Abstract
Background
Liver cysts occur with a prevalence of 4%–7% in the general population. Laparoscopic surgery is effective for solitary cysts and in selected patients with polycystic liver disease (PLD). We present our experience in the laparoscopic management of dysontogenetic cysts.
Patients and methods
Between 1994 and 2002, 36 patients were referred to our centre for the management of dysontogenetic cystic liver disease. Management was laparoscopic in 16 cases. Indications were solitary giant cysts (n=9) and PLD (n=7).
Results
Laparoscopic procedures were completed in 15 patients. Mean operating time was 90 min. There were no deaths. In one case there was an intraoperative complication: bleeding from a superficial hepatic vein necessitated conversion to an open procedure. There were two postoperative complications: one patient with biliary leakage, which was managed conservatively, and one patient with a pneumothorax caused by the cava catheter installed for anaesthesia. Median follow-up was 36 months. There was no symptomatic recurrence.
Conclusion
Laparoscopy can be recommended as the procedure of choice for symptomatic solitary giant cysts and PLD Gigot type I.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver cysts are not uncommon, occurring with a prevalence of 4%–7% in the general population [1]. Although some cysts may attain large sizes and become symptomatic, others remain asymptomatic and frequently present as an incidental finding on ultrasonography or computed tomography (CT). Cystic lesions of the liver represent a wide spectrum of diseases, ranging from simple benign cysts to potentially malignant cysts. Before the era of laparoscopic surgery, open surgical deroofing was the treatment of choice for uncomplicated hepatic cysts. In 1991, the first laparoscopic deroofings of simple giant liver cysts were performed [2–4]. Currently, laparoscopy is widely used in patients with simple giant liver cysts and in selected patients with polycystic liver disease (PLD) [5–14]. We present our experience in the laparoscopic management of these patients.
Patients and methods
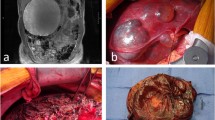
Between 1994 and 2002, 36 patients (28 women, eight men) were referred to our centre for the management of cystic liver disease. Their median age was 57 years (range 30–79 years). There were 18 patients with PLD, 14 with simple giant cysts of the liver, one with a malignant cyst, and three with Caroli’s disease. Laparoscopic management was undertaken in 16 patients with solitary giant cysts (n=9) or PLD Gigot type I (n=7); in the other patients open procedure was chosen due to the extent of the disease. In five patients with simple cysts, open procedure was chosen. In two cases this was due to adhesions resulting from preceding laparotomies; in three further patients it was due to suspicion of neoplasm. All patients underwent preoperative ultrasonography and CT (Table 1). The median size of the solitary simple cysts was 15 cm (range 10–20 cm) [(Fig. 1)]; the median diameter in patients with PLD was 11 cm (range 5–22 cm). All patients were symptomatic; the presenting complaints were abdominal pain or symptoms related to compression of adjacent organs, including nausea, vomiting or early satiety. Three patients with simple cysts showed mild elevation of the liver enzymes glutamine-oxaloacetic-transaminase (GOT), glutamic-pyruvic-transaminase (GPT), and gamma-glutamyl-transpeptidase (γ-GT); these liver enzymes and total bilirubin level were elevated in four patients with PLD. Creatinine was elevated in two patients with PLD and additional polycystic kidney disease (PKD). There were seven patients with PLD and associated PKD.
Operating technique
We insert a 10 mm trocar at the umbilicus and introduce a 30° video-laparoscope. After surveying the intra-abdominal organs, we position a 10 mm trocar in the epigastrium,together with a 5 mm trocar, subxiphoidal and on the right-hand side; a second 5 mm trocar is positioned on this side when we perform a concomitant laparoscopic cholecystectomy (LCHE). The trocars are inserted at a lower level than for LCHE. Initially, we aspirate the fluid from the cysts then we open them with scissors or a harmonic scalpel and aspirate the rest of the fluid. We then explore the cyst cavity for indentations that could indicate neoplasia. In our experience, preoperative imaging is deceptive, and, therefore, we think it is essential to explore the cyst cavity intraoperatively, as this can be done without further trouble and takes minimal time. The cyst wall is removed and sent to pathology for histological examination. It is very important to look for open bile ducts at the edge of the cyst, and, if they are present, to close them with haemostatic clips or suture. Haemostasis is achieved with the argon beam coagulator or electrocautery. We have used the argon beam coagulator in two-thirds of our patients and have had no problems with gas embolism. We generally choose 12 mmHg for the upper limit of abdominal pressure and, if this limit is exceeded, we stop using the argon beam coagulator. Bi-polar cautery is not used. In PLD it is very important to be avoid deep hepatic veins. A drain is placed laterally between liver and diaphragm. The fascia is closed carefully, and the skin is sutured with resorbable material.
Results
Laparoscopic deroofing was successfully completed in 15 patients (Figs. 2 and 3). In one patient with PLD, bleeding from a superficial hepatic vein necessitated conversion to open procedure. The injury was due to coagulation. This patient required 2 units of blood. Two patients had postoperative complications: in one case, pneumothorax due to placement of a cava catheter for anaesthesia required thorax drainage. In the other, biliary leakage was handled conservatively, and the leakage stopped spontaneously. There were no postoperative deaths. In two patients with PLD, and in four patients with simple cysts, laparoscopic cholecystectomy for gallstones was done in the same session. The median operating time was 95 min (range 55–150 min).
Histological examination of surgical specimens revealed that the cysts had a flattened or cuboidal epithelial lining. The supporting connective tissue was usually scanty, except for some larger cysts where it was found to be dense, sometimes hyalinized, and infiltrated by a small number of inflammatory cells, usually lymphocytes. The median length of hospital stay was 10 days (range 7–11 days).
The median follow-up time was 35 months (range 18–60 months). After 1 year, patients with giant cysts alone underwent ultrasonography, and those with PLD underwent CT. There were no symptomatic recurrences.
Discussion and conclusion
Hepatic cysts are quite common and appear more often in women, probably due to the presence of oestrogen [7, 15]. Cysts are classified as true congenital or acquired. The first group includes simple cysts as well as PLD, and the other group comprises tumoral, post-trauma and hydatid cysts.
Simple cysts are the most common cystic lesions of the liver and are often found during routine ultrasonography or CT [5–11]. Patients with these lesions often lack significant symptoms. Small, simple cysts require no active treatment and should be carefully re-assessed yearly by ultrasonography [5, 7–13]. Yearly ultrasonography is not burdensome for the patient and adds considerably to the patient’s feeling of security. Asymptomatic cysts that measure more than 10 cm should be removed surgically, as the risk of internal bleeding, spontaneous perforation or secondary infection is rather high [2–15]. Symptomatic cysts mainly cause pain, nausea, vomiting and jaundice [2–15]. A Mayo clinic study that covered the period between 1907 and 1971 revealed that only 24% of simple cysts are symptomatic [16].
PLD is more prominent in women than in men, and its frequency increases with the woman’s age, usually up to the menopause [6]. Liver cysts will be found in 25%–35% of patients with PKD [17, 18]. There is also an autosomal dominant PLD, which occurs independently of autosomal dominant PKD; it is linked to a locus on chromosome 19p [17, 18]. Gigot et al. [5, 6, 10] have classified PLD into three types: type I has fewer than ten cysts with a diameter more than 10 cm each; type II shows diffuse involvement of liver parenchyma with multiple medium-sized cysts with some liver parenchyma between the cysts, and type III features lesions without liver parenchyma between the cysts.
Routine laboratory values, including those of liver function tests, are generally normal [3, 5–7, 10, 11]. Serological tests for echinococcusspp. should always be done when patients present with cystic liver lesions. When tumour aetiology is uncertain, tumour marker CA 19-9 should be checked; when it is enhanced, it often suggests cystadenoma or cystadenocarcinoma [3, 5, 6, 10–13, 15].
The most accurate imaging modality for diagnosis of hepatic cysts is ultrasonography, with a sensitivity and specificity greater than 90% [19]; it is recommended as the initial diagnostic modality. On CT scans, hepatic cysts are non-enhancing dense fluid lesions with a thin uniform wall. CT scans show the relationship between liver cysts and surrounding structures such as blood vessels, bile ducts and hollow viscera [19]. Magnetic resonance imaging (MRI) shows hepatic cysts as well-circumscribed lesions that produce areas of very low intensity on T1-weighted images and high intensity on T2-weighted images [19].
Surgical procedures have replaced non-operative management because simple aspiration results in 100% recurrence [5, 7–10, 13, 19]. We do not use alcohol on cysts; conflicting data have been published in the literature [5–7, 9, 10]. The goal of surgical management is to decompress the cysts and avoid recurrence. Cyst fenestration, introduced by Lin et al. in 1968 [20], and deroofing are now performed laparoscopically. Simple cysts and Gigot type I lesions can be managed laparoscopically, while type II and III lesions require open fenestration with hepatic resection [5–16]. Bleeding, biliary injury and deep cysts inside the hepatic parenchyma are cause for conversion. In the literature, the recurrence rate ranges from 0% to 23% [5–9, 11–13, 21, 22]. To reduce recurrences, the surgeon could place an omental flap into the cystic cavity. Careful haemostasis of the edge of the cyst is also important. Argon beam coagulator, harmonic scalpel and infrared coagulation can be used [5, 7, 9–11, 13].
Laparoscopic operations combine the advantage of a minimally invasive approach with the effectiveness of open surgery. The clinical recurrence rate is comparable to that of open procedures. Any suspicion of neoplasm is an absolute contraindication for laparoscopy. Repeat laparoscopic fenestration is possible and effective in managing recurrent cysts. In conclusion, laparoscopic surgery can be recommended as the procedure of choice for symptomatic liver cysts.
References
Caremani M, Vincenti A, Benci A, Sassoli S, Tacconi D (1996) Echographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound 21:115–118
Z’graggen K, Metzger A, Klaiber C (1991) Symptomatic simple cyst of the liver: treatment by laparoscopic surgery. Surg Endosc 5:224–225
Paterson-Brown S, Garden OJ (1991) Laser-assisted laparoscopic excision of liver cysts. Br J Surg 78:1047
Lange V, Meyer G, Rau H, Schildberg FW (1992) Minimal invasive Eingriffe bei solitären Lebercysten. Chirurg 63:349
Gigot JF, Legrand M, Hubens G, de Canniere L, Wibin E, Deweer F, Druart ML, Bertrand C, Devrieendt H, Droissart R, Tugilimana M, Hauters P, Vereecken L (1996) Laparoscopic treatment of nonparasitic liver cysts: adequate selection of patients and surgical technique. World J Surg 20:556–561
Gigot JF, Jadoul P, Que F, Van Beers BE, Etienne J, Horsmanns Y, Collard A, Geubel A, Pringot J, Kestens PJ (1997) Is fenestration the most adequate operation for long-term management? Ann Surg 225:286–294
Koperna T, Vogl S, Satzinger U, Schulz F (1997) Nonparasitic cysts of the liver: results and options of surgical treatment. World J Surg 21:850–855
Fabiani P, Mazza D, Toouli J, Bartels M, Gugenheim J, Mouiel J (1997) Laparoscopic fenestration of symptomatic non-parasitic cysts of the liver. Br J Surg 84:321–322
Martin IJ, McKinley AJ, Currie EJ, Holmes P, Garden J (1998) Tailoring the management of nonparasitic liver cysts. Ann Surg 228:167–172
Gigot JF, Metairie S, Etienne J, Horsmanns Y, van Beers BE, Sempoux C, Deprez P, Materne R, Geubel A, Glineur D, Gianello P (2001) The surgical management of congenital liver cysts. Surg Endosc 15:357–363
Berends FJ, Meijer S, Prevoo W, Bonjer HJ, Cuesta MA (2001) Technical considerations in laparoscopic liver surgery. Surg Endosc 15:794–798
Zacherl J, Scheuba C, Imhof M, Jakesz R, Függer R (2001) Long-term results after laparoscopic unroofing of solitary symptomatic congenital liver cysts. Surg Endosc 14:59–62
Ammori BJ, Jenkins BL, Lim PCM, Prasad KR, Pollard SG, Lodge PA (2002) Surgical strategy for cystic diseases of the liver in a Western hepatobiliary center. World J Surg 26:462–469
Tan YM, Ooi LL, Soo KC, Mack POP (2002) Does laparoscopic fenestration provide long-term alleviation for symptomatic cystic disease of the liver. ANZ J Surg 72:743–745
Duca S, Cazacu M, Vlad L, Paraion I, Iancu C, Toganel D, Rusu C (1993) Nonparasitic abdominal serous cysts. A multiple case report. Acta Chir Belg 93:18
Sanfellipo PM, Beahrs OH, Weiland LH (1974) Cystic disease of the liver. Ann Surg 179:922–925
Tahvanainen P, Tahvanainen E, Reijonen H, Halme L, Kaariainen H, Hockerstedt K (2003) Polycystic liver disease is genetically heterogeneous: clinical and linkage studies in eight Finnish families. J Hepatol 38:39–43
Li A, Davila S, Furu L, Quian Q, Tian X, Kamath PS, King BF, Torres VE, Somlo S (2003) Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am J Hum Genet 72:691–703
Cowles RA, Mulholland MW (2000) Solitary hepatic cysts. J Am Coll Surg 191:311–321
Lin TY, Chen CC, Wang SM (1968) Treatment of non-parasitic cystic disease of the liver: a new approach to therapy with polycystic liver. Ann Surg 168:921
Hansen P, Bhoyrul S, Legha P, Wetter A, Way LW(1997) Laparoscopic treatment of liver cysts. J Gastrointest Surg 1:53–47
Diez J, Decoud J, Gutierrez L, Suhl A, Merello J (1998) Laparoscopic treatment of symptomatic cysts of the liver. Br J Surg 85:25–27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kornprat, P., Cerwenka, H., Bacher, H. et al. Minimally invasive management of dysontogenetic hepatic cysts. Langenbecks Arch Surg 389, 289–292 (2004). https://doi.org/10.1007/s00423-004-0506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-004-0506-7