Abstract
Introduction
Gallbladder cancer is the most common malignant tumour of the biliary system with an extraordinarily poor prognosis. In this study, we retrospectively evaluated forty-two patients with histologically proven gallbladder cancer.
Patients and methods
Estimated survival rates were calculated by the Kaplan–Meier method, and differences were assessed using the logrank test. The GKR (combined registry of cancer) and demographic data were used to gain information on community cancer statistics.
Results
In this study, patients with metastases showed poorer survival rates. Furthermore, the survival was significantly better in patients with R0 resections, smaller tumour sizes and without lymph node infiltration. T stage, M stage and R stage were independent prognostic parameters. Sex and age had no significant effect on survival. Also, we found that patients with incidental gallbladder cancer and those with cholecystolithiasis showed significantly better survival rates. Demographic analyses of the study group confirmed a high coverage of our institution for incident cases in our catchment area and no significant regional deviations from the expected incidence of gallbladder cancer.
Conclusion
Despite differences in the incidence in different geographical areas, gallbladder cancer appears to be fairly normally distributed in Western Pomerania, a predominantly rural area of Northeastern Germany. Coverage of incident cases in our catchment area was high. T stage, M stage and R stage were independent prognostic factors in our study. We conclude that, whenever possible, an R0 resection should be the surgical goal in all patients staged resectable before surgery, but heroic resections in patients with highly advanced cancer disease or severe accompanying non-tumour diseases are not warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallbladder cancer is the fifth most common cancer of the gastrointestinal tract and the most common malignant tumour of the biliary system worldwide. It has a poor prognosis with a 5-year survival rate of 5 to 10 % [1]. Symptoms are usually present only in late-stage disease of gallbladder cancer. It affects more females with a male to female ratio of 1:2, commonly diagnosed in the seventh decade of life [2]. There is a broad variation of incidences in different geographical regions as well as in different ethnical groups [3]. Gallstones have been associated with gallbladder cancer [1]. However, whether gallstones are a cause or only bystanders in the pathogenesis of gallbladder cancer remains unclear [4]. Yet, a progression from adenoma to carcinoma has been demonstrated for adenomatous polyps of the gallbladder [5].
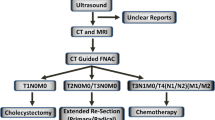
Preoperative diagnosis of early stages of gallbladder cancer can be challenging. Therefore, the diagnosis of T1 and T2 tumours is usually established on histological examination after surgery [5] for symptomatic gallstones. In cases of T1a gallbladder cancer, simple cholecystectomy is sufficient, but careful attention to the surgical margin of the cystic duct is essential [5]. There is no difference in survival or recurrence rates between radical and simple cholecystectomy for T1a tumours [6]. Radical cholecystectomy includes cholecystectomy, regional lymphadenectomy and resection of the gallbladder's fossa [7]. T1b tumours certainly have a higher rate of lymphatic metastases, but it is controversial whether radical cholecystectomy should be performed [6]. Some authors advocate lymphadenectomy of the hepato-duodenal ligament in all patients due to difficulties in distinguishing perioperatively T1 and T2 disease [8]. In T2 or larger tumours, radical tumour resection combined with regional lymphadenectomy and wedge resection of the liver is recommended [9]. The overall goal of surgery is to achieve an R0 situation [6]. In the cases of incidental carcinomas, a second radical operation is recommended depending on the tumour stage.
The aim of this retrospective single-centre study was to evaluate the prevalence of gallstones in gallbladder cancer, the impact of tumour size and lymph node involvement following the International Union Against Cancer (UICC) classification on the prognosis and their role as prognostic factors. Also, we intended to study the incidence of gallbladder cancer at Greifswald University as a tertiary referral centre of Western Pomerania, a predominantly rural area of Northeastern Germany.
Patients and methods
From January 2001 to December 2009, forty-two patients with histologically proven gallbladder cancer were identified by retrospective chart reviews at Greifswald University. This tertiary cancer centre covers an estimated 200,000 people living in a predominantly rural area. Patients' demographic data, as well as type of operation, surgical morbidity and mortality and histopathological classification following the seventh edition of the UICC [10], and survival data were collected in a database for further analyses. Overall survival was calculated starting at the time of primary surgery until the time of death or the last time of follow-up in December 2009. Then, all patients or their family practioners were contacted by phone.
Simple cholecystectomy included removal of the gallbladder only. An extended resection of the gallbladder included cholecystectomy, resection of the gallbladder's fossa as well as a lymphadenectomy including all lymph nodes of the liver hilum, the cystic duct, the hepato-duodenal ligament, the celiac trunk and retro-duodenal tissue. The bile duct was resected only if tumour involvement was present. In the cases of previous laparoscopic cholecystectomies, port sites were only resected if no extraction bags had been used. Patients with unresectable disease were offered with palliative therapies including chemotherapy using gemcitabine.
Standardised incidence ratios were calculated based on the number of study patients per postal code divided by the number of expected cases derived from the GKR (gemeinsames Krebsregister der neuen Bundesländer, i.e. combinded registry of cancer for the new German states) covering all patients with incident gallbladder cancer in Western Pomerania (referral area of the Department of Surgery of the University Medicine Greifswald). Respective population figures of the catchment area were obtained from the federal board of statistics of Mecklenburg Western Pomerania. The time period covered the years from 2001 to 2008.
Assuming that the age group-specific incidence of gallbladder cancer in the study areas of interest (counties) was the same as in the reference population of Mecklenburg Western Pomerania, the expected number of gallbladder carcinomas was calculated using the following formula:
where Erw is the expected number of gallbladder cancers of the area of interest, I′ARi is the age-specific incidence of age group i of the reference population, separate for men and women and Bev i is the population of the age group i, separate for men and women. The standardised incidence ratio was calculated by the ratio of observed and expected incidence of gallbladder cancer:
where SIR is the standardised rate of incidence, Erw is the expected incidence within the area of interest and Beo is the observed incidence within the area of interest. Standardised incidence ratios (SIR) were calculated on a county level (Table 3). An SIR of >1 represented an increased incidence of gallbladder cancer whereas an SIR of <1 represented a decreased incidence.
Survival analyses were performed using the Kaplan–Meier method. Differences in survival were analysed by the logrank test. To determine prognostic factors, the Cox regression analysis was performed. A chi-square test was used to determine differences in frequencies. For all calculations, SPSS software (SPSS, edition 18.0, Chicago, USA) was used. A p value of less than 0.05 was considered statistically significant.
Results
Patients and surgery
Altogether, 42 patients were included in this study. Of these, 35 (83.3 %) were females, and 7 (16.7 %) were males. Mean age was 68.1 ± 9.4 years (range 45–82 years) without significant differences between females and males (68.2 ± 1.6 years for females, 67.1 ± 2.9 years for males). The average time of follow-up was 16.47 ± 3.3 months.
The most common presenting complaint was unspecific upper abdominal pain seen in 15 patients (36 %). Six patients (14.3 %) presented with hyperbilirubinaemia and icterus. Gallstones were present in about half of the patients (19, i.e. 45.2 %). Nine patients (21.4 %) presented with unresectable disease requiring palliative therapy. In these cases, complete resection would not have been possible due to multiple liver metastases, peritoneal metastases, tumour infiltration of large vessels or distant organ metastases.
Thirty-three patients (78.6 %) could be treated surgically. Mean incision to closure time was 252.7 ± 99.7 min ranging from 57 to 442 min. Extended resection was performed in all 33 patients. The bile duct was resected in 13 of 33 patients (39 %) to achieve microscopic complete resection. This was defined as microscopic tumour disease-free resection margins. In these cases, a reconstruction was done by a hepatico-jejunostomy. In six cases, extended resection required an additional gastric wedge resection. In one patient, pylorus-preserving pancreaticoduodenectomy was performed. Following surgery, two patients died of surgery-related complications: one patient died of multi-organ failure due to recurrent bleeding episodes and one patient died of liver failure. A third patient died of pulmonary embolism. Two patients suffered from surgery-related minor complications: one patient developed a hematoma and one a large seroma, both at the margin of liver resection. These were treated by a CT-guided drainage.
In 12 patients (28.6 %), the diagnosis of gallbladder carcinoma was an incidental finding. In 9 of these 12 patients, the gallbladder was without macroscopic pathological findings. In seven of the latter nine patients, the tumour stage was at least T2 (i.e. pT2N0, pT3N0, 4× pT2N1, 2× pT3N1, pT4N1), and re-resection was performed. In the two remaining of these nine patients, tumour stage was T1b, but re-resection was performed because the tumour was localised at the site of the gallbladder's fossa. Both T1 tumours did not show lymph node metastases.
Overall survival
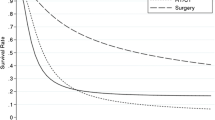
Overall median survival was 9.1 ± 5.1 months (95 % confidence interval (CI) 0–19.1 months). Mean survival was 24.4 ± 5.5 months (95 % CI 13.6–35.3 months). The estimated 1-year survival rate was 48 %; the 3- and 5-year survival rates were both 16 % (Fig. 1). There was a noticeable albeit not statistically significant difference of survival between males and females (6.4 ± 2.4 versus 14.8 ± 5.8 months, p = 0.3); 20 of 42 patients (47.6 %) were younger than 70 years. Again, there was a noticeable but not statistically significant difference in survival rates of patients younger than 70 years compared to those older than 70 years (8.3 ± 1.9 versus 14.8 ± 5.4 months, p = 0.95). The median survival in the palliation group was highly significantly poorer than in the surgically treated group (6.5 ± 0.9 months for the palliation group versus 16.7 ± 7.2 months for the surgically treated patients, p = 0.0043).
Survival curves are depicted. a Overall survival is shown. b Patients with an incidental finding of gallbladder cancer show a better survival than patients with suspected gallbladder cancer. c Patients with lower T stages (T1/T2) show a significantly prolonged survival compared with those suffering from T stages T3/T4. d Patients without metastases (M0) survive significantly longer than those displaying metastases (M1)
M0 versus M1 group
According to the seventh edition of the UICC TNM manual, the following tumours were classified as M1: tumours with distant metastases as well as tumours infiltrating the celiac, periduodenal, peripancreatic or mesenteric lymph nodes. Twenty-theree patients (54.8 %) presented with metastases at the time of primary diagnosis; 14 of these patients were treated surgically, and microscopic complete resection could be performed in 13 (92.9 %) of these cases. Most metastases (n = 17) were found in the liver; one patient had a metastasis within the ovary. Five patients were classified as M1 because of tumour cells found within the above mentioned lymph nodes. Median survival was highly significantly better in the M0 group when compared to the M1 group (47.2 ± 10.8 months, 95 % CI 26.0–68.4 months versus 8.9 ± 2.0 months, 95 % CI 5.0–12.9 months, p < 0.0001; Fig. 1).
R0 versus R1 group
In 29 of the 33 surgically treated patients, an overall R0 (i.e. microscopic complete) resection could be achieved. These cases involved 13 patients with metastases which were completely resected. These patients had a significantly better median survival (31.3 ± 7.1 months, 95 % CI 17.2–45.4 months) when compared to the R1-(microscopic residual disease) resected group (5.4 ± 1.8 months, 95 % CI 1.9–9.0 months, p = 0.03).
T stage
Four (12.1 %) of 33 surgically treated patients had tumour stage T1b. Mean survival within this subgroup was 65.3 ± 16.1 months (95 % CI 33.8–96.9 months). Eight patients (24.2 %) suffered from stage T2 disease. Only three of them died within the analysed interval. Mean survival rate was 47.0 ± 13.5 months (95 % CI 20.6–73.4 months); 12 patients (36.4 %) had stage T3, and 9 patients (27.3 %) had stage T4 disease. Mean survival was 18.3 ± 3.7 months (95 % CI 11.1–25.6 months) for stage T3 and 4.8 ± 1.9 months for stage T4 disease (95 % CI 1.1–8.5 months, Table 1). Survival of stage T4 patients was significantly poorer than survival of stage T3 patients (p = 0.001).
Twelve patients of all surgically treated patients had stage T1 or T2 disease (36.4 %); 21 patients (63.3 %) suffered from stage T3 or T4 disease. Survival rates of patients with stage T1 or T2 disease were significantly better than those of patients suffering from stage T3 or T4 disease (median survival of 35.3 ± 5.3 months, 95 % CI 24.8–45.8 months versus 8.3 ± 2.0 months, 95 % CI 4.8–12.1 months, p = 0.004; Fig. 1). Ten patients (52.6 %) without metastases had low T stage disease (T1/T2), whereas only two patients (14.3 %) showed T1/T2 stage disease in the M1-group. These results suggested a correlation of T and M stages, which could be confirmed statistically (Spearman coefficient 0.39, p = 0.02).
Furthermore, only 42.1 % (8) of M0 patients showed tumour infiltrated lymph nodes. In marked contrast, 92.9 % of the patients suffering from metastases showed positive lymph nodes. Also, these data strongly suggested a correlation of lymph node metastases and M1 stages. This was confirmed statistically (Spearman coefficient 0.52, p = 0.002).
N stage
Twelve of all surgically treated patients (36.4 %) had no lymph node metastases (N0), whereas 21 patients (63.6 %) showed lymph node metastases. Twelve patients (57.2 %) with lymph node metastases displayed tumour involvement of lymph nodes of the cystic duct; 1 patient of the 12 patients also had tumour-positive lymph nodes of the hepato-duodenal ligament. Furthermore, one patient showed infiltration of lymph nodes of the cystic duct, the hepato-duodenal ligament and, additionally, the retro-duodenal lymph nodes. Two patients (9.6 %) of the nodal-positive patients showed isolated tumour involvement of the lymph nodes of the hepato-duodenal ligament. Six patients (28.6 % of the nodal-positive cases) suffered from tumour involvement of the hepato-duodenal ligament and of the retro-duodenal lymph nodes. One patient showed tumour involvement of the retro-duodenal lymph nodes without involvement of local lymph nodes. Mean survival of the patients without positive lymph nodes was 33.4 ± 12.4 months (95 % CI 9.1–57.7 months), whereas survival of the patients with lymph node metastases was significantly shorter with 7.7 ± 1.4 months (95 % CI 5.0–10.4 months; p = 0.016, chi-square 5.8; Table 1).
Tumour grading, blood- and lymphatic vessel invasion
One tumour (3 %) was graded as a G1-tumour; 20 patients suffered from G2-carcinomas (60.6 %), and 12 patients (36.4 %) had a G3-carcinoma. There were no significant differences in survival rates of the patients suffering from G1-, G2- or G3-carcinomas.
In marked contrast, patients suffering from tumours with lymphatic vessel invasion showed significantly poorer survival rates: 23 patients (69.7 %) had lymphatic vessel invasion and 10 patients (30.3 %) had not. Survival rates were 39.7 ± 9.15 months (CI 21.78, 57.64) in patients without lymphatic vessel invasion, whereas survival rates were 8.3 ± 2.78 months in patients with positive lymphatic vessels (p = 0.003).
Twenty-four patients (72.7 %) of all the surgically treated patients did not show blood vessel invasion, whereas nine patients (27.3 %) displayed positive blood vessels. Survival was significantly better in patients without blood vessel invasion (37.67 versus 10.36 months (p = 0.024). Furthermore, there was a very strong correlation of lymph vessel invasion and blood vessel invasion (Spearman coefficient 0.78, p < 0.0001).
Prognostic factors
To test for independent prognostic parameters, Cox regression analyses of the factors T stage, N stage, M stage, L stage, V stage, history of cholecystolithiasis, incidental findings and resection margins were performed. These showed that only T stage, M stage and R stage were statistically significant prognostic factors (p = 0.05 for T stage, p = 0.03 for M stage and p = 0.004 for R stage).
Incidental carcinomas
In 12 patients (28.6 %), the diagnosis of gallbladder cancer was an incidental finding. Seven of these patients had stage T1 or T2 disease, whereas five had stage T3 or T4 disease. In patients with preoperatively suspected gallbladder cancer, 5 patients had stage T1 or T2 disease, whereas 16 patients had T3/T4 disease. Taken together, patients with incidental gallbladder cancer had significantly more often lower T stages (p = 0.047). Furthermore, half of the patients with preoperatively suspected gallbladder cancer displayed distant metastases, whereas only three patients (25 %) with incidental gallbladder cancer suffered from stage M1 disease (p = 0.01, Cramer's V 0.378). In contrast, there were no significant associations with nodal status and/or tumour-free resection margins (data not shown).
Median survival was 22.3 ± 10.1 months (CI 2.6 and 42.1 months) in patients with incidental gallbladder cancer, whereas patients with suspected gallbladder cancer displayed a significantly shorter median survival of 6.7 ± 1.2 months (CI 4.3 and 9.1 months) (p = 0.018, Fig. 1).
Cholecystolithiasis
Nineteen of the 42 patients (45.2 %) had a history of cholecystolithiasis. Of those 19 patients, 2 (10.5 %) were treated intending palliation surgery/therapy. The remaining 17 patients (89.5 %) received surgery intending a complete/curative resection. Of those 17 patients, 10 patients (58.8 %) showed stage T1/T2 disease, and 7 patients (41.2 %) had stage T3/T4 disease. Twenty-three patients (54.8 %) had no history of cholecystolithiasis. Of those, seven patients were treated intending palliation surgery/therapy. Of the remaining 16 patients without a history of cholecystolithiasis, 2 patients (12.5 %) had stage T1/T2 disease, whereas 14 patients (87.5 %) had stage T3/T4 disease. Comparing these results, patients with cholecystolithiasis showed significantly more stage T1/T2 diseases than patients without a history of cholecystolithiasis (p = 0.006, Cramer's V 0.486). At the same time, patients without a history of cholecystolithiasis showed significantly more stage T3/T4 diseases. Furthermore, patients with a history of cholecystolithiasis displayed less metastases (52.2 versus 31.6 %), although this was statistically not significant. Patients with a history of cholecystolithiasis had a median survival of 32.1 ± 8.9 months (CI 14.5 to 40.0 months), those without a history of cholecystolithiasis had a significantly shorter median survival of 15.28 ± 5.0 months (CI 5.5 to 25.1 months) (p = 0.05, chi-square 3.9). Furthermore, gallbladder carcinoma was more often an incidental finding in patients with cholecystolithiasis (p = 0.01, Cramer's V 0.38).
Hyperbilirubinaemia
Six patients (14.3 %) presented with hyperbilirubinaemia. These patients had no differences in survival when compared to patients without hyperbilirubinaemia.
UICC staging
According to the International Union Against Cancer, gallbladder cancer stages range from stage 0 to stage IVB. In this study, 3 of the resected 33 patients (9.1 %) had stage I disease (T1N0M0), 2 patients (6.1 %) had stage II disease (T2N0M0), 6 patients (18.2 %) had stage IIIA disease (T3N0M0) and 6 patients (18.2 %) had IIIB (T1-3N1M0) disease (4 patients T2N1M0, 2 patients T3N1M0). Finally, 2 patients (6.1 %) were diagnosed with stage IVA- (T4 N0-1 M0), and 14 patients (42.3 %) were diagnosed with stage IVB disease (TxNxM1) Mean survival of all patients including their stages is depicted in Table 2.
Epidemiologic analyses
Analysing the demographic data of the study group, we could not detect any unexpected epidemiologic findings within this study group of Western Pomerania (Table 3). Four patients were not included in these investigations since they were diagnosed in the year 2009 for which the GKR is not yet complete. SIR for the different counties in Western Pomerania revealed no significant deviations from 1.0. Observed differences were probably due to normal regional variations. Interestingly, the percentage of death certificate only patients of all observed gallbladder cancers in Mecklenburg Western Pomerania between 2001 and 2008 was much higher (28.2 %) than for most other cancers. A total of 16.3 % of all incident cases in the catchment area were referred to the surgical clinic of the University Medicine, representing a high coverage for our institution.
Discussion
Gallbladder cancer remains an illness with a poor prognosis. Five-year survival rates have been reported as low as 5–10 % [1]. In line with these results, the median overall survival of this study was 9.1 months with a slightly better 5-year survival rate of 16 %. Similarly, gallbladder cancer has been reported as a disease of the elderly [11]; in this study, mean age at the time of diagnosis was 68.1 years. As reported before [12], the majority of our patients was female, too.
In gallbladder cancer, symptoms do usually not occur until advanced tumour stages have developed. Most cases of early gallbladder cancer are detected incidentally [6]. The only chance for cure is a complete resection of the tumour without remnant microscopic disease (R0 resection). In our study, a complete resection was possible in 87.9 % of all the resected patients. Furthermore, 64 % of the resected patients had advanced tumour stages (stage T3/T4 disease). This is in line with other reports [2] and can be explained by the asymptomatic course of the disease in early stages. Correspondingly, patients with incidental cancer of the gallbladder had significantly more often lower T stages (T1/T2). Furthermore, these patients showed significantly less metastases. Accordingly, patients with incidental findings had a significantly better outcome. This clearly shows that more effective early diagnosis strategies are warranted.
Of the study patients, 21.4 % were not resectable at the time of diagnosis. Of those who underwent surgery (33 patients, 78.6 %) nearly 50 % showed UICC stages IVA and IVB with a mean survival of 3.4 and 9.9 months, respectively.
In a retrospective study including 127, patients Schauer et al. [13] could show that the different stages following the UICC/AJCC classification have the strongest predictive value. Previous studies have shown that higher T stage, lymph node involvement and a positive resection margin predict poor outcome in patients with gallbladder cancer [14, 15]. Our study supports these findings as we detected significantly lower survival in stage T3/T4 disease, tumours with lymph node involvement and patients with R1 resections. Due to the small study group, we combined stages T1 and T2 as well as stage T3 and stage T4. The evaluation of survival rates in patients with positive and negative resection margins showed that negative resection margins (R0 resections) were a very strong prognostic marker for better survival. This was confirmed by Cox regression analyses showing that R status, M stage and T stages were independent prognostic factors. Some authors emphasise that lymph node infiltration is one of the strongest predictors for poor survival [16, 17]. We could also show that patients with lymph node infiltrations displayed significantly poorer survival rates. However, this was not an independent prognostic factor as shown by Cox regression analyses. Furthermore, poorer survival of patients with lymph node involvement was mainly due to their associated higher T or M stage disease (T4 and/or M1). In fact, almost all stage IV patients displayed lymph node metastases with an overall survival of only 3.4 months for stage IVA and 11.1 months for stage IVB disease. Since these patients displayed T4 and/or M1 disease, this fact mainly accounted for poorer survival since T and M stages, in contrast to N stage, were independent prognostic factors in this study. Besides, by analysing subgroups stages II, IIIA and IIIB, we found that stage IIIB patients displayed a better mean survival than stages II and IIIA patients. Yet, differences were not significant (p = 0.471). Also, the patient group of stage II disease consisted of only n = 2 patients, making statistical conclusions more difficult. In addition, stage IIIB patients had more often lower T stages (4× T2, 2× T3) whereas stage IIIA patients had more often higher T stages (6× T3), confirming that lymph node involvement is not an independent prognostic marker in this study as well as confirming T stage being more relevant for survival. However, the number of study patients of this single-centre study is not large and may have an impact on these results, too.
Similarly, lymphatic vessel invasion as well as blood vessel invasion was associated with poorer survival. However, neither factor was an independent prognostic factor. Interestingly, tumour grading was not associated with patients' survival.
As expected, resected patients had a significantly better survival than non-surgically treated patients receiving palliation therapy. In addition, resected patients displaying advanced UICC stages also had a significantly longer survival than non-surgically treated patients: resected stage IV patients had a mean survival of 11 months compared to 6.8 months of non-surgically treated stage IV patients receiving only palliation therapy. In this context, it is of importance to note that in all cases of resected stage IV patients, an R0 resection was possible. We conclude that, whenever possible, an R0 resection should be the surgical goal in all patients staged resectable before surgery. Our data, however, do not support heroic resections in patients with highly advanced cancer disease or severe accompanying non-tumour diseases.
Surprisingly, we found that patients with cholecystolithiasis showed significantly better survival. Accordingly, patients with cholecystolithiasis had significantly more often stage T1/T2 disease. This may be explained by the fact that patients with cholecystolithiasis tend to be more often in clinical follow-ups and, if they become symptomatic, are operated on early due to symptomatic cholecystolithiasis [18]. Therefore, gallbladder cancer might be detected earlier in these patients.
Some authors have reported that advanced age is an additional predictor of survival [19]. In our study, we could not detect any statistical differences in survival between patients younger or older than 70 years. A broad variation of incidences in geographical regions as well as in ethnical groups has been published [3] reaching highest incidences in Northern India, the Middle East and North Africa [20, 21]. In this study, we analysed the area of Western Pomerania, a predominantly rural area in Northeastern Germany. In our study group, we did not detect any statistically significant variations of the incidences between counties. The incidence in Western Pomerania, therefore, did not significantly differ from the observed or expected total incidence of the state Mecklenburg Western Pomerania and appears to be similar to the incidence observed in Germany.
Conclusion
The presence of metastases was a strong predictor for poor survival. In surgically treated patients, negative resection margins (R0 resections), M0 stage and lower T stages have been independent predictors of better survival. Interestingly, patients with incidental gallbladder cancer and patients with a history of cholecystolithiasis have been shown to have a better survival. If tumour-free resection margins cannot be achieved in patients with gallbladder cancer, the patient will most likely not benefit from surgery. In these cases, palliation therapy is warranted. Patients with higher risk disease for complete resection should be treated in specialised centres. Although this study has included only a small number of patients, the hypothesis that cholecystolithiasis is a risk factor for gallbladder cancer should be re-evaluated. The incidence of gallbladder cancer in Western Pomerania appears to be similar to the incidence in Germany.
References
Lai CH, Lau WY (2008) Gallbladder cancer—a comprehensive review. Surgeon 6:101–110
Puhalla H, Bareck E, Scheithauer W et al (2002) Therapy of gallbladder carcinoma. Experience of a central hospital. Chirurg 73:50–56
Lemrow SM, Perdue DG, Stewart SL et al (2008) Gallbladder cancer incidence among American Indians and Alaska Natives, US, 1999–2004. Cancer 113:1266–1273
Shrikhande SV, Barreto SG, Singh S et al (2010) Cholelithiasis in gallbladder cancer: coincidence, cofactor, or cause! Eur J Surg Oncol 36:514–519
Miller G, Jarnagin WR (2008) Gallbladder carcinoma. Eur J Surg Oncol 34:306–312
Mekeel KL, Hemming AW (2007) Surgical management of gallbladder carcinoma: a review. J Gastrointest Surg 11:1188–1193
Kondo S, Nimura Y, Kamiya J et al (2002) Mode of tumor spread and surgical strategy in gallbladder carcinoma. Langenbecks Arch Surg 387:222–228
Orth K, Beger HG (2000) Gallbladder carcinoma and surgical treatment. Langenbecks Arch Surg 385:501–508
Goetze TO, Paolucci V (2010) Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German registry. Surg Endosc. doi:10.1007/s00464-010-0914-4
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM Classification of Malignant Tumours, 7th edition, John Wiley and Sons, New York
Jayaraman S, Jarnagin WR (2010) Management of gallbladder cancer. Gastroenterol Clin North Am 39:331–342
Kiran RP, Pokala N, Dudrick SJ (2007) Incidence pattern and survival for gallbladder cancer over three decades—an analysis of 10301 patients. Ann Surg Oncol 14:827–832
Schauer RJ, Meyer G, Baretton G et al (2001) Prognostic factors and long-term results after surgery for gallbladder carcinoma: a retrospective study of 127 patients. Langenbecks Arch Surg 386:110–117
Bartlett DL, Fong Y, Fortner JG et al (1996) Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg 224:639–646
Shimada H, Endo I, Fujii Y et al (2000) Appraisal of surgical resection of gallbladder cancer with special reference to lymph node dissection. Langenbecks Arch Surg 385:509–514
Ito H, Matros E, Brooks DC et al (2004) Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg 8:183–190
Wakabayashi H, Ishimura K, Hashimoto N et al (2004) Analysis of prognostic factors after surgery for stage III and IV gallbladder cancer. Eur J Surg Oncol 30:842–846
Tazuma S, Kajiyama G (2001) Carcinogenesis of malignant lesions of the gall bladder. The impact of chronic inflammation and gallstones. Langenbecks Arch Surg 386:224–229
Foster JM, Hoshi H, Gibbs JF et al (2007) Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol 14:833–840
Lazcano-Ponce EC, Miquel JF, Munoz N et al (2001) Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 51:349–364
Randi G, Franceschi S, La Vecchia C (2006) Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 118:1591–1602
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Katharina Cziupka, Lars Ivo Partecke, Wolfram von Bernstorff, and Albrecht Stier contributed equally and share first/senior authorship.
Rights and permissions
About this article
Cite this article
Cziupka, K., Partecke, L.I., Mirow, L. et al. Outcomes and prognostic factors in gallbladder cancer: a single-centre experience. Langenbecks Arch Surg 397, 899–907 (2012). https://doi.org/10.1007/s00423-012-0950-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-0950-8