Abstract
Purpose
Due to the mechanistic role of myostatin and follistatin in modulating muscle mass, shifts in the follistatin to myostatin ratio (F:M) may help explain changes in muscular size in response to resistance training (RT). The present study examined whether differential responses in follistatin and myostatin occur based on the amount of active musculature in a RT program in middle-aged men.
Methods
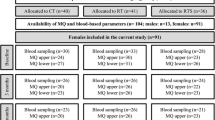
Forty middle-aged men (age = 46.5 ± 3.1 years) were randomly assigned to 1 of 4 groups, upper-body RT (UB; n = 10), lower-body RT (LB; n = 10), combined RT (UB + LB; n = 10) or control (C; n = 10). The training protocol consisted of three exercise sessions per week for 8 weeks. Blood samples were obtained at baseline and 48 h after the final session of the training program.
Results
Muscle mass significantly increased (p < 0.05) following UB = 0.76 ± 0.46 kg, LB = 0.90 ± 0.29 kg, UB + LB = 1.38 ± 0.70 kg, compared to no changes after control. Serum follistatin increased in the LB = 0.24 ± 0.06 ng mL−1, UB = 0.27 ± 0.17 ng mL−1, UB + LB = 0.50 ± 0.18 ng mL−1, while serum myostatin decreased in the LB = − 0.11 ± 0.08 ng mL−1 and UB + LB = − 0.34 ± 0.23 ng mL−1, but not UB = 0.07 ± 0.16 ng mL−1. Further, change in concentration following training was larger between UB + LB and either LB or UB alone for both follistatin and myostatin.
Conclusions
Both UB and LB increase muscle mass and alter the F: M ratio; however, the change in these endocrine markers is approximately twice as large if UB and LB is combined. The endocrine response to RT of myostatin and follistatin may depend on the volume of muscle mass activated during training.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing age is associated with gradual, progressive and spontaneous erosive changes in most physiological systems and functions of the body. It has been well demonstrated that a substantial decrease in muscle strength occurs with aging, which decreases the ability to perform daily activities in middle-age and older adults (Viitasalo et al. 1985). To combat these decrements in muscular performance and functional ability, it is recommended that middle-aged and older adults perform regular resistance training (RT) (Hakkinen et al. 2000). It is well established that RT is an effective modality for increasing muscular strength, hypertrophy, and power (Frontera et al. 1988). Based on available evidence, regular RT in middle-aged adults induces muscle hypertrophy, which positively influences muscular strength and quality of life, both in the immediate period, and ideally into older age (Law et al. 2016). Recently, the influence of RT on myokines and adipo-myokines involved in the stimulation and inhibition of the muscular hypertrophic response has been explored. One notable myokine is myostatin, a powerful negative regulator of muscle size (Elliott et al. 2012). Myostatin circulates in the bloodstream, binds to Activin Type II receptors in the muscle, thereby instigating intercellular signaling pathways that inhibit muscle growth (Gonzalez-Cadavid et al. 1998). Whilst a logical hypothesis would be for an increase in serum myostatin with advancing age, there is currently mixed evidence for this. Indeed, while early work suggested that serum myostatin levels increase with advancing age (Yarasheski et al. 2002), more recent examinations have suggested no difference in circulating myostatin concentration in younger vs older men with and without sarcopenia (Ratkevicius et al. 2011) nor in a cross-sectional study of individuals spanning 18–68 years of age (Barrios-Silva et al. 2018). RT can reduce the expression of myostatin at the mRNA or protein level (Hulmi et al. 2007), leading to exercise-induced muscle hypertrophy in the healthy humans. In fact, many studies have shown that RT can generate a significant decrease in myostatin levels (Allen et al. 2011; Laurentino et al. 2012). For instance, it has revealed that serum myostatin levels decreased 10% by enzyme-linked immunosorbent assay (ELISA) after 12-week arm and leg press RT (Saremi et al. 2010). In addition, it has been reported reduced myostatin mRNA expression in young and old men and women in response to 9 weeks of RT (Roth et al. 2003). The mechanism responsible for the RT-induced blunting of myostatin may involve the myostatin-inhibitor follistatin. This glycoprotein blocks the myostatin receptor, thus decreases myostatin’s effect and increasing muscle mass (Tortoriello et al. 2001). In the presence of follistatin, myostatin is unable to bind to its own receptor, and its atrophic actions are inhibited. In a study, the authors demonstrated that 12-week high-intensity RT increased serum follistatin-like related gene levels, a homolog of follistatin in certain physiological mechanisms, including myostatin inhibition (Willoughby 2004). Thus, the ratio of follistatin to myostatin (F:M) has become a commonly reported important factor in studies examining body composition (i.e., lean mass and fat mass) and the alterations of muscle strength that occur with chronic training (Rodgers and Garikipati 2008; Tortoriello et al. 2001). It was investigated the effect of 8 weeks of two different intensities of RT (low or high) on F:M ratio in 21 sedentary young women. They showed that F:M ratio increased dramatically in high-intensity group (Attarzadeh Hosseini et al. 2017). In addition, it has observed an increase of F:M ratio after 8 weeks of blood flow restriction (KAATSU) training in active men (Laurentino et al. 2012). However, although several studies report an increase in the F:M ratio with RT, such changes have not been found in all investigations (de Souza et al. 2014; Schiffer et al. 2011). This discrepancy might be related to different variables within a RT program, including the amount of active musculature involved. It is likely that the total amount of active musculature plays a role in adaptations to RT, and current recommendations advocate the training of the entire body’s musculature (Medicine ACoS 2009). However, limited information is available concerning the impact of the quantity of active musculature on the F:M ratio. Relative to reports in younger adults, less work has examined the effect of training on myostatin and follistatin in older cohorts. The Vienna Active Ageing cohort reported no change in myostatin following 6 months of RT in a cohort of older women (65–92 years of age). RT was performed twice a week and consisted of various elastic band exercises for the major muscle groups. Intensity was progressed by increasing the resistance of elastic band. (Hofmann et al. 2016). Whilst chronically trained master athletes show similar concentrations of circulating serum myostatin as age-matched untrained controls (Elliott et al. 2017). As myostatin appears unresponsive to acute and chronic training in an older cohort, early and sustained (e.g., young-adult to middle aged) RT interventions may be needed for functional maintenance of muscle mass and strength during aging. Therefore, the purpose of the present investigation was to determine the responses in follistatin and myostatin following RT of differing muscle volumes in middle-aged men. It was hypothesized that the RT program involving the greatest quantity of muscle mass (i.e., full-body training) would elicit greater increases in follistatin and decreases in myostatin as compared to programs involving only select portions of the body’s musculature (i.e., upper-body or lower-body training only).
Methods
Participants
Forty middle-aged sedentary men (40–53 years) participated on the present study. Exclusion criteria included cardiovascular diseases, diabetes, hypertension, sleep disorders or other risk factors based on a doctor’s examination. The participants self-reported via health and exercise history questionnaires performing less than 1 h of exercise per week in the previous year, sleeping at least 7–8 h during the 24-h day and not taking any supplements or medications, including non-steroidal anti-inflammatory drugs. All participants gave written informed consent before their inclusion in the study. The study protocol was approved by the Institutional Human Subject committee and carried out in accordance with the Declaration of Helsinki.
Study design
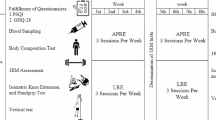
Before baseline measurements, participants were familiarized with the study tests and procedures and were randomly assigned to one of four groups: upper-body RT (UB; n = 10), lower-body RT (LB; n = 10), combined upper- and lower-body RT (UB + LB; n = 10) or control (C; n = 10) groups. Allocation was stratified by BMI (< 25.0 or ≥ 25.0 kg/m2), and the sequence was randomized by a computer. Measurements were collected at baseline and after 8 weeks during the same time of day (− 1 h). Participants were instructed not to alter their regular lifestyle and dietary habits during the study.
Anthropometry and body composition
Upon entering the laboratory, participants were asked to urinate (void) completely within 30 min of the test and then had their body weight measured with a digital scale (lumbar, China) to the nearest 0.1 kg. Participant’s height was measured with a stadiometer (Race industrialization, China) to the nearest 0.1 cm.
BMI, PBF, SkMM and LBM were evaluated by multi-frequency bioelectrical impedance device (Inbody 720, South Korea; Table 1). We instructed the participants to fast for 12 h (an overnight fast, with at least 8 h of sleep) and refrain from physical activity for the previous 36 h before the test. The participants were also instructed to avoid exercising consuming alcohol for 48 h before the test.
Blood sampling and analysis
Fasting blood samples (5 mL) were obtained from the cubital vein using standard procedures. The initial collection occurred 48 h before the baseline training session. Blood samples were clotted for 20 min at room temperature before being centrifuged at 3000 RPM for 20 min. Spun serum was removed from the centrifuge and frozen at − 70 °C for later analysis. Serum myostatin (bound) (human myostatin, Glory Science Co, Del Rio, TX, USA) and follistatin (human follistatin, Glory Science Co, Del Rio, TX, USA) concentrations were measured in duplicate using enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer instructions. The assay’s sensitivity was 0.254 ng mL−1 for myostatin and 0.250 ng mL−1 for follistatin. The intraassay and interassay coefficients of variation were 8.1 and 4.5%, 8.5 and 5.4% for myostatin and follistatin, respectively.
Strength testing
1RM testing
Maximal strength testing took place 24 h after the body composition measurement. 1RM testing was performed to determine training intensity for our resistance training protocol. Before the beginning of the test, the authors explained all the purpose, attendant risks, and discomforts, responsibilities of the participant, benefits, inquiries and freedom of consent for all of them in Ferdowsi university of Mashhad. They were instructed to refrain from alcohol for 48 h, caffeinated drinks for 12 h and food intake for 2 h before the testing session; however, water consumption was allowed. Participants warmed up their bodies for 10 min of general (5 min slow running on treadmill; 3–5 km speed, or elliptical; with 5–10 level) and specific warm-up activities (5 min, e.g., medicine ball twist 1 × 10, medicine ball wood chops 1 × 10, straddled toe touch 2 × 5, Dynamic quadriceps stretch 1 × 5, medicine ball squat 1 × 5–8) before the test. The participants performed two attempts and their highest lifted weight and number of repetitions was recorded. The number of repetitions to fatigue did not exceed ten. There was a 3–5-min rest period between the attempts. There was no arousing stimulus during testing. After the testing session, the participant’s maximal strength was predicted using the following formula:
Isometric quadriceps strength test
The participants stood on the body of the device and bent the knees to 130°–140° and held the trunk upright. The participant grasped the dynamometer handle with the pronation position and adjusted the length of the chain so that the handle of the dynamometer was placed on the thigh. The existing belt attached to the participants shoulders and attached it to each side of the handle of the dynamometer. This allowed the direct quantification of quadriceps isometric force. The participants performed the test three times each time and rested for 3–5 min between each attempt to reduce measurement error due to fatigue. All three attempts were recorded, while the highest was used for the analysis.
Isometric handgrip strength test
The participants held the dynamometer in the hand to be tested, with the arm at right angles and the elbow by the side of the body. The handle of the dynamometer was adjusted as required, with the base resting on first metacarpal (heel of palm), while the handle rested on the middle phalange of the four fingers. When ready the participant contracted the dynamometer with maximum isometric effort, which was maintained for approximately 5 s. No other body movement was allowed. The participants were instructed to give a maximum effort. All three attempts were recorded, while the highest was used for the analysis (Roberts et al. 2011).
Resistance training protocol
Preparatory phase
All the participants performed 1 week of RT, consisting of three exercise sessions, for familiarization before the main training intervention. This phase allowed for supervised instruction of proper lifting technique, familiarization with all exercises, and ensured that the participants initiated the study with a comparable training base. The adaptation phase included a total of six exercises (Table 2). The preparatory phase program was adapted from previous literature in middle-age men (Church et al. 2016).
Training phase
Following the preparatory phase, participants in the UB, LB and UB + LB completed the supervised training three times a week, separated by at least 48 h for 8 weeks. All training sessions were performed between 5 and 6 pm. For each training session, the first 10 min included general and specific warm-up activities (slow running, stretching and light RT). After the general warm-up, participants completed a specific warm-up of 2 sets of 20 repetitions with 30% of 1RM with a 30 s between sets. Following the specific warm-up, training included three sets per exercise in weeks 1–4 and four sets per exercise in weeks 5–8. The rest interval between sets was 30 s in the first and second week, 60 s in the third and fourth week, 75 s in fifth and sixth weeks, and 90 s in seventh and eighth weeks (Table 3). The rest interval between exercises was 2 min throughout the training period.
In each RT program, all exercises included three sets with the intensity of 50–80% 1RM (Table 3). To verify the principle of overload, the following formula was used to predict the 1RM and then determine the exercise load before the start of the training period, the fourth week, and at the end of the eighth week:
The exercises (in order) included in the UB training program were lateral pulldown, chest press, barbell shoulder press, lateral raise, standing barbell biceps curl, and cable triceps pushdown. The exercises included in the LB training program were barbell squat, hack squat, lunges, leg extension, lying leg curls and standing calf raises. The exercises included in the UB + LB program were lateral pulldown, barbell squat, chest press, lunges, lateral raise and standing calf raise. All training sessions were completed under cautious supervision from certified trainers and researchers. The periodized RT programs were adapted from previous literature (Simão et al. 2013), following recommendations by the National Strength and Conditioning Association (Haff and Triplett 2015).
Nutrient intake and dietary analysis
Since the importance of nutrition timing during and around an exercise session, we controlled dietary intake in the hours near (before and after) our training sessions. The participants consumed a banana (0.30–0.35 g of carbohydrate per kilogram of body weight) as a pre-exercise snack ~ 1 h before the training session. In addition, dinner was consumed ~ 1.5–2 h after each training session and was controlled to contain the same amount of carbohydrates (1.7 g/kg of body weight), protein (0.3 g/kg of body weight) and fats (0.4 g/kg of body weight). This was based on the recommendations by the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine for macronutrient distribution (55–65% of total calories from carbohydrates, ˂ 35% of total calories from fats and 10–15% of total calories from protein) and protein timing (0.25–0.3 g of protein/kg of body weight for up to 2 h after resistance training) (Thomas et al. 2016). Other than this small equalization around the exercise sessions, our participants were instructed not to alter their dietary habits during the study. To minimize dietary variability, the participants were required to submit 3-day (2 weekdays and 1 weekend) food records at baseline and at 8 weeks of the assigned intervention. Each dietary item was entered into the program Diet Analysis Plus version 10 (Cengage, Boston, MA, USA) and total energy consumption, and the amount of energy derived from proteins, fats, and carbohydrates was assessed, with paired sample t tests used to confirm a lack of change in dietary factors at pre- and post-time points (Table 4).
Statistical analysis
Normality was confirmed by the Shapiro–Wilk test. Based on previous data (Negaresh et al. 2017; Saremi et al. 2010), it was calculated that ten participants per group would provide 80% power (two-sided α = 0.05) to detect 9% and 7% changes in myostatin and follistatin, respectively. Differences between phenotypic measures, strength measures and endocrine markers were compared by a 4 × 2 ANOVA with repeated measures (group (control × LB × UB × UB + UB) × time (pre × post)). One-way ANOVA was used for ‘change in’ (Δ) scores between times (pre, post) where appropriate. Post hoc testing was performed by Bonferroni corrected t tests. SPSS (Version 24, IBM) was used for all statistical analyses, and all figures were prepared in GraphPad Prism (Version 5.03, GraphPad Software).
Results
RT alters phenotype in a training model-independent manner
Baseline parameters between the two groups were not significantly different (p > 0.05). A significant group × time interaction was noted for SkMM (p < 0.001) and for FM (p < 0.001; Fig. 1a, c, respectively). SkMM had a significantly greater increase [UB = 0.76 kg (95% confidence interval {CI} 0.48–1.05); LB = 0.90 kg (95% CI 0.72–1.08) and UB + LB = 1.38 kg (95% CI 0.95–1.81)] and FM a decreased [UB = − 1.61 kg (95% CI − 1.91 to − 1.29); LB = − 1.32 kg (95% CI − 1.57 to − 1.07) and UB + LB = − 2.26 kg (95% CI − 3.31 to − 1.21)] post-training than did the control (p < 0.05 in training groups, p > 0.05 control group). However, no difference in absolute mass of SkMM gained or FM lost was noted between UB + LB and either UB or LB alone (p > 0.05; Fig. 1b, d).
RT increases skeletal muscle mass and decreases fat mass independent of training modality. a Skeletal muscle mass (SkMM; kg) by group pre- and post-training. b Change in (Δ) SkMM (kg) from pre- to post-training. c Fat mass (FM: kg) by group pre- and post-training. d ΔFM (kg) from pre- to post-training. Error bars represent standard deviation. Individual data points as shown. *Differences from groups as shown, ^differences from control. Upper body (UB), lower body (LB), upper body and lower body combined (UB + LB)
Whole-body RT alters endocrine markers to a greater degree than upper- or lower-body RT alone
A significant group × time interaction was noted for serum concentration of myostatin, follistatin and the myostatin:follistatin concentration ratio (myostatin p < 0.001, follistatin p < 0.001, myostatin: follistatin ratio p < 0.001). Myostatin concentration was shown to be decreased (p < 0.05) in LB [− 0.11 ng mL−1 (95% CI − 0.16 to − 0.06)] and UB + LB [− 0.36 ng mL−1 (95% CI − 0.48 to − 0.19)] groups, but not UB or control (p > 0.05; Fig. 2c, d). Whilst follistatin was increased post-training [UB = 0.22 ng mL−1 (95% CI 0.16–0.38); LB = 0.24 ng mL−1 (95% CI 0.20–0.28) and UB + LB = 0.55 ng mL−1 (95% CI 0.39–0.61)] relative to control groups (p < 0.05 in training groups; Fig. 2a), of more interest was the observation that UB + LB increased follistatin to a greater extent than UB or LB alone (p < 0.05; Fig. 2b). Finally, the follistatin:myostatin ratio was increased in all training groups [UB = 0.07 (95% CI 0.04–0.10); LB = 0.07 ng mL−1 (95% CI 0.06–0.09) and UB + LB = 0.18 (95% CI 0.12–0.24)], relative to both the control group (p < 0.05), and to a greater degree in UB + LB than UB or LB alone (p < 0.05; Fig. 2e, f).
RT alters endocrine factors myostatin and follistatin as a function of the volume of muscle mass trained. a Follistatin (ng mL−1). b Change in (Δ) follistatin pre to post (ng mL−1). c Myostatin (ng mL−1). d Δ myostatin (ng mL−1). e Follistatin:myostatin (F:M) ratio. f ΔF:M (ng mL−1). Error bars represent standard deviation. Individual data points as shown. *Differences from groups as shown, ^differences from control. Upper body (UB), lower body (LB), upper body and lower body combined (UB + LB)
RT improves isometric strength
A significant group × time interaction (p < 0.001, Table 5) was noted for isometric quadriceps which significant increased (p < 0.05) in all training groups [UB = 2.4 kg (95% CI 2.2–2.6); LB = 4.6 kg (95% CI 4.3–5.0) and UB + LB = 3.6 kg (95% CI 3.2–3.9)] compared to no changes after the control group. The LB increased isometric quadriceps strength to a greater extent than UB + LB or LB alone (p < 0.05). There were also significant increases (p < 0.05) in right [UB = 4.6 kg (95% CI 4.3–5.1); LB = 2.4 kg (95% CI 2.1–2.6); and UB + LB = 2.7 kg (95% CI 2.4–2.9)] and left [UB = 3.2 kg (95% CI 2.8–3.6); LB = 1.5 kg (95% CI 1.2–1.8); and UB + LB = 2.3 kg (95% CI 2.1–2.6)] isometric handgrip strength in all training groups but no changes after control. The UB increased right and left isometric handgrip strength to a greater extent than UB + LB or LB alone (p < 0.05).
Discussion
This study aimed to identify the effect of three 8-week RT protocols (LB, UB or UB + LB) on follistatin, myostatin and their ratio in middle-aged men. The key finding we report is that the greatest effects in the response of follistatin and myostatin to chronic RT were observed in the UB + LB group, which might be related to the different volume of muscles recruited in our RT programs. In response to each RT program (UB + LB, UB, and LB), serum follistatin levels increased significantly, and the greatest effect was observed in the group performing both upper- and lower-body RT (i.e., UB + LB). The F:M ratio also increased significantly in all three groups, with the greatest effect observed in UB + LB. Myostatin decreased similarly in all training groups, although not significantly in the UB group following training. As would be expected, all forms of RT resulted in increased muscle mass gain. Indeed, it is well described in the literature that RT results in increase in muscle mass in most healthy individuals (Grgic et al. 2018), and that greater muscle gain results from higher volumes of training (Schoenfeld et al. 2016), higher number of sets completed (Krieger 2010), and indeed specificity of training response to the muscle mass utilized in training (Taniguchi 1997, 1998). The role of myostatin and follistatin in response to RT has been reported prior to this paper, and it is noteworthy that our results here mirror those of others. In particular, Walker et al. (2004) showed that plasma myostatin protein levels decreased by approximately 20% in participants after 10 weeks of twice weekly bouts of RT, in a manner similar to that shown by others (Saremi et al. 2010). Both younger and older men show decreased circulating myostatin and increased follistatin concentration in response to 8 week of RT, in a highly similar manner to the results we report here (Negaresh et al. 2017). However, it is noteworthy, that when challenged with UB + LB, our participants show an endocrine response of myostatin and follistatin that is approximately twice what is seen if UB or LB alone is performed. This novel finding suggests that the volume of muscle mass activated is a key factor in the endocrine portion of the anabolic response to RT. Indeed, RT studies that have examined volume of training report lower volumes produce a lesser endocrine response of anabolic hormones such as growth hormone and testosterone (Goto et al. 2004; Gotshalk et al. 1997). In contrast to these results and the findings we report here, Walker et al. (2004) did not show a difference in plasma myostatin response between a whole body and a UB training protocol like our own, when semi-quantitatively examined by Western blot. We would speculate that this may be due to the measurement technique used, and that current ELISA-based protocols would be more sensitive to changes in concentration than Western blot approaches; however, this has not be objectively tested by ourselves to date. Alternatively, it was examined a relatively light training workload (twice weekly) and muscle mass gain relatively to the load used in our middle-aged men, which may underlie the differences in myostatin response by muscle volume trained (UB vs LB vs LB + UB)(Walker et al. 2004). The single-chain polypeptide follistatin is a member of the larger family of the transforming growth factors (Görgens et al. 2013), that is ubiquitously expressed in all tissues of the human body, including skeletal muscle, and has paracrine and autocrine effects. Previous reports showed that follistatin has anabolic and catabolic effects in skeletal muscle and adipose tissue, respectively (Gilson et al. 2009; Rodino-Klapac et al. 2009), likely via follistatin’s ability to bind and inactivate myostatin (Amthor et al. 2004). Most studies examining RT and follistatin have found increased circulating follistatin concentrations in their research, which is in line with the results we report here (Hofmann et al. 2016; Jang et al. 2016; Negaresh et al. 2017).
The increase in F:M ratio after our UB + LB is related to an improvement in positive muscle growth regulators leading to their predominance in the homeostatic balance of the muscle. In normal conditions, to maintain muscle fiber size, there is a homeostatic balance between positive (such as follistatin and IGF-1) and negative (such as myostatin) regulators of muscle growth; but this balance, when the muscle is atrophic, leads to a dominance in negative regulators; hence, loading the muscle through RT, may lead to the dominance of positive regulators. Although the mechanism of communication between these regulators is not completely clear, this connection seems to be made through a very complex negative feedback loop (Gonzalez-Cadavid et al. 1998). Furthermore, it has been shown that the release rate of myokines and regulatory factors for muscle growth is largely dependent on the intensity and volume of muscles involved in the activity (Motevalli et al. 2015). It appears that the volume of muscle recruited is a key variable in RT, as this promotes anabolic pathways in a larger quantity of tissue (Burd et al. 2010; Terzis et al. 2010). Accordingly, the combined exercise protocol of this study, compared with the upper and lower trunk exercise protocols showed an increase in the F:M ratio in middle-aged men due to the involvement of more muscles in RT. However, at least within the time frames reported here, this did not result in a significant difference in the gains in whole-body muscle mass or decrease in fat mass between training groups. This may be at least partially explained by the short duration of the training protocol. It is generally held that neural adaptations predominate first, while muscle hypertrophy comes to being evident after approximately 8 weeks of training (Haff and Triplett 2015). Therefore, our 8-week intervention might not have warranted enough time for a significant difference in muscle mass gains between training groups (although there was a non-significant tendency for the UB + LB to have higher improvements than the other groups). In addition, somewhat similar training volume was used in all three groups [six exercises (three for each the upper and lower half) in the UB + LB compared to six exercises for the UB and LB groups], which potentially affected the myostatin and follistatin response and hence the muscle mass gain and fat loss in the UB + LB. Myostatin can both inhibit or promote adipogenesis (Deng et al. 2017) which may create some confusion as far as its effects on fat loss in our study. Nonetheless, previous results in rats show that the reduction in fat mass in myostatin-deficient rats is influenced by metabolic changes in skeletal muscle (Guo et al. 2009). Therefore, the changes in fat mass in the UB + LB that tended to be greater (although non-significant) than the LB and UB might be influenced by greater improvement in muscle mass in the UB + LB group. However, future RT studies in different populations are needed to validate our claim.
The interesting findings of our investigation should be understood in the context of the following limitations. We did not measure training motivation and fiber type, which would have strengthened the matching of participants and hence our study design. We were not able to document training data, such as loads and changes in work volume, that might have helped explaining some of our findings. We did not assess electromyography, which would have provided evidence on muscle recruitment and activation and its relationship to myostatin and follistatin changes to our three RT protocols. This study is also limited by short duration and low-moderate intensity (50–80% of 1RM) of our intervention; which warrants further investigations with longer interventions and heavier loads with a focus on understanding the relationship between changes in SkMM and FM, and myostatin as well as follistatin responses after combined upper- and lower-body RT.
In conclusion, the findings of this study show that the use of combined upper- and lower-body RT increases the serum ratio of F:M in middle-aged men over upper- or lower-body training alone, suggesting that volume of muscle mass recruited might influence the magnitude of the serum myostatin and follistatin response. Future research might examine the volume of muscle recruited vs the total amount of work completed, as this research suggests one of these variables underlies the endocrine anabolic response of follistatin and myostatin.
Abbreviations
- RT:
-
Resistance training
- F:M:
-
Follistatin to myostatin ratio
- SkMM:
-
Skeletal muscle mass
- PBF:
-
Percent body fat
- BMI:
-
Body mass index
- LBM:
-
Lean body mass
- FM:
-
Fat mass
- 1RM:
-
One repetition maximum
- ng mL−1 :
-
Nanograms per milliliter
- UB:
-
Upper body
- LB:
-
Lower body
- UB + LB:
-
Upper body + lower body
- mL:
-
Milliliter
- ANOVA:
-
Analysis of variance
- vs:
-
Versus
- IGF-1:
-
Insulin-like growth factor 1
References
Allen DL, Hittel DS, McPherron AC (2011) Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc 43:1828
Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K (2004) Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol 270:19–30
Attarzadeh Hosseini SR, Moeinnia N, Motahari Rad M (2017) The effect of two intensities resistance training on muscle growth regulatory myokines in sedentary young women. Obes Med 2:25–28
Barrios-Silva LV et al (2018) Activin subfamily peptides predict chronological age in humans. Physiol Rep 6:e13823
Burd NA et al (2010) Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol 588:3119–3130
Church DD et al (2016) Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J Appl Physiol 121:123–128
de Souza EO et al (2014) Effects of concurrent strength and endurance training on genes related to myostatin signaling pathway and muscle fiber responses. J Strength Cond Res 28:3215–3223
Deng B et al (2017) The function of myostatin in the regulation of fat mass in mammals. Nutr Metab 14:29
Elliott B, Renshaw D, Getting S, Mackenzie R (2012) The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol 205:324–340
Elliott BT, Herbert P, Sculthorpe N, Grace FM, Stratton D, Hayes LD (2017) Lifelong exercise, but not short-term high-intensity interval training, increases GDF11, a marker of successful aging: a preliminary investigation. Physiol Rep 5:e13343
Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ (1988) Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64:1038–1044
Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen J-P (2009) Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab 297:E157–E164
Gonzalez-Cadavid NF et al (1998) Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci 95:14938–14943
Görgens SW, Raschke S, Holven KB, Jensen J, Eckardt K, Eckel J (2013) Regulation of follistatin-like protein 1 expression and secretion in primary human skeletal muscle cells. Arch Physiol Biochem 119:75–80
Goto K, Nagasawa M, Yanagisawa O, Kizuka T, Ishii N, Takamatsu K (2004) Muscular adaptations to combinations of high-and low-intensity resistance exercises. J Strength Cond Res 18:730–737
Gotshalk LA et al (1997) Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can J Appl Physiol 22:244–255
Grgic J, Mcllvenna LC, Fyfe JJ, Sabol F, Bishop DJ, Schoenfeld BJ, Pedisic Z (2018) Does aerobic training promote the same skeletal muscle hypertrophy as resistance training? A systematic review and meta-analysis. Sports Med 49:1–22
Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC (2009) Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One 4:e4937
Haff GG, Triplett NT (2015) Essentials of strength training and conditioning, 4th edn. Human kinetics
Hakkinen K, Pakarinen A, Kraemer WJ, Newton RU, Alen M (2000) Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. J Gerontol Biol Sci Med Sci 55:B95
Hofmann M et al (2016) Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: the Vienna Active Ageing Study (VAAS). Eur J Appl Physiol 116:885–897
Hulmi JJ et al (2007) Postexercise myostatin and activin IIb mRNA levels: effects of strength training. Med Sci Sports Exerc 39:289–297
Jang KS, Kang S, Woo SH, Bae JY, Shin KO (2016) Effects of combined open kinetic chain and closed kinetic chain training using pulley exercise machines on muscle strength and angiogenesis factors. J Phys Ther Sci 28:960–966
Krieger JW (2010) Single vs. multiple sets of resistance exercise for muscle hypertrophy: a meta-analysis. J Strength Cond Res 24:1150–1159
Laurentino GC et al (2012) Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc 44:406–412
Law TD, Clark LA, Clark BC (2016) Resistance exercise to prevent and manage sarcopenia and dynapenia. Ann Rev Gerontol Geriatr 36:205
Medicine ACoS (2009) American College of Sports Medicine position stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41:687
Motevalli MS, Dalbo VJ, Attarzadeh RS, Rashidlamir A, Tucker PS, Scanlan AT (2015) The effect of rate of weight reduction on serum myostatin and follistatin concentrations in competitive wrestlers. Int J Sports Physiol Perform 10:139–146
Murach KA, Bagley JR (2016) Skeletal muscle hypertrophy with concurrent exercise training: contrary evidence for an interference effect. Sports Med 46:1029–1039
Negaresh R, Ranjbar R, Habibi A, Mokhtarzade M, Fokin A, Gharibvand M (2017) The effect of resistance training on quadriceps muscle volume and some growth factors in elderly and young men. Adv Gerontol Uspekhi Gerontol 30:880–887
Ratkevicius A et al (2011) Serum concentrations of myostatin and myostatin-interacting proteins do not differ between young and sarcopenic elderly men. J Gerontol Ser A Biomed Sci Med Sci 66:620–626
Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40:423–429
Rodgers BD, Garikipati DK (2008) Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 29:513–534
Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR (2009) Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve 39:283–296
Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA (2003) Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med 228:706–709
Saremi A, Gharakhanloo R, Sharghi S, Gharaati M, Larijani B, Omidfar K (2010) Effects of oral creatine and resistance training on serum myostatin and GASP-1. Mol Cell Endocrinol 317:25–30
Schiffer T, Geisler S, Sperlich B, Strüder H (2011) MSTN mRNA after varying exercise modalities in humans. Int J Sports Med 32:683–687
Schoenfeld BJ, Ogborn D, Krieger JW (2016) Effects of resistance training frequency on measures of muscle hypertrophy: a systematic review and meta-analysis. Sports Med 46:1689–1697
Simão R et al (2013) Influence of upper-body exercise order on hormonal responses in trained men. Appl Physiol Nutr Metab 38:177–181
Taniguchi Y (1997) Lateral specificity in resistance training: the effect of bilateral and unilateral training. Eur J Appl Physiol 75:144–150
Taniguchi Y (1998) Relationship between the modifications of bilateral deficit in upper and lower limbs by resistance training in humans. Eur J Appl Physiol 78:226–230
Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E (2010) The degree of p70S6 k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol 110:835–843
Thomas DT, Erdman KA, Burke LM (2016) Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet 116:501–528
Tortoriello DV, Sidis Y, Holtzman DA, Holmes WE, Schneyer AL (2001) Human follistatin-related protein: a structural homologue of follistatin with nuclear localization. Endocrinology 142:3426–3434
Viitasalo J, Era P, Leskinen A-L, Heikkinen E (1985) Muscular strength profiles and anthropometry in random samples of men aged 31–35, 51–55 and 71–75 years. Ergonomics 28:1563–1574
Walker KS, Kambadur R, Sharma M, Smith HK (2004) Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc 36:787–793
Willoughby DS (2004) Effects of an alleged myostatin-binding supplement and heavy resistance training on serum myostatin, muscle strength and mass, and body composition. Int J Sport Nutr Exerc Metab 14:461–472
Yarasheski K, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid N (2002) Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Ageing 6:343–348
Acknowledgements
The authors wish to thank all the participants in this research project.
Author information
Authors and Affiliations
Contributions
AR conceived and designed research. RB conducted the experiments and wrote the paper. AW and JM fixed grammatical mistakes. In addition, AW analyzed data, contributed in writing the paper and preparing revisions. MSM analyzed the nutrition data. BTE analyzed the other data, wrote the result sections and fixed all the section of the paper academically. Finally, all the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by William J. Kraemer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bagheri, R., Rashidlamir, A., Motevalli, M.S. et al. Effects of upper-body, lower-body, or combined resistance training on the ratio of follistatin and myostatin in middle-aged men. Eur J Appl Physiol 119, 1921–1931 (2019). https://doi.org/10.1007/s00421-019-04180-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04180-z