Abstract
Introduction
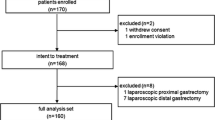
Total gastrectomy is the standard treatment for tumours arising in the proximal stomach and for diffuse cancer according to the Lauren classification. Laparoscopic approach is progressively accepted and provides encouraging results. In order to reduce complications associated to the esophago-jejunal anastomosis, the concept of the 95 % open gastrectomy was developed in Japan, in the early 1980s. This procedure provides the spearing of a small remnant gastric stump of 2 cm and allows performing a gastro-jejunal anastomosis. Unlike the 7/8 gastrectomy, the 95 % gastrectomy allows the complete resection of the gastric fundus and an optimized pericardial lymph node dissection (group 1 and 2). We herein describe, step-by-step, our technique of full laparoscopic 95 % gastrectomy (G95 %), with D2 lymphadenectomy, including complete lymphadenectomy of the cardial nodes.
Discussion
When it is possible to respect the oncologic criteria regarding proximal resection margin, 95 % gastrectomy would offer best short-term results, such as lower anastomotic leak rate and a better quality of life, limiting the effect of disruption of the eso-gastric junction.
Conclusion
In selected patients, laparoscopic G95 % is feasible and safe; it could be performed without any additional technical difficulties. Controlled clinical trials are necessary to confirm the encouraging results of the cases series, recently reported in literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total gastrectomy (TG) is the standard treatment for the tumours arising in the proximal stomach and in all localisations of diffuse type adenocarcinomas, according to Lauren classification (1).
Although the laparoscopic approach is validated for the treatment of the early gastric cancer (EGC), some authors have expanded the indications to the advanced gastric cancer (AGC), confirming the benefits of the less invasive approach in the short-term, without compromising the oncological prognosis of patients (2–8).
However, the leakage rate of esophago-jejunal anastomosis (EJA) remains high, with prevalence between 5 and 17 % out of all TG for cancer, considering both open and laparoscopic approaches (9–11).
In order to reduce the complications associated to the EJA, the concept of a near-total gastrectomy was developed in the early 1980s (12–14).
This technique provides the resection of approximately 95 % of the stomach, preserving a small gastric stump of 2 cm. It allows the performing of a gastro-jejunal anastomosis, instead of an EJA, thus reducing the anastomotic leak rate (14–16).
Unlike the 7/8 gastrectomy, the 95 % gastrectomy (G95 %) provides a complete resection of the gastric fundus and a complete dissection of lymph node stations 1 and 2 (Fig. 1).
In order to improve the benefit of laparoscopy, our team adopted the principles of G95 %, modifying the technique of the standard full laparoscopic TG, which we have been performing since 1993 (1).
We herein describe our technique of full laparoscopic G95 %, with D2 lymphadenectomy, including complete lymphadenectomy of the cardial nodes (Fig. 1), the same as it has been previously described for the full laparoscopic TG (17, 18).
Operative technique
Trocar positioning
The patient is placed in a slight Trendelenburg position with legs apart (Fig. 2). The surgeon is placed between the patient’s legs, with an assistant on each side. Five trocars are placed in an arch around a sixth trocar, which is placed under the xyphoid.
A 0° telescope is placed through the supra-umbilical port for the mid-abdominal dissection and then through the epigastric port for the upper abdominal dissection (Fig. 3).
Perigastric lymphadenectomy
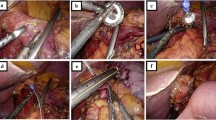
After conducting a complete exploration of the abdominal cavity and of the liver surface, the procedure begins with the section of the gastro-colic ligament, 3–4 cm above the gastro-epiploic vessels, in order to achieve a complete lymphadenectomy of the 4d, 4sb and 4sa lymph node stations (Fig. 4(1)). The access to the lesser cavity is then achieved. The dissection of the adhesions of the posterior aspect of the stomach with the pancreatic capsula allows the mobilization of the antral region of the stomach. A firm grasp on the posterior gastric wall allows the antrum to be toppled upwards.
The gastro-epiploic vessels are divided between clips at their origin, as close as possible to Henle’s trunk and gastroduodenal artery (dissection of the sixth lymph node station). The section of the gastro-epiploic pedicle allows correct exposure of the posterior aspect of the pylorus, and thus, we are able to obtain the retro-duodenal tunnel (Fig. 4(2–4)). The gastroduodenal artery remains in place and it is dissected upward towards the hepatic artery.
The junction between the pars-condensa and the pars-flaccida of the hepato-duodenal ligament is opened, reaching the retro-duodenal dissection (Fig. 4(5)). The right gastric artery is followed and sectioned at its origin from the hepatic artery during the dissection of the fifth lymph node station (Fig. 4(6)). The duodenal bulb, fully dissected, is transected, 2 cm distally to the pylorus, with a 60-mm Echelon Flex® linear stapler (Ethicon Endo-Surgery, Cincinnati, OH, USA) with a blue cartridge of 1.5 mm (Fig. 4(7–8)).
The dissection of the pars flaccida of the lesser omentum continues, as close as possible to the liver, until reaching the right diaphragmatic pillar, where the right paracardial lymph node station (group 1) is dissected.
Lymphadenectomy of the hepatic artery and celiac trunk
The stomach is then tilted to the left in order to expose the hepatic pedicle. The proper hepatic artery is surrounded by a vessel loop used as a non-traumatic retractor (Fig. 4(9–10)). The lymphadenectomy of the hepatic artery (lymph node stations 8a and 8p) is completed with the cranial dissection of the 12a group (Fig. 4: 10–11). The lymph node package thus obtained (8a, 8p and 12a) is tilted medially and the vascular dissection is continued proximally to reach the 9 and the 11p lymph node stations (Fig. 4(12)). The left gastric artery is dissected and sectioned at its origin between clips. The dissection of the seventh group is completed with the section of the left gastric vein, using a Hem-o-lok® Ligation System (Teleflex®, USA) (Fig. 4(13–16)).
Paracardial lymphadenectomy
The dissection continues cranially along the left diaphragmatic pillar, and the second lymph node station is reached, first through a posterior approach and then anteriorly during the dissection of the angle of Hiss.
The right paracardial lymph node station (group 1) was previously removed during the dissection of the pars flaccida of the lesser omentum (Fig. 4(19)).
Unlike what is usually done in the TG procedure, the anterior aspect of the phrenoesophageal membrane is not opened and the distal esophagus is not dissected, in order to spear the vascularization of the gastroesophageal junction (Fig. 4(20)). The arterial supply to the cardia can originate from the esophageal branches of the left gastric artery or from the left inferior diaphragmatic artery (as is shown in the Fig. 4(20)) or both of phrenic and gastric artery.
Gastric section
After the section of the gastro-splenic vessels, the stomach is transected from right to left, 2 cm below the cardia using an Echelon Flex® (Ethicon Endo-Surgery, Cincinnati, OH, USA) linear stapler with a 1.5-mm blue cartridge (Fig. 4(17–18)). A second longitudinal stapling line parallel to the lesser curvature could be calibrated with the help of a 34F gastric tube, drawing a small gastric pouch, about 2-cm high and 3-cm wide (Fig. 4(19–20)).
Omentectomy
In the absence of peritoneal carcinomatosis, no factual evidence exists concerning the modality and the extension of omentectomy. However, total omentectomy is widely associated to a gastrectomy with D2 lymphadenectomy for cancer even though partial omentectomy seems to have encouraging preoperative and postoperative results in patients with EGC (19–21).
At the moment, our team carries out a total omentectomy after the gastrectomy, because of the lack of evidence concerning the benefit of a partial omentectomy, in patients with AGC.
Roux-en-Y jejunal limb
The restoration of intestinal continuity is conventionally performed through a “Roux-en-Y” jejunal limb. The alimentary loop is chosen at the point where there is minimal mesenteric traction: between 20 and 40 cm distal to the ligament of Treitz. The section is done using an Echelon Flex® (Ethicon Endo-Surgery, Cincinnati, OH, USA) linear stapler with a 1.5-mm blue cartridge (Fig. 4(21)). The jejunal mesentery is partially divided for about 3 to 4 cm to facilitate the ascent of the alimentary loop without traction on the mesentery. The jejuno-jejunal anastomosis at the base of the 60-cm alimentary loop is a wide and side-to-side mechanical anastomosis, with an Echelon Flex® (Ethicon Endo-Surgery, Cincinnati, OH, USA) linear stapler with a 1.5-mm blue charger (Fig. 4(21–23)).
The enteral defect is then closed with a running suture using a barbed self-locking V-Loc ® suture (Covidien, USA) (Fig. 4(22)). This anastomosis can be performed after gastrointestinal anastomosis, depending on the preferences.
The transmesocolic crossing
The transverse mesocolon is opened 1 cm above the ligament of Treitz, in its thinnest region (Fig. 4(24)). The alimentary loop is ascended approximately 15 cm. The mesocolic orifice can be closed using a continuous running suture, including the ascended loop, with non-reabsorbable material.
Gastrointestinal anastomosis
The gastro-jejunal anastomosis is a manual end-to-side anastomosis using a knotless triple running suture with 3/0 V-Loc® (Covidien, USA). We perform two posterior and one anterior sutures (Fig. 4(23)). The assistant on the right side holds the jejunal loop by gentle traction towards the left upper quadrant. The first posterior suture includes all sero-muscular layers, both on the jejunal and the gastric side. The stapling line is included in this running suture. The use of a self-locking suture facilitates exposure (22, 23) and does not require a constant traction by the assistant (Fig. 4(25)).
The small intestine is opened above the first suture and the mucosa can be cauterized for haemostasis. The stomach is opened in the same way, with the help of the pressure induced by the gastric tube. After this step, the gastric tube is completely removed (Fig. 4(26)). A second continuous running suture with 3/0 V-Loc® (Covidien, USA) is then carried out to strengthen the posterior plane, to improve haemostasis on the mucosa and to bury the protruding mucosa (Fig. 4(27)). The suture is tightened with the help of the assistant on the right side of the operator to expose the margins of the two digestive segments.
The anterior running suture of this anastomosis is also done with the same V-Loc self-locking system. The suture is started from the left, in contact with the first point of the posterior suture taking large extramucosal steps on the jejunal mucosa and loading the entire wall of the stomach on the gastric side of the suture (Fig. 4(28)). The left grasper of the surgeon is placed in the stomach to keep it open during the needle passages through the gastric wall.
Drainage
A retro-anastomotic Penrose drain is placed and the specimen is extracted through a small Pfannenstiel incision. A radiologic study is routinely performed at the seventh post-operative day (Fig. 5).
Discussion
Laparoscopic gastrectomy is a feasible and reproducible technique for locally advanced gastric cancer. Its advantages are the usual ones of the laparoscopic approach (improved post-operative recovery, avoiding unnecessary laparotomy, fewer wall complications) without compromising the oncological standards (2, 24, 25).
Since the first description of the full laparoscopic gastrectomy for gastric cancer, in 1993 (1), additional technical modifications have been developed.
The major controversies regard eso-jejunal anastomosis, especially regarding its high prevalence of anastomotic leak (AL) rate: between 5 and 17 %, out of all TG for cancer (9–11, 26).
Furthermore, TG may result in post-operative complications such as heartburn, dysphagia, bitter taste and regurgitation, which suggest the presence of reflux esophagitis due to disruption of the gastroesophageal junction (27, 28).
On the other hand, distal gastrectomy (DG), usually indicated in cancers of the distal third of the stomach, is associated with a significantly lower rate of anastomotic leak and better outcomes, at short- and long-term (5).
Thus, DG should be performed when it is possible to achieve the current oncological adequacy, regarding the proximal surgical margins (5).
In this regard, the distance between the proximal gastric section and the upper pole of the tumour remains on a controversial debate. Currently, most of the authors consider 5 cm as a safety distance from the proximal pole of the tumour, in the cases of AGC, and 3 cm for EGC (10, 29–32).
Respecting those conditions, in the current practice, the opportunity to choose a DG is frequently compromised, and a TG is often mandatory. This is particularly true in the Western countries where AGC is more frequent than EGC.
The recent cases series, reported by Jiang and Kim, shows encouraging results of G95 %, which permit to spread a small gastric stump, even when DG is not feasible for the impossibility to respect the adequate oncological margins. Those preliminary experiences with the laparoscopic approach were made with population affected by EGC. More ancient reports describe the open G95 %, performed in a wide panel of indications such as early gastric cancers that have widely spreading, single or multiple lesions, curative or palliative resection of selected patients with advanced gastric cancer and post-vagotomy syndrome (12–16, 33).
Currently, according to the data available in the literature, the oncological indications for the G95 % are very rigorous and may include selected cases of AGC: only in case of intestinal-type neoplasm, according with the Lauren classification, and when it is possible to achieve a margin of more than 5 cm between the proximal gastric section and the upper pole of the tumour. In case of EGC, this margin could be reduced at 3 cm. The negative margins should be verified by the examination of proximal margin frozen section (29, 30).
G95 % could also be employed in palliative resections, severe post-vagotomy syndrome or multi-organ resections for non-gastric neoplasm invading the proximal stomach (12–16, 33).
In all the reported series, G95 % provides an AL rate, estimated to be between 0 and 5 % (12–16, 33).
This lower AL rate could be explicated by the minimisation of the dissection of the gastroesophageal junction respecting the vascular supply of the cardia and providing a gastro-jejunal anastomosis instead of an eso-jejunal anastomosis.
Unfortunately, it is impossible to compare the AL rate of TG and G95 % in terms of evidence-based medicine, because literature lacks in adequate controlled trials and nowadays, just some case series are available.
Conclusion
In selected patients, while respecting the actual oncologic criteria regarding proximal resection margins, laparoscopic G95 % could offer the best short-term results in terms of anastomosis-related complications and early post-operative course, without any additional technical difficulties.
Controlled clinical trials are necessary to confirm that the laparoscopic approach could emphasize the minimal invasiveness of this surgical procedure, without affecting the long-term oncological outcome.
References
Azagra JS, Goergen M, De Simone P, Ibañez-Aguirre J (1999) Minimally invasive surgery for gastric cancer. Surg Endosc 13:351–357
Azagra JS, Ibañez-Aguirre JF, Goergen M, Ceuterick M, Bordas-Rivas JM, Almendral-López ML, Moreno-Elola A, Takieddine M, Guérin E (2006) Long-term results of laparoscopic extended surgery in advanced gastric cancer: a series of 101 patients. Hepatogastroenterology 53:304–308
Ohtani H, Tamamori Y, Noguchi K, Azuma T, Fujimoto S, Oba H, Aoki T, Minami M, Hirakawa KJ (2010) A meta-analysis of randomized controlled trials that compared laparoscopy assisted and open distal gastrectomy for early gastric cancer. Gastrointest Surg 14:958–964
Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R (2013) Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc 27:1509–1520
Mocan L, Tomus C, Bartos D, Zaharie F, Ioana I, Bartos A, Puia C, Necula A, Mocan T, Iancu C (2013) Long term outcome following surgical treatment for distal gastric cancer. J Gastrointestin Liver Dis 22:53–58
Lee MS, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK (2013) Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc 27(7):2598–2605
Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251(3):417–420
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123. doi:10.4172/2161-069×.S1-004
Ben Maamer A, Zaafouri H, Noomene R, Haoues N, Bouhafa A, Oueslati A, Cherif A (2013) Predictive factors of esophagojejunal fistula after total gastrectomy in gastric cancer patients. Tunis Med 91(4):263–268
Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G (2010) Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc 24(10):2475–2479
Liakakos T (2011) Totally laparoscopic total gastrectomy and the challenge of esophagojejunostomy. Surg Endosc 25(10):3468–3469
Saario I, Salo J, Lempinen M, Kivilaakso E (1987) Total and near-total gastrectomy for gastric cancer in patients over 70 years of age. Am J Surg 154(3):269–270
Takagi H, Morimoto T (1984) Near-total gastrectomy. J Surg Oncol 26(1):14–16
Salo JA, Saario I, Kivilaakso EO, Lempinen M (1988) Near-total gastrectomy for gastric cancer. Am J Surg 155(3):486–489
Jiang X, Hiki N, Nunobe S, Nohara K, Kumagai K, Sano T, Yamaguchi T (2011) Laparoscopy-assisted subtotal gastrectomy with very small remnant stomach: a novel surgical procedure for selected early gastric cancer in the upper stomach. Gastric Cancer 14:194–199
Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS (2013) Intracorporeal laparoscopic Roux-en-Y gastrojejunostomy after 95 % gastrectomy for early gastric cancer in the upper third of the stomach: a report on 21 cases. J Laparoendosc Adv Surg Tech A 23(3):250–257
Azagra JS, Goergen M, Arru L, Facy O (2013) Total gastrectomy for locally advanced cancer: the pure laparoscopic approach. Gastroenterol Rep 1(2):119–126
Ibáñez FJ, Azagra JS, Goergen M, Bordas JM, Almendral ML, Erro JM (2005) Cirugía laparoscópica del cáncer gástrico. An Sist Sanit Navar 28(3):21–31
Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T, Tatematsu M (2002) Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer 5(2):69–76
Hagiwara A, Sawai K, Sakakura C et al (1998) Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepatogastroenterology 45(23):1922–1929
Kim MC, Kim KH, Jung GJ, Rattner DW (2011) Comparative study of complete and partial omentectomy in radical subtotal gastrectomy for early gastric cancer. Yonsei Med J 52(6):961–966
Facy O, De Blasi V, Goergen M, Arru L, De Magistris L, Azagra JS (2013) Laparoscopic gastrointestinal anastomoses using knotless barbed sutures are safe and reproducible: a single-center experience with 201 patients. Surg Endosc 27:3841–3845
Facy O, Arru L, Azagra JS (2012) Intestinal anastomosis after laparoscopic total gastrectomy. J Visc Surg 149:e179–e184
Strong VE, Devaud N, Karpeh M (2009) The role of laparoscopy for gastric surgery in the west. Gastric Cancer 12:127–131
Yang Z, Zheng Q, Wang Z (2008) Meta-analysis of the need for nasogastric or nasojejunal decompression after gastrectomy for gastric cancer. Br J Surg 95:809–816
Lang H, Piso P, Stukenborg C, Raab R, Jähne J (2000) Management and results of proximal anastomotic leaks in a series of 1114 total gastrectomies for gastric carcinoma. Eur J Surg Oncol 26(2):168–171
Matei D, Dadu R, Prundus R, Danci I, Ciobanu L, Mocan T et al (2010) Alkaline reflux esophagitis in patients with total gastrectomy and Roux en Y esojejunostomy. J Gastrointestin Liver Dis 19:247–252
Wei HB, Wei B, Zheng ZH, Zheng F, Qiu WS, Guo WP et al (2008) Comparative study on three types of alimentary reconstruction after total gastrectomy. J Gastrointest Surg 12:1376–1382
Bozzetti F, Bonfanti G, Bufalino R, Menotti V, Persano S, Andreola S, Doci R, Gennari L (1982) Adequacy of margins of resection in gastrectomy for cancer. Ann Surg 196(6):685–690
Shin D, Park S-S (2013) Clinical importance and surgical decision-making regarding proximal resection margin for gastric cancer. World J Gastrointest Oncol 5(1):4–11
Squires Iii MH, Kooby DA, Poultsides GA, Pawlik TM, Weber SM, Schmidt CR, Votanopoulos KI, Fields RC, Ejaz A, Acher AW, Worhunsky DJ, Saunders N, Levine EA, Jin LX, Cho CS, Bloomston M, Winslow ER, Russell MC, Cardona K, Staley CA, Maithel SK (2014) Is It time to abandon the 5-cm margin rule during resection of distal gastric adenocarcinoma? A multi-institution study of the U.S. Gastric Cancer Collaborative. Ann Surg Oncol. doi:10.1245/s10434-014-4138-z
Ohe H, Lee WY, Hong SW, Chang YG, Lee B (2014) Prognostic value of the distance of proximal resection margin in patients who have undergone curative gastric cancer surgery. World J Surg Oncol 12:296. doi:10.1186/1477-7819-12-296
Forstner-Barthell AW, Murr MM, Nitecki S, Camilleri M, Prather CM, Kelly KA, Sarr MG (1999) Near-total completion gastrectomy for severe postvagotomy gastric stasis: analysis of early and long-term results in 62 patients. J Gastrointest Surg 3(1):15–21
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Arru, L., Azagra, J.S., Facy, O. et al. Totally laparoscopic 95 % gastrectomy for cancer: technical considerations. Langenbecks Arch Surg 400, 387–393 (2015). https://doi.org/10.1007/s00423-015-1283-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-015-1283-1