Abstract
Background
Low molecular weight heparins (LMWHs) are currently used as a standard for anti-thrombotic therapy. Skin necrosis caused by LMWH is a rare and probably under-reported complication. The aim of our systematic review is to analyse the present literature for cases of LMWH-induced skin necrosis, emphasising the pathogenesis, clinical pattern, and management of this rare side effect.
Methods
We performed a Medline literature search (PubMed database) and manual cross-referencing to identify all articles related to LMWH-induced skin necrosis. Data were analysed for type of LMWH used, time until skin necrosis occurred, localisation, size, laboratory findings, switch anticoagulant, complications, and outcome. Additionally, the case of a patient from our hospital is presented.
Results
We included a total of 20 articles (21 cases) reporting on LMWH-induced skin necrosis. Skin necrosis occurred locally and distant from the injection site. Heparin-induced antibodies were frequently observed (positive 9/11 articles, negative 2/11). However, severe thrombocytopenia (platelet count <100,000 cells/ml) occurred in only four cases, while platelet count remained normal in 50% of the cases. After patients had been switched to other anti-thrombotic drugs, the clinical course was usually benign; however, reconstructive surgery was necessary in two cases.
Conclusion
LMWH-induced skin necrosis may occur as part of the heparin-induced thrombocytopenia (HIT) syndrome, but other pathomechanisms, including allergic reactions and local trauma, may also be involved. When HIT is excluded, unfractionated heparin is a safe switch anticoagulant. Otherwise, non-heparin preparations such as hirudin or fondaparinux should be preferred.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heparin preparations have been successfully used for the prevention and treatment of venous thromboembolism for more than 50 years. Despite several new anticoagulant drugs currently in development, low molecular weight heparins (LMWHs) remain the standard for the prophylaxis of venous thromboembolism in most surgical units [1, 2]. LMWHs have been increasingly advocated on the basis of lower risk of bleeding and easier administration than unfractionated heparin (UFH) [3, 4]. Certain side effects, such as heparin-induced thrombocytopenia, heparin-induced osteoporosis, skin irritations and necrosis, may occur less often with LMWH than with UFH [5–7]. Skin necrosis following LMWH administration is rare, even though the true incidence is probably underestimated due to under-reporting [8].
In this systematic review of the literature, we present all published cases of LMWH-induced skin necrosis and discuss the possible pathomechanisms, clinical appearance and therapeutic options.
Methods
To ensure high methodological quality, we adhered to the criteria for systematic review outlined by McAlister et al. [9]. A Cochrane database search revealed that no comparative trials, no case series and no systematic review of articles had been performed. The Medline/PubMed Database was used in the search for relevant literature on low molecular weight-induced skin necrosis. The present literature review also included EMBASE (Elsevier), which contains journals not covered by Medline. The terms “heparin”, “low molecular weight heparin”, “LMWH”, “skin”, “skin reaction”, and “skin necrosis” were used in various combinations. English, German, and French articles were included. The search terms were identified in the title, abstract, or medical subject heading (MeSH). We also used manual cross-referencing to detect further publications on low molecular weight heparin-induced skin necrosis. We applied the following exclusion criteria: articles on side effects of other anti-thrombotic drugs (unfractionated heparin, warfarin), review articles, skin necrosis caused by underlying immunological diseases (anti-phospholipid syndrome). Additionally, one case at our hospital was included. The articles were analysed for the following data: patient age and gender, previous heparin exposure, type of LMWH, time from first LMWH administration until onset of skin necrosis, dose, site of necrosis (local versus distant), result of skin biopsy, coagulation state, concomitant thrombocytopenia and heparin-induced antibody reaction, thromboembolism, therapy of LMWH-induced skin necrosis, and switch anticoagulant.
Results
Case report
A 57-year-old male patient (obese, body mass index 36 kg/m2) was admitted to our hospital with multiple injuries including mild traumatic brain injury, blunt thoracic trauma with multiple rib fractures and haematopneumothorax on the left side, malleolar fracture, and unstable fracture of the lumbar spine. Past medical history revealed a sleep apnoea syndrome.
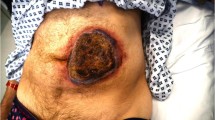
Thromboprophylaxis was started with 15,000 IU/24 h of UFH intravenously after spinal stabilisation (posterior instrumentation with fixateur interne), which was performed on day 3 following admission. After 48 h the patient was transferred from the intensive care unit to the regular ward, and thromboprophylaxis was switched to LMWH (dalteparin, 5,000 IU s.c./24 h). The injection sites were located beside the umbilicus, in the abdominal wall. After 8 days of LMWH administration, we observed a progressive, painful erythematous lesion at the injection sites, and, within hours, areas of necrotic skin developed (Fig. 1). The size of the necrosis was approximately 3 cm in diameter. Coagulation tests and platelet count were normal. Heparin platelet factor-4 (HPF-4) antibody testing was negative by enzyme-linked immunosorbent assay (ELISA). Therefore, we decided to switch anticoagulant therapy to UFH intravenously until the patient’s discharge, since he was at high risk for postoperative thromboembolism. The following course was uneventful, and the necrotic plaques disappeared within 1 week. Subsequently, anterior stabilisation of the spine fracture was successfully performed, and the patient was discharged to a rehabilitation centre without further complications.
Systematic review
The search terms used in various combinations resulted in a total of 247 articles on 20 March 2004. Of these, we identified 20 reported cases of LMWH-induced skin necrosis (Table 1) [10–28]. Thus, a total of 21 cases was reviewed, including the case from our clinic. The articles were published between 1987 and 2003.
LMWH-induced skin necrosis occurred in 11 female and ten male patients (mean age 62±13 years, range 34–87 years; Table 1). Indication for anti-thrombotic therapy was prophylaxis of thromboembolism (n=13; postoperative thromboprophylaxis: n=8/13), treatment of established venous thromboembolism (n=6), acute coronary syndrome (n=1) and atrial fibrillation (n=1).
While in ten articles previous heparin exposure was not reported or was unknown, 11 reports confirmed previous heparin exposure (subcutaneous UFH n=3, systemic intravenous UFH n=4, both subcutaneous and intravenous UFH n=2, LMWH n=2). In most cases, previous exposure to heparin occurred a few days before LMWH administration and consequent onset of skin necrosis; however, in three cases heparin exposure dated back several years [17, 22, 24].
LMWH preparations included dalteparin (9/21), enoxaparin (6/21), tinzaparin (1/21), tedelparin (1/21), certoparin (1/21), nadroparin (1/21) and LMW heparin–dihydroergotamine (1/21). In one case the LMWH preparation was not specified [18]. The daily LMWH dose was reported in 18 articles and ranged from 2,000 IU to 5,000 IU anti-factor Xa (mean dose 4,300±1,355 IU). In one case, a daily dose of anti-factor Xa dalteparin was applied for 5 days because of established deep venous thrombosis [27].
The average time between first administration of LMWH and onset of skin necrosis was 7.6±3.4 days (range 1–17 days). The first symptoms were usually described as erythematous, subcutaneous lesions with oedema and pain at the injection sites. Subsequently, bullous transformation was observed before full-skin necrosis occurred. Biopsies of these urticarial plaques with a necrotic-haemorrhagic centre were obtained in ten cases and showed epidermal necrosis with an inflammatory reaction and microvascular thrombi in dermal vessels. The size of the necrotic areas varied, but generally appeared small and circumscribed, with a maximum diameter of a few centimetres. Occasionally, the skin necrosis enlarged to more than 10 cm [11, 13, 14, 19, 23], in one case involving 6% of the body surface area [24].
Two different patterns of LMWH-induced skin necrosis were observed: The majority of the lesions (16/21) occurred locally at the injection site (abdominal wall, thigh, arm). Less frequently (5/21), other anatomical areas distant from the injection site were affected [13, 18, 19, 26, 27]. These distant manifestations were apparently random in distribution.
The presence of heparin-induced thrombocytopenia was analysed by either an ELISA for HPF-4 antibody (n=10) or a thrombocyte aggregation test [heparin-associated thrombocytopenia (HAT) test], n=1). Heparin-dependent platelet activating antibodies were present in nine out of ten patients. One study showed a negative thrombocyte aggregation test [18].
Platelet count was within the normal range in ten cases, while severe thrombocytopenia occurred in four of 19 patients (thrombocyte concentration <100,000 cells/ml) [13, 18, 20, 21] and mild thrombocytopenia (thrombocyte concentration 100,000 cells/ml–150,000 cells/ml) in five of 19 patients [19, 23–25, 28]. Two articles did not report on platelet concentrations [16, 26].
Other parameters of coagulation (anti-thrombin III, protein s, protein c, prothrombin time, activated partial thromboplastin time) were normal in 15 cases. Five articles did not report on the coagulation state [16, 20, 25–27]. In one case, a patient with a pre-existing anti-thrombin III deficiency and established pulmonary embolism showed a prolonged activated partial thromboplastin time and a positive lupus anticoagulant [18].
After diagnosis of LMWH-induced skin necrosis, anti-thrombotic therapy was switched to coumarin (n=5) [12, 15, 18, 20, 27], unfractionated heparin (n=5, subcutaneous UFH n=2, intravenous UFH n=3) [10, 11, 14, 21, present case], aspirin (n=3) [13, 19, 23], and hirudin (n=2) [25, 28]. In two cases, anti-thrombotic therapy was stopped [22, 24], and in four cases data on anticoagulant switch were not reported [16, 17, 26].
The clinical course of the skin necrosis was uneventful in the majority of the cases (19/21) when LMWH was stopped and changed to alternative anticoagulatory drugs. However, one patient with skin necrosis developed deep venous thrombosis after dalteparin therapy was changed to coumarin therapy [12]. Eventually, UFH was given in this case without further complications. In two cases, surgical therapy was necessary, i.e. debridement of the necrotic skin and soft tissue followed by reconstructive surgery with a skin mesh graft [10, 24].
Discussion
Low molecular weight heparins are heterogeneous preparations with individual molecular weights and pharmacological profiles. They are manufactured through different processes by the cutting down of the molecular structure of unfractionated heparin. The advantages of LMWHs include a predictable anticoagulant response, making daily monitoring unnecessary, improved bioavailability, and a longer half life than UFH. The incidence of skin reactions caused by unfractionated heparin is estimated to be 0.2% [29]. Skin necrosis caused by LMWH is rare and has exclusively been reported in anecdotal cases. Several pathophysiological mechanisms have been proposed: First, LMWH-induced skin necrosis was found to be associated with an established heparin-induced thrombocytopenia (HIT) syndrome. Here, an antibody–platelet–heparin complex leads to an activation of the coagulation cascade that results in microthrombosis of dermal vessels and skin necrosis locally or distant from the injection site [7]. Second, vasculitis of dermal vessels induced by a type III hypersensitivity reaction to the LMWH (Arthus phenomenon with deposit of immunocomplexes on the endothelial structure) has been proposed as an alternative pathomechanism [30]. Third, skin necrosis may be the result of repeated local trauma at the injection site [8]. This non-immunological mechanism is triggered through an incorrect intradermal administration of heparin, with local haemorrhage in a tense dermis leading to pressure on small blood vessels and subsequent necrosis of the overlying skin [8, 31]. In addition, impaired absorption of heparin due to poor vascularisation of adipose tissue may augment local skin damage similar to diabetic lipodystrophy [17].
The switch anticoagulant for patients with LMWH-induced skin necrosis is not standardised. Apparently, UFH may be used as an alternative; however, exclusion of a HIT syndrome is mandatory. Extreme caution has to be taken when a patient is switched to oral anticoagulants, since warfarin (coumarin) may lead to venous gangrene in the presence of a HIT syndrome [32, 33]. Another shortcoming of coumarin therapy is the fact that the anticoagulatory effect is reached only after several days, which is unfavourable for patients with established thrombosis or in patients at high and middle risk. Safe treatment of all patients experiencing anticoagulant-induced skin reactions is feasible, since several anticoagulants are available today [29]. In established HIT syndrome, a switch from heparin to hirudin (a direct thrombin inhibitor) is recommended [34, 35]. Fondaparinux, a selective anti-thrombin-dependent inhibitor of activated factor X, may be used in patients with heparin antibodies because it lacks cross-reactivity and does not lead to platelet activation in serum from patients with established HIT syndrome [36, 37].
In summary, the present review describes the clinical pattern of LMWH-induced skin necrosis. In the majority of cases, heparin–platelet antibodies are detectable, indicating a HIT syndrome, but severe thrombocytopenia with a platelet count below 100,000 cells/ml is rare. Furthermore, there is usually no activation of coagulation, and severe arterial or venous thromboembolic complications associated with fulminant HIT syndrome are exceptional. The outcome of LMWH-induced skin necrosis is usually benign, but, in severe cases, surgical therapy may be necessary.
References
Geerts WH, Anderson JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr, Wheeler HB (2001) Prevention of venous thromboembolism. Chest 119:132–175, 259–265
Hull RD, Pineo GF, Stein P, Mah A, MacIsaac SM, Dahl O, Butcher M, Brant RF, Ghali WA, Bergqvist D, Raskob GE (2001) Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 135:858–869
Kakkar VV, Cohen AT, Edmonson RA, Phillips MJ, Cooper DJ, Das SK, Maher KT, Sanderson RM, Ward VP, Kakkar S (1993) Low molecular weight versus standard heparin for prevention of venous thromboembolism after major abdominal surgery. The Thromboprophylaxis Collaborative Group. Lancet 30:259–265
Litin SC, Heit JA, Mees KA (1998) Use of low molecular weight heparin in the treatment of venous thromboembolic disease: answers to frequently asked questions. Mayo Clin Proc 73:540–550
Leizorovicz A, Simonneau G, Decousus H, Boissel JP (1994) Comparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep venous thrombosis: a meta-analysis. BMJ 309:299–304
Walenga JM, Bick RL (1998) Heparin-induced thrombocytopenia, paradoxical thromboembolism, and other side effects of heparin therapy. Med Clin North Am 82:635–658
Warkentin T (2003) Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol 121:535–555
Wutschert R, Piletta P, Bounameaux H (1999) Adverse skin reactions to low molecular weight heparins: frequency, management and prevention. Drug Saf 20:515–525
McAlister FA, Clark HD, van Walraven C, Straus SE, Lawson FM, Mosher D, Mulrow CD (1999) The medical review article revisited: has the science improved? Ann Intern Med 131:947–951
Cordoliani F, Saiag P, Guillaume JC, Roujeau JC, Wechsler J, Clerici T, Touraine R (1987) Necroses cutanees etendues induites par la fraxiparine (in French). Ann Dermatol Venereol 114:1366–1368
Montserrat I, Lopez D, Zuazu-Jausoro I, Mateo J, Ribera L, Souto JC, Falcon L, Fontcuberta J (1990) Low molecular weight heparin induced skin necrosis. Blood Coagul Fibrinolysis 1:751–752
Ojeda E, Perez MC, Mataix R, Arbalo A, Jimenez S, Campo C, Bal-Da I (1992) Skin necrosis with a low molecular weight heparin. Br J Haematol 82:620
Balestra B, Quadri P, Dermarmels-Biasiutti F, Furlan M, Lämmle B (1994) Low molecular weight heparin-induced thrombocytopenia and skin necrosis distant from injection sites. Eur J Haematol 53:61–63
Koch P, Wagner S, Baum HP (1994) Necroses cutanees a l’heparine et deficit en protein C? Ann Dermatol Venereol 121[Suppl 1]:122
Real E, Grau E, Rubio M, Torrecilla T (1995) Skin necrosis after subcutaneous low molecular weight heparin injection. Am J Hematol 49:253–254
Fried M, Kahanovich S, Dagan R (1996) Enoxaparin-induced skin necrosis. Ann Intern Med 125:521–522
Lefebre I, Delaporte E, Dejobert Y, Catteau B, Piette F, Bergoend H (1997) Enoxaparin-induced cutaneous necrosis localized on insulin lipodystrophies (in French). Ann Dermatol Venereol 124:397–400
Gibson GE, Gibson LE, Drage LA, Garret CR, Gertz MA (1997) Skin necrosis secondary to low molecular weight heparin in a patient with antiphospholipid antibody syndrome. J Am Acad Dermatol 37:855–859
Tietge UJF, Schmidt HH, Jäckel C, Trautwein C, Manns MP (1998) LMWH-induced skin necrosis occurring distant from injection sites and without thrombopenia. J Intern Med 243:313–315
Tonn ME, Schaiff RA, Kollef MH (1997) Enoxaparin-associated dermal necrosis: a consequence of cross-reactivity with heparin-mediated antibodies. Ann Pharmacother 31:323–326
Plath J, Schulze R, Barz D, Krammer B, Steiner M, Anders O, Mach J (1997) Necrotizing skin lesions induced by low molecular weight heparin after total knee arthroplasty. Arch Orthop Trauma Surg 116:443–445
Fureder W, Kyrle PA, Gisslinger H, Lechner K (1998) LMWH-induced skin necrosis. Ann Haematol 77:127–130
Santamaria A, Romani J, Souto JC, Lopez A, Mateo J, Fontcuberta J (1998) Skin necrosis at the injection site induced by low molecular weight heparin: case report and review. Dermatology 196:264–265
Drew PJ, Smith MJ, Milling MA (1999) Heparin-induced skin necrosis and low molecular weight heparins. Ann R Coll Surg Engl 81:266–269
Walder A, Hättenschwiler A, Helfenstein E, Vogt M (2001) Massive Hautnekrosen nach Dalteparin Injektion (in German). Schweiz Med Forum 20/30:773–774
Ang KL, Bose A, Halil O, Cummins D, Amrani M (2003) Low molecular weight heparin (LMWH)-induced skin necrosis in a patient with unstable angina. Int J Cardiol 91:239–240
Payne SM, Kovacs MJ (2003) Cutaneous dalteparin reactions associated with antibodies of heparin-induced thrombocytopenia. Ann Pharmacother 37:655–657
Fontana B, Bodmer A, Gruel Y, Boehlen F, Janer V, Kaya G, Righini M (2004) Skin necrosis is a clinical manifestation of low molecular weight heparin-induced thrombocytopenia. Thromb Haemost 91:196–197
Harenberg J, Hoffmann U, Huhle G, Winkler M, Bayerl C (2001) Cutaneous reactions to anticoagulants. Recognition and management. Am J Clin Dermatol 2:69–75
Ulrick PJ, Manoharan A (1984) Heparin-induced skin reaction. Med J Aust 140:287–289
Lim KB, Tan T (1986) Skin necrosis as a complication of improperly administered subcutaneous heparin. Singapore Med J 27:356–357
Warkentin TE, Elavathil LJ, Hayward CP, Johnston MA, Russett JI, Kelton JG (1997) The pathogenesis of venous limb gangrene associated with heparin-induced thrombocytopenia. Ann Intern Med 127:804–812
Warkentin TE (2001) Venous limb gangrene during warfarin treatment of cancer-associated deep venous thrombosis. Ann Intern Med 135:589–593
Mudaliar JH, Liem TK, Nichols WK, Spadone DP, Silver D (2001) Lepirudin is a safe and effective anticoagulant for patients with heparin-associated antiplatelet antibodies. J Vasc Surg 34:17–20
Hirsh J, Heddle N, Kelton JG (2004) Treatment of heparin-induced thrombocytopenia—a critical review. Arch Intern Med 164:361–369
Amiral J, Lormeau JC, Marfaing-Koka A, Vissac AM, Wolf M, Boyer-Neumann C, Tardy B, Herbert JM, Meyer D (1997) Absence of cross-reactivity of SR90107A/ORG31540 pentasaccharide with antibodies to heparin–PF4 complexes developed in heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis 8:114–117
Parody R, Oliver A, Souto JC, Fontcuberta J (2003) Fondaparinux (ARIXTRA) as an alternative anti-thrombotic prophylaxis when there is hypersensitivity to low molecular weight and unfractionated heparins. Haematologica 88:ECR32
Acknowledgements
The authors thank PD Dr. T. Bombeli, Division of Haematology, Department of Internal Medicine, University Hospital of Zurich, for assistance and correction of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Handschin, A.E., Trentz, O., Kock, H.J. et al. Low molecular weight heparin-induced skin necrosis—a systematic review. Langenbecks Arch Surg 390, 249–254 (2005). https://doi.org/10.1007/s00423-004-0522-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-004-0522-7