Abstract
Background and aims
We investigated the immune status in 32 pancreatic cancer patients (PC) in comparison with healthy controls (HC).
Materials and methods
Using flow cytometry, peripheral blood lymphocytes (PBL) were characterized by the expression of surface markers for T helper cells (CD4), T suppressor cells (CD8), B cells (CD19) and NK cells (CD56). The blastogenic response of PBL was analyzed after stimulation with concavalin A (ConA), phytohemagglutinin (PHA), pokeweed mitogen (PWM) and anti-CD3 antibodies. The serum levels of TNF-α, IL-1β, IL-2, IL-10, IL-12, IL-18, IL-1RA, sIL-2R and TGF-β were determined by ELISA.
Results
No differences in the distribution of peripheral immunocytes in PC were found, whereas the blastogenic response of peripheral blood lymphocytes (PBL) after stimulation with PHA or anti-CD3 antibodies was significantly decreased in PC. In PC, we found reduced serum levels of IL-2 and significantly elevated levels of TNF-α, TGF-β1, IL-10, IL-2R, IL-1β and IL-1RA.
Conclusion
These data provide evidence for a systemic immune dysfunction in pancreatic cancer patients characterized by a shift towards a T helper cell type 2 cytokine profile, a significant elevation of substances related to T cell suppression and a reduced blastogenic response to PHA and anti-CD3 antibodies of PBL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is the fifth leading course of cancer deaths in the Western world, and is associated with a poor prognosis and a 5-year survival rate of less than 4% [1]. Clinically, pancreatic cancer is characterized by rapid tumor progression, early metastization and unresponsiveness to most conventional treatment modalities [1]. On the molecular genetic level, mutations in oncogenes like K-ras or tumor-suppressor genes like p53 are frequently found [2]. Furthermore, pancreatic cancers are known to produce a wide variety of growth factors, which promote tumor growth like EGF or PDGF or act as tumor-promoting agents and immunosuppressive substances like TGF-β [2].
Cancers, including those of the pancreas, are vulnerable to immune effector cells such as natural killer cells (NK), lymphokine-activated killer cells and tumor-specific cytotoxic T lymphocytes [3]. However, most cancers can evade this immune surveillance by several distinct mechanisms like production of immunosuppressive factors (e.g. TGF-β), inactivation of infiltrating lymphocytes by the FAS/FAS ligand system or by downregulation of the CD3 ζ-chain via caspase-3 induction [4].
We have recently shown that pancreatic carcinoma cells and surrounding tumor-infiltrating lymphocytes express caspase-1 and caspase-3 [5] and that pancreatic cancer patients with elevated markers of macrophage activity like neopterin or sCD44v6 have a highly significant improved prognosis than patients with depressed serum levels of these factors [6, 7]. To test whether the distribution or function of peripheral immunocompetent blood cells is reduced in pancreatic cancer patients in terms of a tumor-driven immunosuppression, we determined (1) the distribution, (2) the blastogenic response of these cells and (3) the serum concentrations of various immunosuppressive, Th1-related or Th2-related cytokines and growth factors in pancreatic cancer patients and compared these data with a gender-matched and age-matched group of healthy volunteers.
Materials and methods
Patients
Between June 1997 and June 1999, 32 patients diagnosed as having pancreatic cancer were enrolled into the prospective study. Three patients were classified UICC stage I, 2 patients were UICC stage II, 15 patients were UICC stage III and 12 patients were UICC stage IV. Fifteen patients underwent resection of the tumor. In 17 patients, palliative operations were performed due to irresectability or distant metastization of the tumor. In all patients, the diagnosis of adenocarcinoma of the pancreas was confirmed by histological examination. The mean age was 65.5 years ranging from 51 to 83 years. All patients enrolled into this study had no hyperbilirubinaemia at the time of investigation. Of the 32 patients, 20 were male and 12 were female. In all patients, 15 ml of heparinated blood was drawn before operation and immediately proceeded for the examinations concerning the immune status. Furthermore, 10 ml of blood was drawn and serum was obtained by centrifugation, aliquoted and stored at −80°C until the determination of the cytokine concentrations. The control group comprised 24 healthy volunteers (mean age 64.7 years ranging from 48 to 79 years), 8 were female and 16 were male.
All patients gave informed consent to the study, which was performed according to the guidelines of the local ethics community.
Phenotyping of peripheral blood lymphocytes
For the identification of peripheral blood lymphocytes in whole blood, we used a four color flow cytometric method as described previously [8]. The lymphocyte sub-sets were counted using antibodies from BD Biosciences, Heidelberg, Germany, to CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), CD45 (clone 2D1), CD16 plus CD56 (clones B73.1 and NCAM16.2), and CD19 (clone SJ25C1) conjugated to flourescein isothiocyanate (FITC), peridinin-chlorophyll protein (PerCP), phycoerythrin (PE), or allophycocyanin (APC). Isotypic immunoglobulin G1 (IgG1) and isotypic immunoglobulin G2 (IgG2) served as background control. Fifty microliters of EDTA-anticoagulated blood was incubated with the appropriate mixture of antibodies for 15 min at room temperature. After incubation for 10 min with 2 ml of FACS lysis solution, cells were washed twice with PBS-0.3% bovine serum albumin and fixed with PBS-1% paraformaldehyde. At least 5,000 cells were analyzed on the flow cytometer (FACSCalibur, Becton Dickinson, Heidelberg, Germany) after gating on lymphocytes for all antibody combinations. Sub-set analysis was performed using the CellQuest software (Becton Dickinson).
Determination of proliferative capacity of peripheral blood lymphocytes
The proliferation capacity of peripheral blood lymphocytes after stimulation with phytohemagglutinin (PHA), concavalin A (ConA), pokeweed mitogen (PWM) and anti-CD3 antibodies was determined by using a commercially available kit (Blastest, YLEM, Roma, Italy). Immediately after puncture, 250 μl of heparinated whole blood were incubated in 3 ml of culture medium and incubated at 5% CO2 atmosphere at 37°C in a glass vial. Every blood sample consisted of five stimulation cultures, namely, PHA-stimulation, ConA-stimulation, PWM-stimulation, anti-CD3-stimulation and control. After a 72 h incubation period, nuclei were isolated and stained with propidium iodide according to the manufacturer’s instructions. The nuclei were analysed on a FACScan (Becton Dickinson, Germany) using the CellQuest program. The corrected stimulation index (CSI) was calculated by the following formula:
Determination of serum concentrations of cytokines
Serum concentrations of the cytokines TNF-α, IL-1β, IL-2, IL-10, IL-12 (total), IL-18 the IL-1RA (receptor antagonist), the soluble IL-2 receptor (IL-2R) and TGF-β1 were determined by commercially available ELISA kits (DPC Biermann, Bad Nauheim, Germany and R&D Systems, Oxford, UK). As markers for monocyte/macrophage release, IL-12, IL-18, TNF-α, the proinflammatory IL-1β and its receptor antagonist IL-1RA were chosen. As markers for lymphocyte activation, the soluble cleaved interleukin-2 receptor CD25 (IL-2R) was measured. For evaluation of Th1 and Th2 lymphocyte activities, IL-2 and IL-10 were determined, respectively. For the estimation of immunosuppressive potential, the serum levels of TGF-β1 were determined.
Statistics
All statistics were calculated using the Medcalc® Software Package. Significance was set at p < 0.05. Significance was calculated using the Student’s t-test.
Results
The function but not the distribution of peripheral blood immunocytes is altered in pancreatic cancer patients.
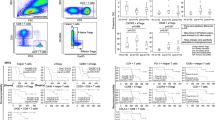
Immunofluorescence analysis of the peripheral blood of pancreatic cancer patients revealed a distribution of immunocytes very similar to the distribution found in the healthy volunteer group (Table 1). T lymphocytes (CD3+/CD4+ and CD3+/CD8+), NK cells (CD56+) and B lymphocytes (CD19+) did not differ significantly between cancer patients and healthy volunteers. The CD4+/CD8+ ratio was also not different between these two groups (Table 1). It is interesting to note that the blastogenic response of the peripheral immunocytes was significantly reduced in pancreatic cancer patients compared to the healthy controls. The blastogenic response of the peripheral blood immunocytes was comparable in both groups after stimulation with ConA and PWM, whereas the blastogenic responses after stimulation with PHA or anti-CD3 antibodies were significantly diminished in pancreatic cancer patients, which can either be due to a primary dysfunction of the T cells or the immunosuppressive activity of monocytes (Fig. 1).
Blastogenic response of peripheral blood lymphocytes from pancreatic cancer patients and healthy controls after stimulation with concavalin A (ConA), phytohemagglutinin (PHA), pokeweed mitogen (PWM) or anti-CD3 antibodies (anti-CD3). The corrected stimulation indices (CSIs) in pancreatic cancer patients were significantly lower after stimulation with PHA or anti-CD3 compared to the healthy controls
Pancreatic cancer patients exhibit an immunosuppressive cytokine profile
Determination of the cytokines revealed that in pancreatic cancer patients TNF-α was significantly elevated compared to the control group (p < 0.05, Fig. 2). Similarly, TGF-β1, which is supposed to be involved in immunosuppressive mechanisms in cancer was significantly higher in pancreatic cancer patients than in the control group (p < 0.05 and p < 0.005, respectively, Fig. 2). IL-2, mainly produced by type 1 T helper cells (Th1) cells, was significantly reduced in pancreatic cancer patients (p < 0.005), whereas IL-10 reflecting the type 2 T helper cell (Th2) activity was significantly elevated in cancer patients (p < 0.05) pointing to a predominance of Th2 cells (Fig. 2). This imbalance in T cell-related activity was also reflected in increased levels of IL-2R in pancreatic cancer patients resulting in a significant decrease in the IL-2/IL-2R ratio in these patients (pancreatic cancer: 0.22 (SEM 0.05); healthy controls: 1.29 (SEM 0.4), p < 0.001). The cytokine IL-1β and its receptor antagonist IL-1RA as markers for monocyte/macrophage cytokine release were also significantly higher in pancreatic cancer patients (p < 0.05 and p < 0.005). However, IL-12 and IL-18 levels did not differ between pancreatic cancer patients and healthy controls (Fig. 2).
The reduced blastogenic response in pancreatic cancer patients correlates with increased serum concentrations of IL-1β, TGF-β, TNF-α and IL-2R and reduced concentrations of IL-2
To evaluate the relationship between the reduced blastogenic response observed in pancreatic cancer patients and the increased serum concentrations of the cytokines described above, we performed correlation analyses between the serum concentrations of the cytokines and the CSIs from the blastogenic response assays (Table 2). IL-2 serum concentrations correlated positively with the blastogenic response after stimulation with PHA or anti-CD3 antibodies, whereas elevated serum concentrations for IL-1β, TGF-β, TNF-α and IL-2R were associated with low proliferation rates after stimulation of peripheral blood with PHA or anti-CD3 antibodies, which supports the hypothesis of a cytokine-driven immune dysfunction in these patients.
The reduced blastogenic response of peripheral blood after stimulation with anti-CD3 antibodies and the increased levels of TGF-β1 are associated with advanced stages of pancreatic cancer
To evaluate whether the immune dysfunction in pancreatic cancer patients increases with the extent of the disease, we performed sub-group analyses correlating the clinicopathological features with the investigated parameters. No correlation was found between tumor stage or metastization and serum levels of TNF-α, IL-1β, IL-1RA, IL-2, IL-2R, IL-10, IL-12 or IL-18. However, pancreatic cancer patients with UICC stage IV tumors had significantly higher serum concentrations of TGF-β1 than patients with UICC stage I–III tumors (UICC stage I–III: 5.8 ng/ml, SEM 0.5 ng/ml; UICC stage IV: 16.3 ng/ml, SEM 5.8 ng/ml, p < 0.05). The same correlation was found with regard to the reduced blastogenic response after anti-CD3 antibody stimulation and advanced tumors that already had spread into distant organs (UICC stage I–III: 8.25%, SEM 1.1%; UICC stage IV: 4.4%, SEM 1.2%, p < 0.05).
Discussion
In recent years, it has become clear that host defense against tumors are controlled by several immunological mediators including cytokines that play an important role in tumor/immune system conflict [9]. Evidence was provided that tumor cells are able to produce growth factors such as TGF-α, IGF-1 [10], or TGF-β1 [11], which on the one hand promote the growth of the tumor cells themselves and on the other hand like TGF-β are able to influence tumor-infiltrating lymphocytes e.g. by downregulation of T cell receptor ζ-chain [4]. Furthermore, a variety of cytokines can be produced by the tumor cells themselves, disturbing the machinery of immune-activating mechanisms. Recently, Bellone et al. demonstrated that pancreatic carcinoma cells express and secrete the proinflammatory cytokines IL-8 and IL-18, whereas IL-2 and IFNγ are not expressed. On the other hand, the anti-inflammatory cytokines TGF-β1, TGF-β2 and TGF-β3 were also expressed and secreted by the tumor cells [12]. Halak et al. were able to show that IL-10 can be produced by tumor cells leading to an inhibition of type 1 immune responses directed at a tumor antigen and non-tumor antigens present at the tumor site [13]. Evidence was provided indicating that cytokines produced by both Th1 (T helper 1) and Th2 (T helper 2) cells play an important role during the development of an immune response and its regulation. While Th1 cells producing IL-2 and IFNγ induce a cellular type immune response, Th2 cells secrete IL-4, IL-5, IL-6 and IL-10 and promote humoral immune response. A correlation between the progression of some diseases, such as human infectious and inflammatory autoimmune diseases, and the balance between Th1 and Th2 responses was suggested. In human cancer diseases, few studies identifying the Th1/Th2 balance were described in non-small cell lung cancer and colorectal cancer patients [14, 15]. In the bone marrow and the blood of pancreatic cancer patients, high numbers of tumor-reactive T cells secreting TH2 cytokines upon stimulation with tumor antigens were found suggesting a shift towards a TH2 profile [16]. In our study, we also found significantly elevated levels of IL-10 and significantly reduced levels of IL-2 in the serum of pancreatic cancer patients compared to a sex-matched and age-matched control group. Furthermore, we found significantly elevated levels of TGF-β1 and TNF-α, both of which are suggested to be actively involved in the immune dysfunction in cancer patients [16]. The cytokine IL-1β and its receptor antagonist IL-1RA were also significantly elevated in cancer patients, whereas IL-12 and IL-18 representing in part the monocytic lineage did not differ significantly compared to the control patients, although serum levels of these cytokines were slightly elevated in pancreatic cancer patients compared to the control group. Anyhow, the problem of identification of the source of certain cytokine production remains difficult, especially in view of the fact that many tumors are able to produce several cytokines like IL-10, IL-1β or the growth factor TGF-β isoforms themselves [13, 17, 18]. Nevertheless, in our group of pancreatic cancer patients, we found a shift in the cytokine profile from a Th1-like type towards a Th2-like type. These cytokine profiles were also reflected in the blastogenic response of peripheral blood to stimulation with ConA, PWM, PHA or anti-CD-3 antibodies. The blastogenic response of cells of the B cellular lineage was not affected in pancreatic cancer patients, whereas the CSIs after stimulation with PHA or anti-CD3 antibodies were significantly diminished in pancreatic cancer patients. It is interesting to note that the number and the distribution of peripheral immunocytes did not differ between the control group and pancreatic cancer patients, whereas the proliferation capacity of peripheral blood immunocytes after PHA or anti-CD3 antibody stimulation was dramatically diminished, pointing to a qualitative but not quantitative suppression of immunocytes in pancreatic cancer patients. These results are supported by the observation of Yanagimoto et al. who found an impaired function of circulating dendritic cells in patients with pancreatic cancer [19]. Our data underline the hypothesis that the immune response in cancer patients is altered [3]. However, our observations in pancreatic cancer patients are clearly different from the generalized immunosuppression seen in patients receiving high doses of corticosteroids or chemotherapy. As described by Finke et al., the term immune dysfunction seems to be more adequate to describe the observed changes in immune function in cancer patients. One possible underlying mechanism could be the interaction of TGF-β and the downregulation of the T cell receptor ζ-chain [3]. Gastman et al. were able to demonstrate that the TCR ζ-chain is downregulated by stimulation with TGF-β via induction of caspase-3 in T cells [4]. Our data support this observation from the clinical point because TGF-β1 levels correlated significantly with the blastogenic response of blood from pancreatic cancer patients to anti-CD3 antibodies. Furthermore, TGF-β1 serum levels and the unresponsiveness after stimulation with anti-CD3 antibodies increased with increasing tumor stages. Together with the elevated serum levels of TGF-β1 and the depressed levels of IL-2, these observations could also explain in part the decrease in blastogenic T cell response in pancreatic cancer patients and could be one explanation for the success of combined immunochemotherapy in this disease [20].
In conclusion we could demonstrate an extensive systemic immune dysfunction in pancreatic cancer patients. On the serum cytokine level, we found a shift towards a Th2 type cytokine profile and a significant elevation of substances related to T cell suppression like TGF-β1, whereas serum levels of IL-2 were significantly decreased. The hypothesis of the immune dysfunction in pancreatic cancer patients is further supported by a significantly diminished blastogenic response of peripheral blood lymphocytes after stimulation with PHA or anti-CD3 antibodies. These observations could be the background for the further development of immunotherapeutic strategies in this disease.
References
Warshaw AL, Fernandez-del Castillo C (1992) Pancreatic carcinoma. N Engl J Med 326:455–465
Gansauge S, Gansauge F, Beger HG (1996) Molecular oncology in pancreatic cancer. J Mol Med 74:313–320
Finke J, Ferrone S, Frey A, Mufson A, Ochoa A (1999) Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today 20:158–160
Gastman BR, Johnson DE, Whiteside TL, Rabinowich H (1999) Caspase-mediated degradation of T-cell receptor zeta-chain. Cancer Res 59:1422–1427
Gansauge S, Gansauge F, Yang Y, Muller J, Seufferlein T, Ramadani M, Beger HG (1998) Interleukin 1beta-converting enzyme (caspase-1) is overexpressed in adenocarcinoma of the pancreas. Cancer Res 58:2703–2706
Birk D, Gansauge F, Gansauge S, Schwarz A, Beger HG (1999) Levels of serum neopterin are increased in pancreatic cancer patients and correlate with the prognosis. Eur J Med Res 4:156–160
Gansauge F, Gansauge S, Rau B, Scheiblich A, Poch B, Schoenberg MH, Beger HG (1997) Low serum levels of soluble CD44 variant 6 are significantly associated with poor prognosis in patients with pancreatic carcinoma. Cancer 80:1733–1739
Manfras BJ, Reuter S, Wendland T, Kern P (2002) Free in PMC increased activation and oligoclonality of peripheral CD8(+) T cells in the chronic human helminth infection alveolar echinococcosis. Infect Immun 70:1168–1174
Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY (1997) The host–tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today 18:493–497
Betsholtz C, Bergh J, Bywater M, Pettersson M, Johnsson A, Heldin CH, Ohlsson R, Knott TJ, Scott J, Bell GI et al (1987) Expression of multiple growth factors in a human lung cancer cell line. Int J Cancer 39:502–507
Smith JJ, Derynck R, Korc M (1987) Production of transforming growth factor alpha in human pancreatic cancer cells: evidence for a superagonist autocrine cycle. Proc Natl Acad Sci USA 84:7567–7570
Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecci A, Pirisi M, Emanuelli G (2006) Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother 55:684–698
Halak BK, Maguire HC Jr, Lattime EC (1999) Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res 59:911–917
Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM (1995) Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res 55:3847–3853
Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU (1996) Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother 42:1–8
Schmitz-Winnenthal FH, Volk C, Z’graggen K, Galindo L, Nummer D, Ziouta Y, Bucur M, Weitz J, Schirrmacher V, Buchler MW, Beckhove P (2005) High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res 65:10079–10087
Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D, Chouaib S, Mami-Chouaib F (1998) Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int J Cancer 77:7–12
Kurtzman SH, Anderson KH, Wang Y, Miller LJ, Renna M, Stankus M, Lindquist RR, Barrows G, Kreutzer DL (1999) Cytokines in human breast cancer: IL-1alpha and IL-1beta expression. Oncol Rep 6:65–70
Yanagimoto H, Takai S, Satoi S, Toyokawa H, Takahashi K, Terakawa N, Kwon AH, Kamiyama Y (2005) Impaired function of circulating dendritic cells in patients with pancreatic cancer. Clin Immunol 114:52–60
Lygidakis NJ, Berberabe AE, Spentzouris N, Dedemadi G, Kalligas T, Loukas G, Sotiropoulou V (1998) A prospective randomized study using adjuvant locoregional chemo-immunotherapy in combination with surgery for pancreatic carcinoma. Hepatogastroenterology 45:2376–2381
Acknowledgement
This work was supported by Grant SFB 518 (C1) from the Deutsche Forschungsgemeinschaft to F.G.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bertram Poch and Errki Lotspeich contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Poch, B., Lotspeich, E., Ramadani, M. et al. Systemic immune dysfunction in pancreatic cancer patients. Langenbecks Arch Surg 392, 353–358 (2007). https://doi.org/10.1007/s00423-006-0140-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-006-0140-7