidence of AL in patients with or without transanal drainage tubes showed no difference (5.8 vs. 10.7 %, p = 0.078) (Table 3). There were no differences in AL which needed therapeutic intervention (5.8 vs. 9.2 %, p = 0.205) or re-laparotomy (5.2 vs. 7.9 %, p = 0.278) between the two groups. However, the transanal tube group experienced less grade C AL with definite peritonitis (1.9 %) than the no transanal tube group (6.0 %, p = 0.047). After propensity score matching, the incidence of AL in patients with and without transanal drainage tube was 5.8 and 9.1 %, respectively (p = 0.278). There was a tendency for reduced rates of grade C AL with acute symptoms of peritonitis in patients with transanal drainage tube (1.9 vs. 5.8 %), although this was not statistically significant (p = 0.077).
Table 4 demonstrates the comparison of postoperative complications between the two groups. The overall complication rates were 13.6 and 14.1 %, respectively, which showed no significant difference (p = 0.880). However, in the transanal drainage tube group, one patient experienced iatrogenic colonic perforation due to the transanal tube at postoperative day 5. There was no 30-day mortality in either groups.
Discussion
In the present study, 9.3 % of patients developed AL after low anterior resection. Male sex, high ASA score, and neoadjuvant chemoradiation were independent predictors of AL. We found that transanal drainage tubes tended to be associated with grade C AL with definite peritonitis, although not related to the development of AL.
Anastomotic leakage is one of the most important complications after rectal cancer surgery. It brings about prolonged hospital stays, increased postoperative morbidity and mortality, and increased healthcare costs [4]. Researchers have tried to identify patients who are at high risk of AL in advance of the operation, but there is no clear consensus to date. A recent systematic review categorized the risk factors for AL as follows: nonadjustable preoperative risk factors such as male sex, distal anastomosis, large tumor size, advanced stage, high ASA score, diabetes; potentially adjustable preoperative risk factors such as smoking, obesity, excess alcohol consumption; and intraoperative risk factors such as long operation time, blood loss, and blood transfusion [4]. In the present study, male sex, high ASA score, and neoadjuvant chemoradiation were significant predictors of AL after rectal cancer surgery. This is consistent with the findings of previous studies.
Several studies have suggested surgical techniques that might reduce the incidence of AL after rectal cancer surgery. Fecal diversion is one of the most widely used methods to prevent AL. However, the role of a diverting stoma in preventing AL is still debatable. Some randomized controlled trials reported that diverting stomas could reduce the incidence of symptomatic AL [7, 20], while a recent large multicenter cohort study using propensity score matching analysis demonstrated that diverting stomas were not associated with symptomatic AL [8]. Others have suggested that transanal or intracorporeal reinforcing sutures could effectively reduce AL after low anterior resection, but the results of these studies are not conclusive [10, 11]. We previously explored the impact of fibrin glue on AL and reported that fibrin glue has a protective effect on the anastomosis, without any oncologic advantages [12].
There have also been many studies on intraluminal devices aimed at preventing AL after rectal cancer surgery [21]. Some studies report the use of rectal tubes or transanal rubber drains for transanal decompression, while others utilized transanal stents or colonic prostheses for intracolonic bypass [21]. However, to date, none of these devices have been widely accepted [21]. The transanal drainage tube, which was reported to reduce rectal resting pressure [14], has been studied as a tool for preventing AL after rectal cancer surgery, but the results are inconsistent [13–18], with some studies demonstrating favorable outcomes [14, 17, 18], while other studies reported nonsignificant results [15] or even an increase in AL with transanal tubes [16]. These previous studies had limitations such as lack of control group [13], marked selection bias between the two groups [16, 18], or small sample size [15, 17]. Although Xiao at al. recently reported a randomized controlled trial on the effectiveness of transanal drainage tubes for reducing AL, these results were not reliable because they included patients with sigmoid colon cancer and patients who had undergone hand-sewn anastomoses [14]. A recent meta-analysis concluded that placement of a transanal drainage tube is effective to decrease the rate of AL after an anterior resection [22]. However, nonrandomized design, different inclusion criteria, and selection bias of included studies [14, 15, 17, 18] made it hard to draw solid conclusions. In contrast, the present study has several strengths, namely that we included a homogenous population of rectal cancer patients who underwent low anterior resection without diverting stoma, that our sample size was relatively large, and that we minimized selection bias by using the propensity score matching method. Therefore, we believe that the present study is of value although our results were opposite to the result of the meta-analysis [22].
In the present study, we found that transanal drainage tubes may lessen the clinical severity of AL, preventing the development of generalized peritonitis. The transanal tube functions by keeping the anal sphincter open, thereby decreasing the intraluminal pressure of the neorectum and the anastomosis [21]. We believe that the more favorable clinical course was owing to the decrease in the intraluminal pressure of the neorectum caused by the transanal tube. However, we also encountered a case of iatrogenic colonic perforation at postoperative day 5. The patient underwent urgent re-laparotomy, and we found a 1-cm-sized perforation at the right lateral side of the neorectum, 10 cm proximal to the anastomosis, which was thought to be caused by the transanal drainage tube. We performed massive irrigation, primary repair, and diverting ileostomy. A previous study also reported a similar complication in that they experienced perforation by the transanal tube at the posterior wall of neorectum [17]. Therefore, we should be mindful of this possible complication when applying a transanal drainage tube.
Our study has several limitations. First, the retrospective study design might result in selection bias, although we utilized propensity score matching analysis to minimize this. Most of the applications of the transanal drainage tube were done in the later period of the study. A possibility exists that the accumulated experience of the surgeon might bring about the decrease in AL in the later period. However, all of the surgeries were performed by a single skilled surgeon who already had experience of more than 50 cases of rectal cancer surgery before the study period. Considering that the cutoff point of the learning curve for laparoscopic TME is suggested to be 50 cases [23], we believe that the surgeon’s experience had very little effect on the main result. Next, we used a different diameter of drainage tube (10 Fr) with a different protocol (maintained for 3 days after surgery) from previous studies (26–28 Fr catheters for 5–7 days) [15, 17]. The differences in the tube diameter and the duration of its application may have influenced the conflicting results. Finally, not all patients were routinely screened for AL by CT, which could affect the results by missing some patients with grade A AL.
In summary, placement of a transanal drainage tube may lessen the grade C AL with definite peritonitis and may have a positive effect on the clinical course, although it was not associated with a reduction in the total incidence of AL after low anterior resection for rectal cancer. Well-designed randomized controlled trials are needed to draw a solid conclusion on this issue.
References
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
Kim NK, Kim YW, Min BS, Lee KY, Sohn SK, Cho CH (2009) Operative safety and oncologic outcomes of anal sphincter-preserving surgery with mesorectal excision for rectal cancer: 931 consecutive patients treated at a single institution. Ann Surg Oncol 16:900–909
Shirouzu K, Ogata Y, Araki Y (2004) Oncologic and functional results of total mesorectal excision and autonomic nerve-preserving operation for advanced lower rectal cancer. Dis Colon Rectum 47:1442–1447
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102:462–479
Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H (2013) Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 257:665–671
Frasson M, Flor-Lorente B, Ramos Rodriguez JL, Granero-Castro P, Hervas D, Alvarez Rico MA, Brao MJ, Sanchez Gonzalez JM, Garcia-Granero E (2015) Risk factors for anastomotic leak after colon resection for cancer: multivariate analysis and nomogram from a multicentric, prospective, national study with 3193 patients. Ann Surg 262:321–330
Matthiessen P, Hallbook O, Rutegard J, Simert G, Sjodahl R (2007) Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 246:207–214
Shiomi A, Ito M, Maeda K, Kinugasa Y, Ota M, Yamaue H, Shiozawa M, Horie H, Kuriu Y, Saito N (2015) Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg 220:186–194
Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, Rutten HJ, van de Velde CJ (2005) Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 92:211–216
Baek SJ, Kim J, Kwak J, Kim SH (2013) Can trans-anal reinforcing sutures after double stapling in lower anterior resection reduce the need for a temporary diverting ostomy? World J Gastroenterol 19:5309–5313
Maeda K, Nagahara H, Shibutani M, Ohtani H, Sakurai K, Toyokawa T, Muguruma K, Tanaka H, Amano R, Kimura K, Sugano K, Ikeya T, Iseki Y, Hirakawa K (2015) Efficacy of intracorporeal reinforcing sutures for anastomotic leakage after laparoscopic surgery for rectal cancer. Surg Endosc Published Online First: 12 February 2015. doi: 10.1007/s00464-015-4104-2
Kim HJ, Huh JW, Kim HR, Kim YJ (2014) Oncologic impact of anastomotic leakage in rectal cancer surgery according to the use of fibrin glue: case-control study using propensity score matching method. Am J Surg 207:840–846
Sterk P, Schubert F, Gunter S, Klein P (2001) Anastomotic protection with a transanal tube after rectum resection and total mesorectal excision. Zentralbl Chir 126:601–604
Xiao L, Zhang WB, Jiang PC, Bu XF, Yan Q, Li H, Zhang YJ, Yu F (2011) Can transanal tube placement after anterior resection for rectal carcinoma reduce anastomotic leakage rate? A single-institution prospective randomized study. World J Surg 35:1367–1377
Zhao WT, Hu FL, Li YY, Li HJ, Luo WM, Sun F (2013) Use of a transanal drainage tube for prevention of anastomotic leakage and bleeding after anterior resection for rectal cancer. World J Surg 37:227–232
Cong ZJ, Fu CG, Wang HT, Liu LJ, Zhang W, Wang H (2009) Influencing factors of symptomatic anastomotic leakage after anterior resection of the rectum for cancer. World J Surg 33:1292–1297
Nishigori H, Ito M, Nishizawa Y, Nishizawa Y, Kobayashi A, Sugito M, Saito N (2014) Effectiveness of a transanal tube for the prevention of anastomotic leakage after rectal cancer surgery. World J Surg 38:1843–1851
Hidaka E, Ishida F, Mukai S, Nakahara K, Takayanagi D, Maeda C, Takehara Y, Tanaka J, Kudo SE (2015) Efficacy of transanal tube for prevention of anastomotic leakage following laparoscopic low anterior resection for rectal cancers: a retrospective cohort study in a single institution. Surg Endosc 29:863–867
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Buchler MW (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147:339–351
Chude GG, Rayate NV, Patris V, Koshariya M, Jagad R, Kawamoto J, Lygidakis NJ (2008) Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure? A prospective randomized study. Hepatogastroenterology 55:1562–1567
Morks AN, Havenga K, Ploeg RJ (2011) Can intraluminal devices prevent or reduce colorectal anastomotic leakage: a review. World J Gastroenterol 17:4461–4469
Shigeta K, Okabayashi K, Baba H, Hasegawa H, Tsuruta M, Yamafuji K, Kubochi K, Kitagawa Y (2015) A meta-analysis of the use of a transanal drainage tube to prevent anastomotic leakage after anterior resection by double-stapling technique for rectal cancer. Surg Endosc Published Online First: 20 June 2015. doi: 10.1007/s00464-015-4237-3
Bege T, Lelong B, Esterni B, Turrini O, Guiramand J, Francon D, Mokart D, Houvenaeghel G, Giovannini M, Delpero JR (2010) The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution’s experience. Ann Surg 251:249–253
Conflicts of interest
The authors received no financial support for this study. The authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was exempted because this retrospective study was harmless to the patients and contained no personal data. It was approved by the institutional review board of our institution.
Authors’ contributions
H.R.K. was responsible for the study conception and design. S.Y.L. and C.H.K. contributed to the acquisition of data; S.Y.L., C.H.K., and Y.J.K. to the analysis and interpretation of data; and S.Y.L. and H.R.K. to the drafting of the manuscript. Y.J.K. and H.R.K. were responsible for the critical revision of manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.Y., Kim, C.H., Kim, Y.J. et al. Impact of anal decompression on anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis. Langenbecks Arch Surg 400, 791–796 (2015). https://doi.org/10.1007/s00423-015-1336-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1336-5