Abstract
Background and aims
In gastric cancer, regional lymph node metastasis verified by histopathological examination is the most important prognostic factor after complete surgical tumor resection (R0). However, the prognostic value of immunohistochemically identifiable disseminated tumor cells in lymph nodes without histopathological tumor burden in patients with gastric cancer is still controversially discussed. The aim of the study was to assess the frequency and prognostic impact of minimal tumor cell spread to lymph nodes in these patients.
Patients–methods
One hundred sixty lymph nodes judged as “tumor free” on routine histopathology obtained from 58 patients with gastric adenocarcinoma were analyzed immunohistochemically using the monoclonal anti-EpCAM antibody Ber-EP4 for occult disseminated tumor cells.
Results
Tumor cells in lymph nodes were detected in 62 (38.8%) of the 160 “tumor-free” lymph nodes obtained from 39 (67.2%) patients. Multivariate Cox regression analysis confirmed the presence of disseminated tumor cells in “tumor-free” lymph nodes as an independent prognostic factor for both a significantly reduced relapse-free survival (p = 0.008) and overall survival (p = 0.009).
Conclusions
The frequent occurrence and prognostic impact of minimal disseminated tumor cells in lymph nodes of patients with gastric carcinoma support the need for a refined staging system of excised lymph nodes, which should include immunohistochemical examination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early metastatic relapse after the complete resection of an apparently localized primary tumor indicates an occult tumor cell dissemination or micrometastatic disease, undetectable by current staging procedures. Therefore, more sensitive immunohistochemical and immunocytochemical assays have been developed that are able to detect occult disseminated tumor cells in bone marrow [1–4] and lymph nodes classified as “tumor free” by conventional histopathologic examination [5–17].
In gastric cancer, the strongest predictor for long-term survival after complete tumor resection (R0) is the absence of regional lymph node metastases verified by histopathological examination (pN0) [18–21]. Nevertheless, a relevant number of these patients with stages I and II disease (22–66%) die as a result of local or distant relapse despite complete surgical tumor resection with tumor-free resection margins (R0) [22]. Recent studies in a variety of solid tumors could demonstrate a high incidence of immunohistochemically detectable disseminated tumor cells in lymph nodes classified as “tumor free” on routine histopathological examination [15–17, 23]. Moreover, in several malignant diseases including carcinoma of the esophagus, colorectum, or breast, the presence of disseminated tumor cells in “tumor-free” lymph nodes has been associated with a poorer postoperative prognosis [7–13, 15, 16, 23]. In patients with gastric carcinoma, the prognostic significance of these minimal tumor cell deposits in lymph nodes remains controversial. The wide differences of applied tumor cell detection protocols contribute to this confusion significantly.
In the present study, we performed our standardized immunohistochemical approach with a monoclonal anti-EpCAM antibody for the identification of disseminated tumor cells in lymph nodes in patients with gastric cancer. This antibody has been shown to be more specific for the detection of ectopic epithelial-derived tumor cells in lymphatic tissue compared with anticytokeratin (CK) antibodies, which were normally used in this context [24, 25]. The immunohistochemical findings were correlated with clinical follow-up data to validate the clinical relevance of the detected cells. Moreover, a critical appraisal of the recent literature revealed that there is an urgent need for standardization of the current tumor cell detection protocols to resolve the question of the prognostic value of disseminated tumor cells in lymph nodes in patients with resectable gastric carcinoma.
Materials and methods
Patients
This study was approved by the ethics committee of the chamber of physicians in Hamburg. Informed consent was obtained from all the patients before their inclusion in the study. Between November 1992 and June 2002, lymph nodes were prospectively sampled from 109 consecutive patients with gastric carcinoma who underwent gastric resection or gastrectomy at the Department of Surgery, University Hospital Hamburg-Eppendorf. Patients with histopathologically verified metastasis in all sampled lymph nodes, advanced peritoneal carcinosis, and/or additional malignancies were excluded. Thus, 58 patients with adenocarcinoma of the stomach undergoing intentionally curative (n = 48) or palliative (n = 10) surgical procedure were enrolled in the present study. None of the 58 study patients received neoadjuvant chemotherapy or radiotherapy.
All tumors were classified according to the TNM classification of the International Union Against Cancer [26, 27]. The histological classification into intestinal-, diffuse-, or mixed-type carcinoma is based on the classification of Laurén [28].
For survival analyses, patients without complete tumor resection (R1; n = 3), patients who died during the hospital stay (n = 2), and patients with overt distant metastasis (n = 10) were excluded. Four additional patients were lost to follow-up. The median length of postoperative observation period of the remaining 39 patients was 28 months (range, 3 to 111 months).
Lymph node preparation and immunohistochemical tumor cell detection
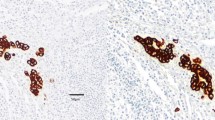
During the systematic lymphadenectomy, lymph nodes that were macroscopically inconspicuous were randomly collected and were divided into two parts as described previously [23]. One part of each lymph node was embedded in paraffin for routine histopathological examination, whereas the other part was snap-frozen in liquid nitrogen and stored at −80°C for immunohistochemical analysis. If a lymph node was “positive” on routine histopathology, it was excluded from the immunohistochemical analysis. The remaining nodes that were “negative” on routine histopathology were then analyzed immunohistochemically. From each of these nodes, cryostat sections of 6- to 8-μm thickness were cut at three different levels. Two consecutive samples obtained at each level were stained immunohistochemically with the monoclonal antibody (mAb) Ber-EP4 (IgG1; Dako, Hamburg Germany and Carpinteria, CA, USA) for the presence of disseminated tumor cells. This mAb detects the epithelial cell adhesion molecule (EpCAM), also known as 17-1A or EPG40, which is frequently expressed by epithelial cells and epithelial-derived tumors [12, 16, 25]. We and other groups previously demonstrated that immunohistochemical analysis with anti-EpCAM antibodies is a sensitive and specific method for detecting disseminated tumor cells in lymph nodes in a variety of solid epithelial tumors [9, 10, 12, 16, 23, 24]. Visualization of antibody binding was performed by the alkaline phosphatase-antialkaline phosphatase technique as described previously [16]. In addition, one adjacent section of each collected lymph node was incubated instead of the anti-EpCAM antibody with an isotype-matched, irrelevant murine monoclonal antibodies (MOPC-21; Sigma, Germany) for negative control. Sections of normal colonic mucosa consistently expressing EpCAM served as positive controls. The immunostained slides were evaluated in a blinded fashion by two observers working independently. Lymph nodes were interpreted as positive for disseminated tumor cells if they contained EpCAM-positive single cells or cell clusters and were negative on the negative controls.
Statistical analysis
Associations between categorical parameters were assessed via the χ 2 test and whenever appropriate with the Fisher’s exact test. To analyze survival and recurrence events, we used log rank tests for univariate analysis. For comparison purposes, log-rank tests were performed. Cox’s proportional hazards models were fitted for multivariate analysis. Relative risk and 95% confidence limits are presented. Differences between groups are considered to be significant if the p values were less than 0.05 for a two-tailed test (software SPSS 10.0, SPSS Inc. 1999).
Results
Routine histopathological examination of the in total 1,413 resected lymph nodes (mean 24.4 lymph nodes per patient; range six to 49 lymph nodes per patient) revealed lymph node metastases in 309 (21.9%) lymph nodes obtained from 38 (65.5%) of the 58 patients. For the immunohistochemical analysis, a total of 225 lymph nodes from these 58 patients were sampled. Of these sampled lymph nodes, 171 (76%) were judged as “tumor free” on routine histopathology and were further analyzed by immunohistochemistry. In 11 (6.4%) of these 171 lymph nodes, immunohistochemical analysis revealed immunostained cells that were also detectable in the negative control stainings. These single lymph nodes were therefore excluded as false positive. In the remaining 160 lymph nodes (mean 2.8 lymph nodes per patient; range one to eight lymph nodes per patient) EpCAM-positive tumor cells were found in 62 (38.8%) of these lymph nodes from 39 (67.2%) of the 58 patients (Table 1). These minimal tumor cell deposits presented as single cells (n = 10 patients; Fig. 1), small cell clusters (n = 18 patients), or both single cells and cell clusters (n = 11 patients). Half of the patients classified as pN0 on routine histopathology displayed EpCAM-positive tumor cells in lymph nodes (Table 1). The distribution of EpCAM-positive cells in the three lymph node compartments (D1–3) was not statistically different (p = 0.579) and is summarized in Table 2.
There were significant correlations between the presence of EpCAM-positive cells in lymph nodes and primary tumor classification (pT category; p = 0.006), lymph node classification (pN category; p = 0.042), and the presence of intraoperatively detected distant metastases (p = 0.012; Table 1).
For Kaplan–Meier survival analysis, a total of 39 patients undergoing curative tumor resection (R0) were available. Relapse-free and overall survival was significantly associated with the presence of lymph node metastases (pN1–3; p = 0.0215 and p = 0.0324, respectively) and higher age (p = 0.0299 and p = 0.0050, respectively; Table 3). Moreover, after a median observation period of 28 months (range, 3 to 111 months), the presence of EpCAM-positive cells in lymph nodes was associated with both a significantly reduced relapse-free survival (p = 0.0149) and overall survival (p = 0.0215; Table 4, Figs. 2 and 3). Eleven (47.8%) of 23 patients with EpCAM-positive cells in lymph nodes developed recurrence within a mean time of 41 months and 12 (52.2%) of them died within a mean time of 40 months compared with two (12.5%) and three (18.8%) patients without EpCAM-positive cells, respectively, within a mean time of 97 and 92 months, respectively. The estimated 2- and 5-year survival rates were both 81.3% for patients without and 65.2% and 47.8% for patients with EpCAM-positive cells in lymph nodes. Detailed analysis of the relapse-free survival revealed that the presence of EpCAM-positive cells in lymph nodes predicted for both a significantly reduced local recurrence-free survival (p = 0.0228) and distant metastasis-free survival (p = 0.0359; Table 4). Remarkably, none of the patients without EpCAM-positive cells developed local recurrence compared with five (21.7%) patients displaying EpCAM-positive cells in their lymph nodes. Multivariate Cox regression analysis confirmed the presence of EpCAM-positive cells in “tumor-free” lymph nodes as an independent prognostic factor for both a significantly reduced relapse-free survival (p = 0.008) and overall survival (p = 0.009). Consequently, patients with EpCAM-positive tumor cells in lymph nodes showed a 8.7 times increased risk for tumor relapse and a 6.1 times increased risk for shorter survival compared to patients without such cells (Table 5).
Discussion
In gastric cancer, the number and the level of lymph node metastasis identified on routine histopathological examination is the most important prognostic factor after curative tumor resection with tumor-free resection margins (R0) [18, 20, 21]. Recent studies in various solid tumors, including gastric cancer, demonstrated high incidences of disseminated tumor cells in the regional lymph nodes previously judged as “tumor free” on routine histopathology using sensitive immunohistochemical assays [5–12, 14–17, 23, 29]. However, especially in gastric cancer, the prognostic relevance of these cells remains unclear. Several studies found no impact of the presence of disseminated tumor cells in lymph nodes on postoperative outcome [30–33], whereas other groups described that the detection of these cells predicted for a significantly reduced postoperative survival [34–40]. However, only two of six published studies that described a prognostic impact of immunohistochemically detected tumor cells in lymph nodes confirmed their results by a multivariate analysis [37, 40]. Our study provides evidence that the presence of disseminated tumor cells in lymph nodes of gastric cancer patients are independent prognostic factors for both a significantly reduced relapse-free survival (p = 0.008) and overall survival (p = 0.009). Remarkably, none of the patients who were found to be free of disseminated tumor cells in lymph nodes developed local recurrence within the median observation period of 28 months.
An explanation for these discrepant results might be due to the lack of any standardization in tumor cell detection protocols (Table 6), which might also explain tumor cell detection rates in lymph nodes of gastric cancer patients that range between 10% [33] and 49% [37] of the analyzed patients.
First, there are differences in the applied tumor cell detection antibodies (Table 4). Although all of these studies applied mAb against CK, different antibody clones against different CK components were used. Some investigators applied the mAb AE1/AE3, which is directed against a broad spectrum of CK components, including CK 1–6, 8, 10, 14–16, and 19 [32, 36, 37, 39], whereas others used the mAb CAM 5.2, which detects the CK components 8 and 18 [34, 38, 40], the mAb MNF 116, which is directed against CK 5, 6, 8, 17, and probably CK 19 [33], a mAb against CK 8 [30], or a cocktail of mAb CAM 5.2 combined with an anti-CEA antibody [35] (Table 6). In this context, it becomes obvious that the application of different antibody clones can result in different tumor cell detection rates due to their different targets. Fukagawa et al. [31] proved this point testing the sensitivities and specificities of three different anti-CK antibodies (AE1/AE3, KL-1, and CAM5.2) using primary tumor tissues of gastric cancer patients and found that mAb AE1/AE3 was the most sensitive one. On the other hand, described irregular CK expression (e.g., CK 18) in normal lymphatic reticulum cells [41], endothelial cells, or extrafollicular dendritic cells [42, 43] can lead to false positive results. Therefore, we used a mAb against the EpCAM, also known as 17-1A or EPG40 [12], which is frequently expressed by epithelial cells and epithelial-derived tumors and which seems to be a more specific target for tumor cell detection in lymph nodes since it is not expressed in lymphatic tissues [12, 16, 24, 25]. Applying this approach, we and other groups were able to demonstrate that detection of EpCAM-positive cells in “tumor-free” lymph nodes was of independent prognostic significance for a worse postoperative prognosis in patients with carcinoma of the pancreas [10, 29], esophagus [9, 10, 23], and lung [12, 16].
In addition, there are differences in the number of immunohistochemically analyzed sections per lymph node (Table 6). Some investigators evaluated only one section per lymph node [30, 32, 34], whereas others analyzed two [31] or three consecutive sections per lymph node [40]. Others [33] performed a serial sectioning of the entire lymph node on three different levels at intervals of 150 μm or do not give any information about the number of analyzed sections [35, 36, 38].
Another problem is the different criteria in the evaluation of the immunohistochemical findings. Most investigators defined all events of both immunostained single cells or cell clusters that were unidentifiable by routine hematoxylin and eosin staining as disseminated tumor cells or micrometastases (MM) [31, 33–35, 38, 40], whereas others differentiated between “real MM”, defined as single tumor cell or tumor cell cluster with a surrounding stromal reaction, and “tumor cell microinvolvement,” defined as tumor cells without a stromal reaction [36,39]. Making this differentiation, Nakajo et al. [39] demonstrated that immunostained tumor cells in lymph nodes in the absence of a stromal reaction do not appear to affect survival, whereas the presence of “MM” was significantly correlated with a worse postoperative outcome. However, as described above, these authors did not confirm their results by a multivariate analysis. Furthermore, negative control immunostainings as described in the present study were only performed by a minority of these investigators [30, 32, 35, 39] (Table 6). Negative control staining, in our experience, is an essential component of immunohistochemistry. It lead to the exclusion of 11 (6.4%) of the analyzed lymph nodes in our study defined as “false positive”.
Another difference of our study compared to previously published studies is the study design. All the described studies had a retrospective design reevaluating paraffin-embedded material. This study is prospectively analyzing intraoperatively harvested and randomly collected lymph nodes. We realize that the number of analyzed lymph nodes is low (n = 160) and that this could introduce a sampling error. However, we and other groups were able to demonstrate that the described approach applying the anti-EpCAM antibody and analyzing low numbers of prospectively collected lymph nodes provides sufficient information [9, 10, 12, 16, 23, 29]. Furthermore, analyzing all resected “tumor-free” lymph nodes would not be routinely feasible since it is very costly and time-consuming.
As an alternative to immunohistochemistry, nucleic-acid-based detection of occult tumor cells has recently received considerable attention, which allows hypothetically the evaluation of an entire lymph node. In gastric cancer, several groups applied reverse-transcriptase polymerase chain reaction assays for epithelial (e.g., MUC2, CEA, CK20) or tumor marker (e.g., MAGE-3) transcript detection in lymph nodes [44–47]. However, the specificity of these ultrasensitive molecular assays is limited by the lack of any morphological correlate, the heterogeneity of genetic alterations, and the absence of suitable detection markers on mRNA or DNA level exclusively found in carcinoma cells [48–51]. Moreover, up to now, there are no reports evaluating the prognostic impact of occult tumor cells in lymph nodes detected by nucleic-acid-based methods in a valid number of patients with gastric cancer.
Conclusion
Our data indicate that the described immunohistochemical approach using an anti-EpCAM antibody can be used to refine the staging procedure for gastric cancer and help to identify patients at a high risk of tumor recurrence, which cannot be cured by surgery alone. Further studies with larger numbers of patients are needed to resolve the question of the prognostic value of disseminated tumor cells in lymph nodes in patients with resectable gastric carcinoma. However, there is an urgent need for standardization of the current tumor cell detection protocols and study designs in gastric cancer patients. This should include sufficient negative controls and a multivariate survival analysis, before immunohistochemical lymph node staging can be implemented into clinical practice. The detection of the earliest manifestations of tumor cell dissemination is an extremely promising approach, which might enable us to identify suitable candidates for adjuvant treatment strategies, for instance with humanized therapeutic anti-EpCAM antibodies [52].
References
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353:793–802 doi:10.1056/NEJMoa050434
Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmuller G (1992) Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet 340:685–689 doi:10.1016/0140-6736(92)92230-D
Seeliger H, Spatz H, Jauch KW (2003) Minimal residual disease in gastric cancer. Recent Results Cancer Res 162:79–87
van Heek NT, Tascilar M, van Beekveld JL, Drillenburg P, Offerhaus GJ, Gouma DJ (2001) Micrometastases in bone marrow of patients with suspected pancreatic and ampullary cancer. Eur J Surg Oncol 27:740–745 doi:10.1053/ejso.2001.1209
Bonavina L, Ferrero S, Midolo V, Buffa R, Cesana B, Peracchia A (1999) Lymph node micrometastases in patients with adenocarcinoma of the esophagogastric junction. J Gastrointest Surg 3:468–476 doi:10.1016/S1091-255X(99)80099-7
Calaluce R, Miedema BW, Yesus YW (1998) Micrometastasis in colorectal carcinoma: a review. J Surg Oncol 67:194–202 doi:10.1002/(SICI)1096-9098(199803)67:3<194::AID-JSO11>3.0.CO;2-2
Cote RJ, Peterson HF, Chaiwun B, Gelber RD, Goldhirsch A, Castiglione-Gertsch M et al (1999) Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet 354:896–900 doi:10.1016/S0140-6736(98)11104-2
Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW Jr (1994) Identification of occult micrometastases in pericolic lymph nodes of Duke’s B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer 73:563–569 doi:10.1002/1097-0142(19940201)73:3<563::AID-CNCR2820730311>3.0.CO;2-D
Hosch S, Kraus J, Scheunemann P, Izbicki JR, Schneider C, Schumacher U et al (2000) Malignant potential and cytogenetic characteristics of occult disseminated tumor cells in esophageal cancer. Cancer Res 60:6836–6840
Hosch SB, Knoefel WT, Metz S, Stoecklein N, Niendorf A, Broelsch CE et al (1997) Early lymphatic tumor cell dissemination in pancreatic cancer: frequency and prognostic significance. Pancreas 15:154–159 doi:10.1097/00006676-199708000-00007
Komukai S, Nishimaki T, Suzuki T, Kanda T, Kuwabara S, Hatakeyama K (2002) Significance of immunohistochemical nodal micrometastasis as a prognostic indicator in potentially curable oesophageal carcinoma. Br J Surg 89:213–219
Kubuschok B, Passlick B, Izbicki JR, Thetter O, Pantel K (1999) Disseminated tumor cells in lymph nodes as a determinant for survival in surgically resected non-small-cell lung cancer. J Clin Oncol 17:19–24
Liefers GJ, Cleton-Jansen AM, van de Velde CJ, Hermans J, van Krieken JH, Cornelisse CJ et al (1998) Micrometastases and survival in stage II colorectal cancer. N Engl J Med 339:223–228 doi:10.1056/NEJM199807233390403
Lindblom A (1998) Improved tumor staging in colorectal cancer. N Engl J Med 339:264–265 doi:10.1056/NEJM199807233390410
McGuckin MA, Cummings MC, Walsh MD, Hohn BG, Bennett IC, Wright RG (1996) Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer 73:88–95
Passlick B, Izbicki JR, Kubuschok B, Nathrath W, Thetter O, Pichlmeier U et al (1994) Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with non-small-cell lung cancer. J Clin Oncol 12:1827–1832
Trojani M, de Mascarel I, Coindre JM, Bonichon F (1987) Micrometastases to axillary lymph nodes from invasive lobular carcinoma of breast: detection by immunohistochemistry and prognostic significance. Br J Cancer 56:838–839
Adachi Y, Kamakura T, Mori M, Baba H, Maehara Y, Sugimachi K (1994) Prognostic significance of the number of positive lymph nodes in gastric carcinoma. Br J Surg 81:414–416 doi:10.1002/bjs.1800810331
Bozzetti F, Bonfanti G, Morabito A, Bufalino R, Menotti V, Andreola S et al (1986) A multifactorial approach for the prognosis of patients with carcinoma of the stomach after curative resection. Surg Gynecol Obstet 162:229–234
Maruyama K, Okabayashi K, Kinoshita T (1987) Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 11:418–425 doi:10.1007/BF01655804
Siewert JR, Bottcher K, Stein HJ, Roder JD (1998) Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 228:449–461 doi:10.1097/00000658-199810000-00002
Lim L, Michael M, Mann GB, Leong T (2005) Adjuvant therapy in gastric cancer. J Clin Oncol 23:6220–6232 doi:10.1200/JCO.2005.11.593
Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A et al (1997) Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med 337:1188–1194 doi:10.1056/NEJM199710233371702
Hosch SB, Stoecklein NH, Izbicki JR (2003) Molecular markers and staging of early esophageal cancer. Langenbecks Arch Surg 388:77–82
Latza U, Niedobitek G, Schwarting R, Nekarda H, Stein H (1990) Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol 43:213–219 doi:10.1136/jcp.43.3.213
Hermanek P, Sobin LH (eds) (1992) In: UICC: TNM classification of malignant tumours. 4th edn. Springer, Berlin 2nd rev
Sobin LH, Wittekind C (eds) (1997) In: TNM classification of malignant tumors. 5th edn. Wiley, New York
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Scheunemann P, Stoecklein NH, Rehders A, Bidde M, Metz S, Peiper M et al (2008) Occult tumor cells in lymph nodes as a predictor for tumor relapse in pancreatic adenocarcinoma. Langenbecks Arch Surg 393:359–365
Choi HJ, Kim YK, Kim YH, Kim SS, Hong SH (2002) Occurrence and prognostic implications of micrometastases in lymph nodes from patients with submucosal gastric carcinoma. Ann Surg Oncol 9:13–19 doi:10.1245/aso.2002.9.1.13
Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K et al (2001) Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer 92:753–760 doi:10.1002/1097-0142(20010815)92:4<753::AID-CNCR1379>3.0.CO;2-5
Kikuchi Y, Tsuchiya A, Ando Y, Yoshida T, Takenosita S (1999) Immunohistochemical detection of lymph node microinvolvement in node-negative gastric cancer. Gastric Cancer 2:173–178 doi:10.1007/s101200050042
Morgagni P, Saragoni L, Scarpi E, Zattini PS, Zaccaroni A, Morgagni D et al (2003) Lymph node micrometastases in early gastric cancer and their impact on prognosis. World J Surg 27:558–561 doi:10.1007/s00268-003-6797-y
Cai J, Ikeguchi M, Maeta M, Kaibara N, Sakatani T (1999) Clinicopathological value of immunohistochemical detection of occult involvement in pT3N0 gastric cancer. Gastric Cancer 2:95–100 doi:10.1007/s101200050030
Ishida K, Katsuyama T, Sugiyama A, Kawasaki S (1997) Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer 79:1069–1076 doi:10.1002/(SICI)1097-0142(19970315)79:6<1069::AID-CNCR3>3.0.CO;2-B
Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Higashi H, Watanabe T et al (2003) Clinical impact of micrometastasis of the lymph node in gastric cancer. Am Surg 69:573–577
Lee E, Chae Y, Kim I, Choi J, Yeom B, Leong AS (2002) Prognostic relevance of immunohistochemically detected lymph node micrometastasis in patients with gastric carcinoma. Cancer 94:2867–2873 doi:10.1002/cncr.10562
Maehara Y, Oshiro T, Endo K, Baba H, Oda S, Ichiyoshi Y et al (1996) Clinical significance of occult micrometastasis lymph nodes from patients with early gastric cancer who died of recurrence. Surgery 119:397–402 doi:10.1016/S0039-6060(96)80138-3
Nakajo A, Natsugoe S, Ishigami S, Matsumoto M, Nakashima S, Hokita S et al (2001) Detection and prediction of micrometastasis in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol 8:158–162 doi:10.1007/s10434-001-0158-6
Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S (2002) Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol 9:771–774
Franke WW, Moll R (1987) Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation 36:145–163 doi:10.1111/j.1432-0436.1987.tb00189.x
Domagala W, Bedner E, Chosia M, Weber K, Osborn M (1992) Keratin-positive reticulum cells in fine needle aspirates and touch imprints of hyperplastic lymph nodes. A possible pitfall in the immunocytochemical diagnosis of metastatic carcinoma. Acta Cytol 36:241–245
Gown AM, Boyd HC, Chang Y, Ferguson M, Reichler B, Tippens D (1988) Smooth muscle cells can express cytokeratins of “simple” epithelium. Immunocytochemical and biochemical studies in vitro and in vivo. Am J Pathol 132:223–232
Kubota K, Nakanishi H, Hiki N, Shimizu N, Tsuji E, Yamaguchi H et al (2003) Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with real-time RT-PCR: a comparative study with immunohistochemistry. Int J Cancer 105:136–143 doi:10.1002/ijc.11031
Matsumoto M, Natsugoe S, Ishigami S, Nakashima S, Nakajo A, Miyazono F et al (2002) Lymph node micrometastasis and lymphatic mapping determined by reverse transcriptase-polymerase chain reaction in pN0 gastric carcinoma. Surgery 131:630–635 doi:10.1067/msy.2002.124632
Okada Y, Fujiwara Y, Yamamoto H, Sugita Y, Yasuda T, Doki Y et al (2001) Genetic detection of lymph node micrometastases in patients with gastric carcinoma by multiple-marker reverse transcriptase-polymerase chain reaction assay. Cancer 92:2056–2064 doi:10.1002/1097-0142(20011015)92:8<2056::AID-CNCR1545>3.0.CO;2-L
Sonoda H, Yamamoto K, Kushima R, Okabe H, Tani T (2004) Detection of lymph node micrometastasis in gastric cancer by MUC2 RT-PCR: usefulness in pT1 cases. J Surg Oncol 88:63–70 doi:10.1002/jso.20143
Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R et al (1998) Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol 16:2632–2640
Jung R, Petersen K, Kruger W, Wolf M, Wagener C, Zander A et al (1999) Detection of micrometastasis by cytokeratin 20 RT-PCR is limited due to stable background transcription in granulocytes. Br J Cancer 81:870–873 doi:10.1038/sj.bjc.6690778
Ko Y, Klinz M, Totzke G, Gouni-Berthold I, Sachinidis A, Vetter H (1998) Limitations of the reverse transcription-polymerase chain reaction method for the detection of carcinoembryonic antigen-positive tumor cells in peripheral blood. Clin Cancer Res 4:2141–2146
Zippelius A, Kufer P, Honold G, Kollermann MW, Oberneder R, Schlimok G et al (1997) Limitations of reverse-transcriptase polymerase chain reaction analyses for detection of micrometastatic epithelial cancer cells in bone marrow. J Clin Oncol 15:2701–2708
Liljefors M, Nilsson B, Fagerberg J, Ragnhammar P, Mellstedt H, Frodin JE (2005) Clinical effects of a chimeric anti-EpCAM monoclonal antibody in combination with granulocyte-macrophage colony-stimulating factor in patients with metastatic colorectal carcinoma. Int J Oncol 26:1581–1589
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scheunemann, P., Stoecklein, N.H., Hermann, K. et al. Occult disseminated tumor cells in lymph nodes of patients with gastric carcinoma. A critical appraisal of assessment and relevance. Langenbecks Arch Surg 394, 105–113 (2009). https://doi.org/10.1007/s00423-008-0369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-008-0369-4