Abstract
Purpose
Limited data suggest that second resections for colorectal cancer metastases may improve survival, but no study has compared surgery with chemotherapy in this setting. Therefore, we retrospectively compared the clinical outcome of potentially resectable patients who received a second metastasectomy with those who did not in our single-centre experience.
Methods
We retrospectively reviewed the clinical records of all patients treated for metastatic colorectal cancer in our centre over a period of 12 years. We selected patients who relapsed after radical resection of metastases from colorectal cancer and were deemed resectable again by our multidisciplinary team. We then compared the clinical outcome of those who received a second operation with those who refused surgery and also evaluated the role of prognostic factors.
Results
We identified 60 patients fulfilling the inclusion criteria. Twenty-nine underwent a second resection and 31 refused surgery. Median overall survival rates were 58.7 and 24.0 months, median times to progression were 14.4 and 6.6 months. Patients who received surgery plus perioperatory chemotherapy (18/29) had a significantly better outcome; 4/29 achieved long-term disease-free survival.
Conclusions
Our study suggests that in highly selected metastatic colorectal cancer patients, a multimodal treatment plan, including a second resection, can achieve longer survival with respect to medical therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies and accounts for 8 % of cancer-related deaths worldwide [1]. Europe is considered a high-incidence region, with more than 400,000 new cases and over 200,000 deaths in 2006. After radical surgery, 20 % of patients with stage II and 50 % with stage III CRC will develop metastatic lesions that in the majority of cases involve the liver. Furthermore, 25 % of patients are diagnosed with stage IV disease. In this setting, chemotherapy prolongs survival to about 2 years [2].
It is now widely accepted that in selected patients hepatic [3] or pulmonary [4] resection can achieve long-term survival and perhaps cure. Currently, studies report a 5-year overall survival (OS) rate of 25–50 % and median OS often over 30 months, results far better than those achieved with chemotherapy alone. This is true only in the case of radical surgery; partial or palliative resection generally provides no benefit in terms of survival.
Nevertheless, the majority of patients will eventually relapse even after radical surgery. In this case, chemotherapy is usually the treatment of choice, although limited retrospective data suggest that a second resection can achieve results comparable to those of initial surgery [5–12]. However, no study has evaluated the results of aggressive multimodal management with respect to outcomes achieved with medical therapy alone. This work aimed to compare these strategies in our single-centre experience.
Patients and methods
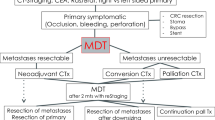
We retrospectively reviewed the prospectively maintained medical records of CRC patients seen in our institution between August 1995 and July 2007. By our policy, patients undergoing radical (i.e. R0) metastasectomy are followed-up every 3–4 months with at least a chest X-ray and an abdominal ultrasound for 2 years, and every 6 months thereafter. Among those who relapsed, we selected a subset fit for a second operation and with radically resectable lesions, as assessed by our multidisciplinary team of medical oncologists and surgeons.
All patients had been evaluated on an individual basis after disease staging by contrast-enhanced computed tomography (CT) scan of the chest, abdomen, pelvis and other sites if clinically indicated. In case of diagnostic doubts, positron-emission tomography or contrast-enhanced ultrasonography was performed if available. Patients were considered candidates for repeat surgery if all the lesions could be resected with radical intent (i.e. with an adequate safety margin). Furthermore, in case of liver surgery, a remnant liver parenchyma of at least 30 % was required. In case of lung surgery, resectability was assessed after pulmonary function testing (spirometry and diffusing capacity for carbon monoxide). A thorough evaluation of comorbidities and life expectancy was also performed.
For the purpose of the present study, right hemihepatectomy was defined as anatomical resection of liver segments V, VI, VII and VIII; left hemihepatectomy as anatomical resection of liver segments II, III and IV; liver segmentectomy as anatomical resection of an entire liver segment. Pulmonary lobectomy was defined as anatomical resection of an entire pulmonary lobe. Resections of less than an entire liver segment or pulmonary lobe were considered non-anatomical resections.
All candidates for second resection, lacking strong evidence supporting aggressive management, had been exhaustively informed about both therapeutic options (multimodal treatment plan including surgery or chemotherapy alone) and the specific risks of the surgical procedure, following our centre’s policy. The final decision was left to the patient, who was requested to sign a written informed consent form, in accordance with institutional guidelines. Some patients who agreed to surgery also received perioperatory chemotherapy for about 6 months.
In case of further relapses amenable for surgery, the same therapeutic approach was followed. Patients who did not accept surgery received medical therapy only, with restaging CT scans and serum markers (carcinoembryonic antigen (CEA), CA19.9) every 3–4 months of treatment. Once the others became disease-free, they were followed-up according to the guidelines detailed above; further imaging studies, including CT scan, were performed upon clinical indication.
Statistical analysis was performed with KyPlotTM 2.0 software (KyensLab Incorporated, Tokyo, Japan) running on the Windows® XP operating system (Microsoft Corporation, Redmond, WA, USA). The tool and related methodology have been described elsewhere [13]. Patients’ characteristics in the two groups were compared by Pearson’s chi-square test, Student’s t test or Kolmogorov–Smirnov test as appropriate. The primary objective was to compare overall survival, estimated by the Kaplan–Meier method, between patients who underwent a second metastasectomy and those who did not, computed from first evidence of relapse after initial surgery for metastatic disease (intention to treat analysis). Secondary objectives were to compare progression-free survival and identify prognostic factors and patient subgroups that gained more benefit from each treatment modality. In particular, patients were stratified by AJCC stage at diagnosis (metastatic or not), number of lesions at second relapse (one or more), disease volume (less than 10 cm3 or more) and perioperatory chemotherapy (only in the second surgery group); differences were assessed by log-rank test. The independent prognostic value of the factors identified was assessed by multivariate analysis using the Cox proportional hazards model.
Results
Patient characteristics
We identified 105 patients who underwent radical resection of metastases from colorectal cancer. Seventy-five patients were evaluated for second surgery at relapse. Of these, 60 had potentially resectable disease and, after thorough discussion, 29 chose to undergo a second metastasectomy. Eighteen of these patients also received perioperatory medical treatment with fluoro-folate plus irinotecan (eight patients) or fluoro-folate plus oxaliplatin (ten patients; one of these had actually been scheduled for FOLFOXIRI, but irinotecan was withheld after the first cycle because of severe gastrointestinal toxicity). Medical treatment was usually administered for 3 months before and 3 months after surgery. Responses, evaluated per RECIST 1.0 criteria, were as follows: no complete responses, 5 partial responses, 10 stable disease and 3 progressive disease. No patient progressed to an extent preventing surgery with radical intent during preoperatory therapy.
The remaining 31 patients did not receive a second resection. Nineteen patients declined surgery because a benefit was not demonstrated, nine preferred not to face the potential morbidity and mortality associated with a second resection and the remaining three did not give reason for their decision. Except for a single patient who refused any therapy, all other patients were treated with a fluoro-folate-based regimen until progression, unacceptable toxicity or patient’s refusal. No prolonged treatment breaks were allowed. Upon progression, the patients were offered further medical treatment if a reasonable benefit was expected.
Patients’ characteristics are summarized in Table 1. Both groups are comparable, in particular with respect to age (p = 0.11) and time to relapse from first surgery for metastatic disease (p = 0.88). On average, patients in the chemotherapy group received two treatment lines for advanced disease (range, 1–5), detailed in Table 2, which included at least oxaliplatin or irinotecan except two patients. Sixteen of these received further treatments beside the first two lines.
Table 3 shows the first resections for metastatic disease performed in both groups. The second resections (performed or planned in case of the no surgery group) are shown in Table 4 and did not differ significantly between the two groups. Surgical margin status was positive in 4/29 patients. On average, 1.7 metastasectomies beyond the first one (range, 1–7) were performed in this group, since ten patients underwent further resections after second relapse. When surgery was no longer feasible or accepted, most of them received chemotherapy for advanced disease.
Overall survival
After a median follow-up of 77.7 months, 40 survival events were observed, 16 in the second surgery group and 24 in the chemotherapy group. Median OS, evaluated since relapse after first metastasectomy, was 58.7 months (95 % confidence interval (CI), 35.2–82.2 months) in the surgery arm and 24.0 months (95 % CI, 16.7–31.3 months) in the chemotherapy group for 5-year survival rates of 48.9 % and 11.4 %, respectively. The difference observed is highly significant (p < 0.001). Among patients in the second surgery group, those who did not receive perioperatory therapy had a significantly worse survival rate (30.3 months, p = 0.003). Kaplan–Meier plots are depicted in Fig. 1.
Among the prognostic factors, only a second metastasectomy was significantly associated with survival both at univariate and multivariate analysis (hazard ratio for death, 0.3; p = 0.002 at multivariate analysis), as seen in Table 5. No treatment-related death occurred in either group.
Progression-free survival
Fifty-five progression events were observed, 25 in the second surgery group and 30 in the chemotherapy group. Median time to progression (or relapse in case of a second surgery) was 14.4 months (95 % CI, 3.5–25.3 months) and 6.6 months (95 % CI, 4.3–8.8 months), respectively; the difference observed is statistically significant (p < 0.001). The number of lesions at relapse, total volume of lesions and stage at diagnosis were not significantly associated with prognosis. These data were also confirmed by multivariate analysis, shown in Table 6.
Among the patients who underwent a second resection, those who received perioperatory therapy had a significantly better time to progression (21.6 months; 95 % CI, 0–49.6 months) than the others (6.7 months; 95 % CI, 0–13.4 months; p = 0.002). Interestingly, all four patients who did not experience disease relapse are in this good-prognosis group. Kaplan–Meier plots of progression-free survival are shown in Fig. 2.
Discussion
Many retrospective studies show that radical resection of hepatic metastases from colorectal cancer is associated with improved survival among selected patients. Therefore, it is agreed that when a radical resection is feasible, this strategy should be considered despite the absence of prospective randomized studies [2, 14]. Likewise, in selected cases, patients with lung metastases also benefit from an aggressive therapeutic strategy including surgery, although this is the subject of some controversy [4, 15]. Some retrospective reports suggest that in some instances metastases to other sites can also be radically resected achieving long-term survival [16].
Most studies identified factors significantly related to prognosis, although there is no consensus on their relevance. Therefore, a surgical indication is generally evaluated on an individual basis, taking into account many factors such as disease-free interval, disease burden, age, comorbidity, response to chemotherapy, probability of radical resection and type of surgical procedure required. This complex evaluation should ideally be made by a multidisciplinary team.
However, most patients will eventually relapse even after radical surgery. This situation is becoming increasingly common with the growing number of metastasectomies performed. Optimal management is debated since it is not known whether a second resection, when feasible, is beneficial with respect to chemotherapy alone. Especially in the case of R0 surgery, a relapse means that the disease is no longer limited but likely systemic and therefore the chance of obtaining a second long-term remission is low. If this were the case, a systemic treatment would be necessary to achieve disease control regardless of surgery. Thus, the fact that initial hepatic resections afford a survival advantage cannot be uncritically transferred to further resections. Moreover, modern chemotherapy regimens achieve good survival rates in this setting [17, 18] and are associated with lower morbidity than major surgery.
Several retrospective studies, summarized in Table 7, evaluated the survival of 819 patients who underwent a second resection of metastases from colorectal cancer, reporting interesting results and good 3-year overall survival rates (46–60 %), at least comparable to those achieved with chemotherapy alone [5–12, 19, 20]. In some patients, a third and even a fourth resection has been reported, with a few cases of long disease remission.
A second metastasectomy is associated with low perioperative mortality (6/819 patients, 0.7 %), with six studies reporting no perioperative mortality. No perioperative mortality occurred in our series, further confirming literature data. Another recent retrospective study on the outcome of liver resection for metastatic colorectal cancer reported a better survival in relapsed patients who underwent a second resection with respect to the others, but the disease burden between the groups may be quite different and bias the result. Indeed the work was not specifically tailored to answer this question [21].
Surgery-associated morbidity is more difficult to estimate. Two studies do not report data; the others report a complication rate of 15–28 %, but events might have been assessed differently. In any case, a second resection appears feasible and is likely associated with a complication rate not much higher than a first hepatic metastasectomy [3].
In summary, limited evidence suggests that for some highly selected well-informed patients a second surgical resection for metastasis from colorectal cancer could be a reasonable therapeutic option if potentially radical. No study, however, has compared the outcomes of this aggressive clinical approach with a control group of patients who received only medical therapy. To answer this important question a randomised controlled prospective trial would be required. However, such a study could be challenging and have poor accrual, since patients may prefer to share this important therapeutic choice with their physician and refuse randomisation. Therefore, we designed this retrospective study, the first, to our knowledge, performing a direct comparison.
We report the clinical outcomes of patients who underwent a second (or further) resection and those of patients who received only chemotherapy. The control group comprised resectable patients who refused surgery. This is a possible source of selection bias, but the groups do not significantly differ for many factors reported to have an impact on prognosis after first metastasectomy [22, 23] also including the surgical procedures effectively performed (second surgery group) or planned (no second surgery group).
CEA preoperatory level was not considered among the prognostic factors due to conflicting literature data, as many studies do not associate it with survival [24–29]. A recent work evaluating prognostic scores for patients undergoing liver resections for CRC metastases, some of which do include CEA level, supports its independent prognostic significance for disease-specific survival, but did not evaluate OS. Furthermore, as the authors acknowledge, the clinical value of prognostic scoring systems is still unclear [30].
The resectability rate in our study is indeed high and is likely due to early diagnosis of relapse. In fact, intensive follow-up, including regular imaging studies, is common practice at our centre, although its impact on survival has not been demonstrated.
As previously stated, the expected risks and benefits of each treatment modality (i.e. a second resection for metastatic disease or chemotherapy alone) were discussed with every patient. This could have introduced a further bias, considering that the curing physician’s convictions can lead to partial or unbalanced information and therefore guide the patient’s decision. A recent work, however, reported that most decisions on cancer treatment with uncertain evidence are indeed patient-controlled or shared, while only 15.7 % are physician-controlled [31], suggesting that such influence is negligible.
Although not a primary objective, our study also suggests that perioperatory chemotherapy has an important role. It was associated with significantly better overall survival and disease-free survival, and in some cases, long-term remission was also achieved. These data are consistent with the results of a recent trial which demonstrated an advantage for perioperatory chemotherapy with respect to surgery alone [32]. This approach should therefore be considered in the majority of patients, despite its potential disadvantages [33], also allowing a better selection of patients for resection [34, 35].
The clinical outcomes of the 31 patients in the chemotherapy group (median OS, 24 months) were comparable to data reported in contemporary clinical trials of chemotherapy, suggesting that it represents a reliable control population [18, 36, 37]. This is likely due to the progressive introduction of new drugs and therapeutic schedules with good activity, leading to a meaningful improvement in the prognosis of advanced colorectal cancer patients.
The major limitation of the study is the small number of patients, which reduces its clinical and statistical significance. Inclusion criteria are indeed stringent, and the monocentric design allows a rigorous evaluation of a highly selected patient population homogeneously treated and followed-up, uncommon characteristics among retrospective studies.
In conclusion, this is the first retrospective study to compare the clinical outcomes of colorectal cancer patients treated in the same oncology centre by second surgery for metastatic disease with a control group. The control group comprised of patients fit for surgery who relapsed after radical metastasectomy and refused a second resection even though lesions were deemed resectable with radical intent. The study shows a clear advantage in survival and time to progression, confirmed by multivariate analysis, for the second surgery group: some patients (4/29; 13.8 %) even achieved long-term disease remission. It also suggests that to achieve best results, surgery should be integrated with perioperatory chemotherapy.
Therefore, in highly selected patients relapsing after radical surgery for metastatic colorectal cancer, a second metastasectomy can be considered, especially as part of a multimodal therapeutic strategy. Treatment-related mortality and morbidity are acceptable and counterbalanced by a substantial survival advantage, with some patients experiencing long-term disease remission.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917. doi:10.1002/ijc.25516
Van Cutsem E, Nordlinger B, Cervantes A, Group ESMOGW (2010) Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol 21(Suppl 5):v93–v97
Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M (2006) Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 94:982–999. doi:10.1038/sj.bjc.6603033
Pfannschmidt J, Dienemann H, Hoffmann H (2007) Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 84:324. doi:10.1016/j.athoracsur.2007.02.093
Nordlinger B, Vaillant JC, Guiguet M, Balladur P, Paris F, Bachellier P, Jaeck D (1994) Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. L’Association francaise de chirurgie. J Clin Oncol 12:1491–1496
Fernández-Trigo V, Shamsa F, Sugarbaker PH (1995) Repeat liver resections from colorectal metastasis. Repeat hepatic metastases registry. Surgery 117:296–304
Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, Abascal A, Majno P, Engerran L (1997) Repeat hepatectomy for colorectal liver metastases. Ann Surg 225:51–60, discussion 60–2
Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Moriya Y, Sugihara K (1999) Repeat liver resection for recurrent colorectal liver metastases. Am J Surg 178:275–281
Petrowsky H, Gonen M, Jarnagin W, Lorenz M, DeMatteo R, Heinrich S, Encke A, Blumgart L, Fong Y (2002) Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis. Ann Surg 235:863–871
Ogata Y, Matono K, Hayashi A, Takamor S, Miwa K, Sasatomi T, Ishibashi N, Shida S, Shirouzu K (2005) Repeat pulmonary resection for isolated recurrent lung metastases yields results comparable to those after first pulmonary resection in colorectal cancer. World J Surg 29:363–368. doi:10.1007/s00268-004-7537-7
Nishio H, Hamady ZZR, Malik HZ, Fenwick S, Prasad KR, Toogood GJ, Lodge JPA (2007) Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol 33:729–734. doi:10.1016/j.ejso.2006.07.005
Welter S, Jacobs J, Krbek T, Krebs B, Stamatis G (2007) Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 84:203–210. doi:10.1016/j.athoracsur.2007.03.028
Yoshioka K (2002) Kyplot–a user-oriented tool for statistical data analysis and visualization. Comput Stat 17:425–437
Parks R, Gonen M, Kemeny N, Jarnagin W, D’Angelica M, DeMatteo R, Garden OJ, Blumgart LH, Fong Y (2007) Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg 204:753–761. doi:10.1016/j.jamcollsurg.2006.12.036, discussion 761–3
Fiorentino F, Hunt I, Teoh K, Treasure T, Utley M (2010) Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med 103:60–66. doi:10.1258/jrsm.2009.090299
Mahmoud N, Dunn KB (2010) Metastasectomy for stage iv colorectal cancer. Dis Colon Rectum 53:1080–1092. doi:10.1007/DCR.0b013e3181dcadbc
Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase iii study. J Clin Oncol 26:2013–2019. doi:10.1200/JCO.2007.14.9930
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chien CRC, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417. doi:10.1056/NEJMoa0805019
Miller G, Biernacki P, Kemeny NE, Gonen M, Downey R, Jarnagin WR, D’Angelica M, Fong Y, Blumgart LH, DeMatteo RP (2007) Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg 205:231–238. doi:10.1016/j.jamcollsurg.2007.04.039
Mise Y, Imamura H, Hashimoto T, Seyama Y, Aoki T, Hasegawa K, Beck Y, Sugawara Y, Makuuchi M, Nakajima J, Kokudo N (2010) Cohort study of the survival benefit of resection for recurrent hepatic and/or pulmonary metastases after primary hepatectomy for colorectal metastases. Ann Surg 251:902–909. doi:10.1097/SLA.0b013e3181c9868a
D’Angelica M, Kornprat P, Gonen M, Dematteo RP, Fong Y, Blumgart LH, Jarnagin WR (2011) Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol 18:1096–1103. doi:10.1245/s10434-010-1409-1
Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D (1996) Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 77:1254–1262
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318, discussion 318–21
Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, Adson MA (1992) Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 216:493–504, discussion 504–5
Sugihara K, Hojo K, Moriya Y, Yamasaki S, Kosuge T, Takayama T (1993) Pattern of recurrence after hepatic resection for colorectal metastases. Br J Surg 80:1032–1035
Scheele J, Stang R, Altendorf-Hofmann A, Paul M (1995) Resection of colorectal liver metastases. World J Surg 19:59–71
Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, Yamamoto J, Imamura H (2000) Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg 231:487–499
Ercolani G, Grazi GL, Ravaioli M, Cescon M, Gardini A, Varotti G, Gaudio MD, Nardo B, Cavallari A (2002) Liver resection for multiple colorectal metastases: influence of parenchymal involvement and total tumor volume, vs number or location, on long-term survival. Arch Surg 137:1187–1192
Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto T, Okinaga K (2004) A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg 11:79–83. doi:10.1007/s00534-002-0778-7
Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, Müller SA, Schemmer P, Büchler MW, Weitz J (2009) Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol 16:3279–3288. doi:10.1245/s10434-009-0654-7
Keating NL, Landrum MB, Arora NK, Malin JL, Ganz PA, van Ryn M, Weeks JC (2010) Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol 28:4364–4370. doi:10.1200/JCO.2009.26.8870
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Cutsem EV, Scheithauer W, Gruenberger T, Group EORTCGITC, UK CR, und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO) AL, (AGITG) AGITG, de Cancérologie Digestive (FFCD) FF (2008) Perioperative chemotherapy with folfox4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (eortc intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–1016. doi:10.1016/S0140-6736(08)60455-9
Benoist S, Nordlinger B (2009) The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol 16:2385–2390. doi:10.1245/s10434-009-0492-7
Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y (2003) Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg 7:109–115, discussion 116–7
Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D, Castaing D (2008) Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol 26:1635–1641. doi:10.1200/JCO.2007.13.7471
Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) Folfiri followed by folfox6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237. doi:10.1200/JCO.2004.05.113
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342. doi:10.1056/NEJMoa032691
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandi, G., Corbelli, J., de Rosa, F. et al. Second surgery or chemotherapy for relapse after radical resection of colorectal cancer metastases. Langenbecks Arch Surg 397, 1069–1077 (2012). https://doi.org/10.1007/s00423-012-0974-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-0974-0