Abstract
Background
Cellular stress during reoxygenation is a common phenomenon in solid organ transplantation and is characterized by production of reactive oxygen species. Herein, we studied in isolated tubular segments of rat kidney cortex the impact of oxygen radical scavengers and an iron chelator on post-hypoxic recovery.
Methods
Tubules, suspended in Ringer’s solution containing 5 mM glycine, underwent 30 min hypoxia and 60 min reoxygenation. Untreated tubules served as controls. Hypoxia–reoxygenation injury was measured by membrane leakage, lipid peroxidation and cellular functions. In hypoxia–reoxygenated-isolated tubular segments, protective effects of different scavengers and of the iron chelator deferoxamine on hypoxia–reoxygenation injury were analyzed.
Results
Scavengers protected isolated tubular segments from hypoxia–reoxygenation-induced cellular disintegration and dysfunction. Deferoxamine was found to exert the most distinct protection. It was further found to exert a dose-dependent protection on hypoxia–reoxygenation damage in isolated tubular segments, which was critically mediated by chelating tissue and bond iron.
Conclusions
Our data demonstrate that radical scavengers effectively protect from hypoxia–reoxygenation injury in isolated tubular segments and that the iron chelator deferoxamine is especially a potent inhibitor of iron ion-mediated hypoxia–reoxygenation damage. Thus, inclusion of this iron chelator in organ storage solutions might improve post-transplant organ function and protect from reperfusion injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organ preservation seeks to ensure the functional viability of transplanted organs. The principle of preservation during ischemia includes measures to prevent acidosis, to maintain cell volume, and to optimize by utilizing anaerobic energy reserves. Our previous studies have demonstrated that apart from the ischemic damage, additional tissue injury evolves as a result of reperfusion and reoxygenation [1]. Oxygen radicals are central mediators of the cellular injury, which occurs upon post-ischemic reperfusion. Hypoxia and reoxygenation of renal cells in vitro and kidney ischemia-reperfusion injury in vivo are strongly associated with cell death [2]. The hydroxyl radical (OH), a metabolite of phospholipids, centrally participates in cell death signaling pathways [3]. However, recent experiments indicate that despite a remarkable prevention of pathological increases in plasma membrane permeability, reoxygenated ITS may develop severely compromised energetic function [4].
Thus, ITS, which had been incubated for 60 min at low oxygen tension in the presence of 5 mM glycine [5], for example, exhibited an impaired capability for adenosine triphosphate (ATP) restoration and intracellular K+ accumulation during reoxygenation despite a suppression of cytoplasmic membrane leakage [6]. This insufficiency of aerobic energy metabolism may have derived from an increased permeability of the inner mitochondrial membrane. Besides physiological factors which induce mitochondrial permeability changes, reactive oxygen species (ROS) may have also altered the structure of the inner membrane [7]. Under conditions of reperfusion or reoxygenation, the activity of cellular protective enzymes and the capacity of oxygen radical scavenging cell constituents become insufficient. Increased levels of univalent reduced oxygen from the mitochondrial electron transport system or via increased activity of xanthine oxidase may then react with peroxide ions to form OH, which finally oxidize cellular macromolecules and permeabilize the inner mitochondrial membrane. Intracellular accumulation of superoxide (O2 −) and of peroxide anions (O2 2−) from the mitochondrial electron transport chain and of O2 − via increased xanthine oxidase activity then leads to formation of OH under the catalytic action of chelated Fe2+ or Fe3+ [8]. Thus, the reported impairment of energetic function in glycine-protected, reoxygenated ITS may have been mediated, at least in part, by reactive oxygen species.
To test this hypothesis, we investigated in reoxygenated isolated renal tubulus segments in the presence of 5 mM glycine the effect of antioxidants and protective enzymes on the formation of thiobarbituric acid-reactive substances (TBA-RS), the liberation of cytoplasmic and mitochondrial marker enzymes, and the post-hypoxic reversibility of ATP-dependent gluconeogenesis (GNG) and K+-pumping activity (K+). To avoid secondary, mechanical damages in the intact kidney by the transfer of filtered hemoglobin in tubular lumina, tubulus segments (ITS) were isolated in an in vitro preparation.
Material and methods
Animals
Wistar rats of either sex (300–350 g) were housed in single cages at 22–24°C with a 12–12 h dark–light cycle and were kept on water and standard chow ad libitum. After approval by the local animal care committee, the experiments were conducted according to the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH publication 86–23, revised 1985).
Isolation of tubulus segments
Animals were anesthetized by an intraperitoneal injection of pentobarbital sodium (60 mg/kg; Narcoren, Merial, Hallbergmoos, Germany). After a median laparotomy, ligation of the superior mesenteric artery and celiac trunc and cannulation of the distal aorta, the kidneys were flushed with 25 ml chilled, oxygenated medium I (Table 1). The inferior vena cava was incised and 2 ml of chilled collagenase solution (Worthington CLS II, Freehold, USA; Table 1) were injected. After decapsulating both resected kidneys, the cortex was cut in 1.0 mm thick slices and the marrow was separated. The kidney cortex was chopped up homogeneously and the resulting particles were continuously stirred in 30 ml oxygenated collagenase solution (37°C). After 30 min, the tubule segments were filtered through a sieve (pore size 1 mm). Subsequently, the suspension was centrifuged at 50×g, the sediment resuspended and washed once more in medium I, centrifuged, and finally oxygenated (95% O2/5% CO2 = carbogen) in ice-cold medium II (Table 1). Under moderate shaking, the isolated tubules were then gassed for 30 min with carbogen at 37°C for recovery from the preceding collagenase treatment [5, 6]. Subsequently, the tubule suspension was centrifuged twice at 4°C for 30 s at 50×g (Kühlzentrifuge Heräus Christ, Osterode, Germany) and the cell precipitation was resuspended in chilled medium II. The precipitate, replenished with medium II to 6.5 ml, finally served as a standard for ITS in the following experiments.
Incubation media
For incubation of the ITS a modified Krebs Ringer bicarbonate solution was used (Table 1). With exception of medium I, all solutions contained the physiological buffer system CO2/H2CO3/HCO3 −/H+, excluding an influence of organic buffer molecules on reactive oxygen metabolites. Medium I was used merely for first-time washing of the cells and during processing, the cells in the collagenase treatment solution (2 mg/ml collagenase or 350 U/ml, Worthing CLS, Freehold, USA). For pH stabilization, hydroxyethyl piperazinyl ethansulfonacid (HEPES buffer) was added. Medium II contained 10 mM lactate as a substrate for gluconeogenesis and additionally 0.5% of albumin. The raised calcium concentration in the collagenase solution catalyzed collagenase activity [9].
The pH in medium I was set up at 4°C, in medium II and the collagenase solution at 37°C by a Schott-glass electrode (Polzin, Kiel, Germany) on pH 7.35. All suspensions were supplemented with 5 mmol/l glycine [5, 6, 10]. With addition of other substances, the equivalent molar mass of NaCl was lowered in the media. Thus, a steady isosmotic extra-cellular concentration existed in all solutions.
Testing procedure
After preincubation and repeated washing processes, ITS were resuspended at 37°C in 50 ml flasks in 5 ml deoxygenated medium II at a concentration of 1 mg cell protein/ml. Consecutively, 30 min hypoxia were performed (pO2 < 1 mmHg) by gassing with a nitrogen-containing gas mixture (95% N2/5% CO2). After hypoxia, reoxygenation was performed for 60 min with carbogen.
Measuring methods
Samples were taken before hypoxia, after 30 min hypoxia, and after 60 min reoxygenation and then centrifuged immediately for 6 s at 20,000×g (Zentrifuge 3200, Eppendorf, Hamburg, Germany). The precipitate was used for K+ and protein determination, the supernatant served for measurement of enzyme activities (lactate dehydrogenase (LDH) or glutamate dehydrogenase (GLDH)), glucose concentration, and the content of TBA-RS. The suspension was mixed immediately with 0.5 ml ice-cold 10% trichloracetic acid (TCA). All used reagents were from p.A. quality and were purchased from Merck (Darmstadt, Germany) and Serva (Heidelberg, Germany).
For ascertainment of the intracellular K+ concentration, the precipitate was mixed with 1 ml aqua destillatum, shaken, and centrifuged for 30 min with a glass bead on a rotation mixer (5432 Eppendorf). The K+ concentration in the supernatant was determined in a flame photometer (K 701 A, Eppendorf) according to a linear standard curve from 0.1–0.5 mmol K+/l.
Enzyme activities and glucose concentration in the supernatant were measured spectrophotometrically with standard test kits (Boehringer, Mannheim, Germany). Precipitates were analyzed for protein content by a modified Lowry method according to Gronow et al. [6, 11]. Oxidative degradation of unsaturated fatty acids caused by oxygen radicals was determined by the formation of TBA-RS: 0.5 ml cell suspensions were homogenized with 0.5 ml 10% TCA, then added to 0.5 ml TBA (0.6%) and heated for 20 min at 95°C.
During this procedure, adducts from the fatty acid oxidation formed a red color complex with TBA. After cooling the mixture to 20°C, the red color complex was extracted in 1.5 ml N-butanol [11] and extinction was measured spectrophotometrically at 500 nm and 535 nm. The difference in absorbance yielded in a range between 1–4 nmol/ml the TBA-RS content according to a linear calibration curve made up with 1,1,3,3-tetraethoxypropan (TEP, Fluka, Neu-Ulm, Germany).
Statistics
All data are expressed as mean ± SD. Statistical comparison between two groups was performed by a paired t-test, multiple comparisons were performed by one way analysis of variance with Dunnett post-hoc correction using a statistical software (Sigma Stat, Jandel Scientific, CA, USA). A p-value < 0.05 was assumed to indicate a significant difference.
Reagents and solutions
All reagents were products of the highest purity grade available. Scavengers were present in the incubation medium in a final concentration of 1 mM ascorbic acid (AA), benzoate (BA), mannitol (MAN), α-tocopherol (TF) and deferoxamine (DFO).
Results
Cellular integrity
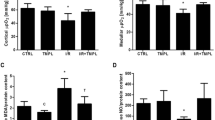
To determine the impact of hypoxia–reoxygenation on cellular integrity and permeability of the inner mitochondrial membrane of ITS, losses of LDH and GLDH were analyzed by measuring the enzyme activity in the incubation medium. In a first series of experiments, the effects of different antioxidative substances in a concentration of 1 mM were examined under conditions of carbogen gassing and under conditions of hypoxia–reoxygenation. ITS without addition of any scavenger served as carbogen gassing and hypoxia–reoxygenated controls (Fig. 1). During carbogen gassing, no relevant protection by all examined scavengers except of TF and DFO could be monitored (Fig. 1a,c; p < 0.05). In comparison to MAN, DFO exerted a significant protection of the mitochondrial membrane (Fig. 1c; p < 0.05). All analyzed scavengers significantly lowered LDH loss from hypoxia–reoxygenated ITS (Fig. 1b; p < 0.05). Further, all scavengers except MAN prevented hypoxia–reoxygenation-induced impairment of mitochondrial integrity, as indicated by a significantly reduced GLDH activity in the medium (Fig. 1d; p < 0.05). Interestingly, the most distinct protection of plasma membrane and mitochondrial membrane integrity was achieved by the iron chelator DFO, which exerted significantly more cellular protection than all other tested antioxidants (Figs. 1b,d; p < 0.05).
Membrane integrity of ITS, measured by LDH (a, b) and GLDH (c, d) concentrations in culture medium, during 90 min carbogen gassing (a, c), as well as after 30-min extreme hypoxia and 60-min reoxygenation (b, d). ITS were incubated in the presence of different radical scavengers (gray bars), such as BA, MAN, AA, TF and DFO. ITS without additional scavengers served as controls (ctrl). Data are given as mean ± SD; *p < 0.05 vs. sham, **p < 0.05 vs. control, ***p < 0.05 vs. DFO; n = 15
Cellular functions
Active metabolic functions in ITS were assessed by measurement of intracellular K+ accumulation and gluconeogenesis. Under carbogen gassing, all scavengers ameliorated the cellular function. The iron chelator DFO clearly demonstrated an additional capacity of ITS for K+ content when compared to MAN, AA, and TF (Fig. 2a; p < 0.05). Compared to TF and MAN, DFO also significantly increased the GNG (Fig. 2c; p < 0.05). During reoxygenation, only MAN and TF in the scavenger group effectively increased intracellular K+ concentration (Fig. 2b; p < 0.05), whereas all antioxidants distinctly increased gluconeogenesis in ITS when compared to controls (Fig. 2d; p < 0.05). Again, DFO showed the highest protective effects and was even significantly more potent to reduce hypoxia–reoxygenation-induced impairment of active cellular functions than the other antioxidants (Figs. 2b,d; p < 0.05).
Cellular function of ITS, measured by intracellular K+ content (a, b) and GNG (c, d) during 90 min carbogen gassing (a, c) as well as after 30 min extreme hypoxia and 60 min reoxygenation (b, d). ITS were incubated in the presence of different radical scavengers (gray bars), such as BA, MAN, AA, TF and DFO. ITS without additional scavengers served as controls (ctrl). Data are given as mean ± SD; *p < 0.05 vs. sham, **p < 0.05 vs. control, ***p < 0.05 vs. DFO; n = 15
Lipid peroxidation
All tested substances were also effective to reduce lipid peroxidation, as indicated by a reduction of TBA-RS formation (Figs. 3a,b; p < 0.05). Under carbogen gassing, DFO induced significantly less TBA-RS formation when compared to controls and MAN treatment (Fig. 3a; p < 0.05). Among the investigated scavengers, TF was found to most effectively prevent hypoxia–reoxygenation-induced TBA-RS formation, whereas MAN was by far less effective. According to previous observations [1], benzoate significantly reduced TBA-RS formation to 60.1% (Fig. 3b; p < 0.05).
Lipid peroxidation in ITS, measured by thiobarbituric reactive substances formation (a, b) during 90 min carbogen gassing (a), as well as after 30 min extreme hypoxia and 60 min reoxygenation (b). ITS were incubated in the presence of different radical scavengers (gray bars), such as BA, MAN, AA, TF and DFO. ITS without additional scavengers served as controls (ctrl). Data are given as mean ± SD; *p < 0.05 vs. sham, **p < 0.05 vs. control, ***p < 0.05 vs. DFO; n = 15
Iron chelator deferoxamine
Because DFO proved to exert the most distinct protection, we hypothesized that this protection was due to its iron-chelating properties. Thus, to determine whether the endogenous tissue-bonded iron contributes to reoxygenation-induced cell injury in ITS, protective effects of increasing concentrations of the iron chelator DFO were examined (Figs. 4, 5, 6).
Spectrophotometric analyses of LDH and GLDH demonstrated that concentrations of 0.0001 and 0.001 mM DFO had no effects on enzyme loss. However, from a threshold of 0.01 mM to 1.0 mM DFO, increasing concentrations dose-dependently caused a significant decrease of LDH and GLDH, which was most distinct at 0.1 mM. An increase of DFO concentration to 1 mM showed no additional protective effects. Higher concentrations of 10 mM induced major membrane disintegration (Figs. 4a,b; p < 0.05). Quantitative analysis of active cell functions revealed that DFO exerted no effect on intracellular K+ and GNG at low concentration, i.e., ≤0.001 mM, whereas from concentrations above 0.01 mM, the active cell functions were found to be significantly improved with a maximum at 0.1 mM and 1.0 mM. Similarly to membrane stability, higher concentrations up to 10 mM induced a significant reduction of cellular function (Figs. 5a, b; p < 0.05). In parallel to enzyme loss, TBA-RS formation was also found to be significantly reduced by DFO at concentrations above 0.01 mM (Fig. 6; p < 0.05).
Discussion
For liver and kidney procurement, perfusion with University of Wisconsin or histidine–tryptophan–ketoglutarate solution followed by hypothermic preservation in the same solution remains the most common technique. However, hypothermic organ storage is associated with oxygen deprivation, which inevitably leads to some degree of ischemia-reperfusion injury upon transplantation. During the cold ischemia time ATP is degraded to hypoxanthine. The catabolism of adenine nucleotides results in an accumulation of hypoxanthine in ischemic cells [12]. Ischemia is also associated with the proteolytic conversion of xanthine dehydrogenase to xanthine oxidase. When xanthine oxidase converts hypoxanthine to xanthine in the presence of molecular oxygen, superoxide radicals (O2 −) and hydrogen peroxide (H2O2) are generated [13, 14]. During reperfusion or reoxygenation a large amount of ROS, especially OH, are produced by the reentry of oxygenated blood into the ischemic organ. ROS, which are generated during reoxygenation in Fe2+-catalyzed reactions, take a central role in cellular dysfunction and exert direct tissue damage, including lipid peroxidation, protein denaturation, and DNA oxidation [15–17]. Free iron catalytically exacerbates oxygen stress, and it has been proposed that superoxide-mediated genotoxicity is a function of its ability to liberate protein-bound iron [18, 19]. Although protein oxidation has been demonstrated at the level of the peptide backbone and amino acids, there has been relatively little scrutiny of differences between proteins in their sensitivities so far [20]. In the context of long term single organ transplantation, an aspect of oxygen toxicity is its promotion by some metals and by elevated O2 partial pressure. Iron and copper catalyze the cleavage of ROS (Fenton’s reaction), leading to the generation of OH [21, 22]. OH is the most reactive oxidant, reacting at diffusion-limited rates. The catalytic properties of iron and copper explain why cells possess metal-chelating proteins such as ferritin and transferrin, which reduce the concentration of redox-active metals. In humans, the body content of iron increases with age (in men throughout their lives, and in women after menopause), and it has been suggested that this accumulation may increase the chronic oxidative damage of transplanted organs with age [23–25].
Despite addition of 5 mM glycine to incubation media, whose cytoprotective qualities have already been presented in numerous studies [1, 4, 5, 26–28], we found distinct cell damages after reoxygenation as indicated by raised enzyme levels and decreased cell functions. The presence of hypoxia–reoxygenation injury in ITS, which occurred even in the presence of glycine, indicates that hypoxia–reoxygenation damage is, at least to some extent, glycine-insensitive [4]. Comparing different scavengers with DFO, it could be evidenced that both a radical-directed and a Fe2+-directed approach significantly attenuates hypoxia–reoxygenation injury. However, DFO could be shown to exert the strongest antioxidant effect as well to best preserve membrane integrity and cellular function.
Oxygen is a highly reactive molecule and can be partially reduced to form a number of chemically reactive radicals so that an enhanced electron flux via mitochondrial electron transfer chain can be obtained. Even under carbogen gassing, DFO and TF stabilized the mitochondrial integrity, as indicated by a decreased GLDH loss. In parallel, the scavengers, but in particular the iron chelator, could significantly enhance the K+ content and the GNG. These findings indicate some improvement by the scavengers and DFO. At least, both did not exert harmful effects on ITS under carbogen gassing.
It can be assumed that under continuous oxygen supply, oxygen radicals arise, which particularly are catalyzed via Fe2+. That would assert the better performance of DFO during oxygen tension in mitochondrial (GLDH) compared to cytosolic membrane integrity (LDH).
Considerably, more distinct were the effects under reoxygenation conditions.
Not only exogenous iron but also iron ions from intracellular storage can contribute under reoxygenation conditions to the generation of reactive oxygen species [29]. Accordingly, even without addition of exogenous iron the addition of 0.01–1.0 mM DFO significantly diminished lipid peroxidation and membrane leakage, and cell function was found to be markedly improved. Even low-dose 0.1 mM DFO was effective to protect ITS from hypoxia–reoxygenation injury by binding intracellular iron ions probably liberated from tissue sources. Kidney tissue contains up to 10 mg iron/kg wet weight [30]. Calculated to tissue dry weight, this amount equals ~15.6 mg iron/kg protein, which in turn corresponds to 0.28 nmol iron/mg protein [31]. Obviously, less than 0.1% of 0.1 mM DFO taken up by the cells would have been sufficient to bind the entire iron quantity of the tubular cells.
As shown in the literature, other iron complex chelators, as iron-ethylenediamine-N,N-diacetate (EDDA), are less effective than DFO [32]. EDDA binds a more insufficient complex and tends during reoxygenation conditions, like intracellular iron accumulators, to generate ROS und to enhance reoxygenation damages. This potency of DFO is underlined by our findings that even low doses of 0.1 mM DFO reduce hypoxia–reoxygenation damage of untreated ITS by approximately 50% to 100%.
Our results are of limited impact on clinical situations because they are obtained in an in vitro model with isolated cells. But our data are in line with several other reports showing protective effects of DFO both ex vivo on ischemia or reperfusion injury in isolated perfused lungs and hearts and in vivo on hemorrhagic shock-induced liver injury [33–35].
Conclusion
Based on these experimental findings, the effect of DFO should be tested in clinical studies against post-hypoxic formation of reactive oxygen species in kidney transplantation with long cold and short warm ischemic times. Actually, DFO is used clinically for the treatment of hemochromatosis and the beta-thalassemia in a limited dosage 40 mg/kg body weight, which corresponds to a concentration of more than 1 mM. In organ preservation, DFO could reduce ischemia-reperfusion-induced organ dysfunction after transplantation as well as the fatal consequences of persisting blood within the transplanted donor organ during organ preservation.
References
Gronow G, Moussavian M, Malyusz M (1999) Effect of hydroxyl radical scavengers in renal cortical cells. Adv Exp Med Biol 471:345–351
Vinas JL, Sola A, Hotter G (2006) Mitochondrial NOS upregulation during renal I/R causes apoptosis in a peroxynitrite-dependent manner. Kidney Int 69:1403–1409
Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Amoscato AA et al (2007) Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther 320:1050–1060
Weinberg JM, Roeser NF, Davis jA, Venkatachalam MA (1997) Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52:140–151
Gronow G, Malyusz M, Niedermayer W, Klause N (1994) Diminution of histidine-induced reoxygenation damage by glycine in posthypoxic renal cells. Adv Exp Med Biol 1345:717–722
Gronow G, Klause N, Malyusz M (1994) Restriction of hypoxic membrane defect by glycine improves mitochondrial and cellular function in reoxygenated renal tubules. Adv Exp Med Biol 361:585–589
Plin C, Tillement JP, Berdeaux A, Morin D (2005) Resveratrol protects against cold ischemia–warm reoxygenation-induced damages to mitochondria and cells in rat liver. Eur J Pharmacol 528:162–168
Zager RA, Burkhart K (1997) Myoglobin toxicity in proximal human kidney cells: roles of Fe, Ca2+, H2O2, and terminal mitochondrial electron transport. Kidney Int 51:728–738
Hashimoto T, Hirata M, Itoh T, Kanmura Y, Kuriyama H (1986) Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol 370:605–618
Gronow GH, Cohen JJ (1984) Substrate support for renal functions during hypoxia in the perfused rat kidney. Am J Physiol 247:618–631
Bertermann H, Gronow G, Weiss C (1975) An improved technique for metabolic studies on isolated cortical cells and tubules of the rat kidney. Curr Probl Clin Biochem 4:76–78
Knox CD, Pierce JM, Nicoud IB, Belous AE, Jones CM, Anderson CD et al (2006) Inhibition of phospholipase C attenuates liver mitochondrial calcium overload following cold ischemia. Transplantation 81:567–572
Cherry PD, Omar HA, Farrell KA, Stuart JS, Wolin MS (1990) Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol 259:1056–1062
Salvemini D, Doyle TM, Cuzzocrea S (2006) Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans 34:965–970
Khalid MA, Ashraf M (1992) Maximal OH production is seen upon reoxygenation of viable anoxic cultured cardiomyocytes but not of compromised cells. Am J Cardiovasc Pathol 4:245–255
Kopnin PB, Agapova LS, Kopnin BP, Chumakov PM (2007) Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species upregulation and genetic instability. Cancer Res 67:4671–4678
Ozer MK, Parlakpinar H, Cigremis Y, Ucar M, Vardi N, Acet A (2005) Ischemia-reperfusion leads to depletion of glutathione content and augmentation of malondialdehyde production in the rat heart from overproduction of oxidants: can caffeic acid phenethyl ester (CAPE) protect the heart? Mol Cell Biochem 273:169–175
Martin LJ, Chen K, Liu Z (2005) Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci 25:6449–6459
Chen L, Lee HM, Greeley GH Jr, Englander EW (2007) Accumulation of oxidatively generated DNA damage in the brain: a mechanism of neurotoxicity. Free Radic Biol Med 42:385–393
Barondeau DP, Kassmann CJ, Tainer JA, Getzoff ED (2006) Understanding GFP posttranslational chemistry: structures of designed variants that achieve backbone fragmentation, hydrolysis, and decarboxylation. J Am Chem Soc 128:4685–4693
Zecchina A, Rivallan M, Berlier G, Lamberti C, Ricchiardi G (2007) Structure and nuclearity of active sites in Fe-zeolites: comparison with iron sites in enzymes and homogeneous catalysts. Phys Chem Chem Phys 9:3483–3499
Maiti D, Sarjeant AA, Karlin KD (2007) Copper(II)-hydroperoxo complex induced oxidative N-dealkylation chemistry. J Am Chem Soc 129:6720–6721
Droge W, Schipper HM (2007) Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 6:361–370
de Vries B, Walter SJ, von Bonsdorff L, Wolfs TG, van Heurn LW, Parkkinen J et al (2004) Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation 77:669–675
Wang XH, Wang K, Zhang F, Li XC, Li J, De W et al (2005) Heme oxygenase-1 alleviates ischemia-reperfusion injury in aged liver. World J Gastroenterol 11:690–694
Weinberg JM, Davis JA, Abarzua M, Kunkel R (1990) Protection by glycine of proximal tubules from injury due to inhibitors of mitochondrial ATP production. Am J Physiol 258:1127–1140
Paller MS, Patten M (1992) Protective effects of glutathione, glycine, or alanine in an in vitro model of renal anoxia. J Am Soc Nephrol 2:1338–1344
Moussavian MR, Slotta JE, Kollmar O, Menger MD, Schilling MK, Gronow G (2007) Hemoglobin induces cytotoxic damage of glycine-preserved renal tubules. Transpl Int 20:884–894
Nakamura J, Purvis ER, Swenberg JA (2003) Micromolar concentrations of hydrogen peroxide induce oxidative DNA lesions more efficiently than millimolar concentrations in mammalian cells. Nucleic Acids Res 31:1790–1795
Grassmann E, Reichlmayr-Lais AM, Kirchgessner M, Kim JJ (1983) Iron concentration in various organs of rats following administration of various iron and protein loads. Z Ernahrungswiss 22:195–204
Paller MS (1994) The cell biology of reperfusion injury in the kidney. J Investig Med 42:632–639
Liu M, Okada S (1994) Induction of free radicals and tumors in the kidneys of Wistar rats by ferric ethylenediamine-N,N′-diacetate. Carcinogenesis 15:2817–2821
Zhao G, Ayene IS, Fisher AB (1997) Role of iron in ischemia-reperfusion oxidative injury of rat lungs. Am J Respir Cell Mol Biol 16:293–299
Shadid M, Van Bel F, Steendijk P, Dorrepaal CA, Moison R, Van Der Velde ET, Baan J (1999) Effect of deferoxamine on post-hypoxic-ischemic reperfusion injury of the newborn lamb heart. Biol Neonate 75:239–249
Bauer C, Marzi I, Larsen R (1997) Deferoxamine-conjugated hydroxyethyl starch reduces reperfusion injury to the liver following hemorrhagic shock. Anaesthesist 46:53–56
Author information
Authors and Affiliations
Corresponding author
Additional information
German Society of Surgery, Surgical Forum 2008, Best of Abstracts.
Rights and permissions
About this article
Cite this article
Moussavian, M.R., Slotta, J.E., Kollmar, O. et al. Post-hypoxic cellular disintegration in glycine-preserved renal tubules is attenuated by hydroxyl radical scavengers and iron chelators. Langenbecks Arch Surg 393, 303–310 (2008). https://doi.org/10.1007/s00423-008-0287-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-008-0287-5