Abstract
Purpose
To examine the effect of acute quercetin (Q) ingestion on neuromuscular function, biomarkers of muscle damage, and rate of perceived exertion (RPE) in response to an acute bout of resistance training.
Methods
10 young men (22.1 ± 1.8 years, 24.1 ± 3.1 BMI) participated in a randomized, double-blind, crossover study. Subjects consumed Q (1 g/day) or placebo (PLA) 3 h prior to a resistance training session which consisted of 3 sets of 8 repetitions at 80% of the one repetition maximum (1RM) completed bilaterally for eight different resistance exercises. Electromyographic (EMG) signals were recorded from the knee extensor muscles during maximal isometric (MVIC) and isokinetic voluntary contractions, and during an isometric fatiguing test. Mechanical and EMG signals, biomarkers of cell damage, and RPE score were measured PRE, immediately POST, and 24 h (blood indices only) following the resistance exercise.
Results
After a single dose of Q, the torque–velocity curve of knee extensors was enhanced and after the resistance exercise, subjects showed a lower MVIC reduction (Q: 0.91 ± 6.10%, PLA: 8.66 ± 5.08%) with a greater rate of torque development (+ 10.6%, p < 0.005) and neuromuscular efficiency ratio (+ 28.2%, p < 0.005). Total volume of the resistance exercises was significantly greater in Q (1691.10 ± 376.71 kg rep) compared to PLA (1663.65 ± 378.85 kg rep) (p < 0.05) with a comparable RPE score. No significant differences were found in blood marker between treatments.
Conclusions
The acute ingestion of Q may enhance the neuromuscular performance during and after a resistance training session.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resistance training is important for inducing physiological muscular adaptation and maximizing muscle strength production (Schoenfeld et al. 2014). However, the previous studies have reported that a bout of resistance training induces an excessive increase in reactive oxygen species (ROS) production which could be implicated in the reduction in skeletal muscle force generating capacity observed during the exercise (Bloomer et al. 2005; Goldfarb et al. 2005). In addition, the ensuing inflammatory response might increase muscle damage, decrease the normal muscular function activity, accelerate the onset of fatigue, and delay muscle recovery (Goldfarb et al. 2005; Powers et al. 2011). Anti-inflammatory nutrients, by definition, reduce levels of inflammatory markers in the blood and thereby may contribute to a reduction in post-exercise muscular damage (Goldfarb et al. 2011; Ackerman et al. 2014). Extensive research has been carried out on antioxidant and anti-inflammatory properties of these nutrients in relation to exercise (Sureda et al. 2014; Braakhuis and Hopkins 2015). The results are still inconclusive, conceivably due to the difficulties in controlling a number of confounding factors such as the type, timing, dose of the supplement, and training factors (Urso and Clarkson 2003).
Quercetin (Q) is the most frequently consumed flavonoid with levels as high as ~ 350 ppm in the archetypical western diet (Harwood et al. 2007), and one of the most prominent dietary antioxidants (Boots et al. 2008). Food sources for Q include green tea, red and white onions, apples, peppers, blueberries, and dark green vegetables (Q content per 100 mg of selected foods: 2.7; 33; 21; 4.7; 15; 5.1; 4.1 mg/100 gm edible portion, respectively) (Chun et al. 2007; USDA NASS 2009). It has been observed a relatively high interindividual variability in plasma Q concentrations absorption after oral supplementation in homogeneous humans’ study population (ranged from 0 to over 50% of the administered dose)(Hollman et al. 1996).
It has been suggested that Q may be a possible ergogenic aid (MacRae and Mefferd 2006), may reduce the perceived effort of exercise, and improve fatigue by acting on adenosine receptors (Alexander 2006). Moreover, the existence of the hydroxyl and catechol-type B-ring in Q may promote a radical scavenging effect which, in turn, could mitigate the oxidative stress (Davis et al. 2009).
A large body of scientific literature has investigated the effects of a chronic Q supplementation on exercise performance (MacRae and Mefferd 2006). Most of the studies have focused on endurance exercise and reported that Q increases the maximal endurance capacity and voluntary physical activity, an effect that is associated with increased mitochondrial biogenesis in muscle (Davis et al. 2009). On the other hand, there are only two studies that have examined the acute effects of Q administration (e.g., administered on the day of the exercise). Moreover, one of these studies was focused on the acute effect of Q on endurance exercises (Cheuvront et al. 2009) and the other one used Q in combination with other antioxidant compounds (Konrad et al. 2011). To the best of our knowledge, there are no studies investigating whether acute Q supplementation may have an effect on alterations in neural drive that occur immediately after an intense resistance training session.
Accordingly, the aim of this study was to investigate the effect of an acute dose of Q ingested before a standardized resistance training session on parameters of neuromuscular function, oxidative stress and classical muscle damage markers, RPE, and muscle soreness. To this end, participants underwent a complete neuromuscular function evaluation of the knee extensors muscles as already described (Bazzucchi et al. 2009, 2011, 2016).
Studies on other flavonoid-rich products such as blueberry, dark chocolate, pomegranate, and phenolic-rich juice have observed significant effects following acute supplementation of appropriate doses consumed within a few hours before an intensive resistance exercise (García-Alonso et al. 2006; MacRae and Mefferd 2006; Davison et al. 2012; Sureda et al. 2014), but none of these studies was interested in assessing changes in the neuromuscular function. It was hypothesized that an acute dose of Q assumed before a resistance training session could be able to attenuate the detrimental oxidative stress and to reduce the subsequent strength loss.
Methods
Subjects
Ten healthy trained non-smoker male volunteers (mean ± SD age, height, body mass and BMI were 22.1 ± 1.8 years, 1.79 ± 0.09 m, 77.5 ± 8.7 kg, and 24.1 ± 3.1 kg/m2, respectively) took part in this study. The following inclusion criteria were adopted: (a) sedentary to recreationally active individuals (no more than 2 times per week); (b) aged 18–30 years; (c) free from current health issues as verified through a general health questionnaire. The exclusion criteria were: (a) symptoms and/or positive history for endocrine, metabolic, renal, cardiac, or neurological diseases; (b) undertaking regular resistance training in the 6 months prior to participation; (c) negative response to the Physical Activity Readiness Questionnaire. For the duration of the study, participants were asked to continue their habitual exercise and nutritional routines. In addition, from 1 week before and during the 2-week study period volunteers were requested to avoid the ingestion of Q and other antioxidants containing foods, nutritional supplements, and ergogenic aids. Subjects were also asked to refrain from taking anti-inflammatory medications while taking part in the study.
Dosage and timing of the supplement were based on findings from the previous studies (Kaushik et al. 2012; Larson et al. 2012). Accordingly, in the present investigation a single oral dose of 1000 g per day of Q was used, since it has been shown to produce rapid and persistent elevations in circulating levels of Q sufficient to exert its biological properties that are likely to improve physical performance. Moreover, plasma Q has been shown to remain elevated for 8 h after a single oral consumption of Q-rich foods (Harwood et al. 2007).
Ethical approval was granted for this study from the local University Human Research Ethics Board. Participants were informed about the nature and aim of this study and informed written consent was provided by each subject before testing and data collection.
Study design
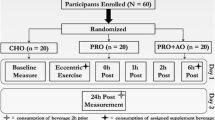
The investigation used a randomized, double-blind, crossover design consisting of 3 visits: a familiarization followed by 2 test visits. During the first visit (BASELINE test), volunteers familiarized with the experimental procedures, performed all the tests and no treatment capsules were ingested. Upon arrival, subjects were tested for one repetition maximum (1RM) to determine the resistance training loads. Participants then returned to the laboratory on 2 additional days separated by at least 1 week and performed the experimental protocol before (pretest, PRE) and after (posttest, POST) ingesting 2 capsules of 500 mg each of Q or 2 placebo (PLA) capsules (500 mg of magnesium stearate + cornstarch), which were administered in a random order. In each experimental session, the neuromuscular function tests were assessed before acute supplementation and immediately after performing a supervised resistance training session. Subjects consumed their capsules 3 h before performing each resistance training session. The two condition sessions (Q and PLA) were separated by a 1-week wash-out period to negate the effects of training and supplementation (Fig. 1). All test visits were scheduled on the same day of the week at the same time in the morning. The subjects were instructed to refrain from caffeine, Q containing foods and/or energy drinks for 24 h prior to resistance training. Subjects recorded their diet and were asked to replicate the same dietary intake 24 h prior to each session.
At BASELINE, the 1RM for each exercise of the resistance training sessions was assessed. Afterward, participants underwent a complete neuromuscular evaluation of the knee extensor muscles.
During 2 experimental testing days, the neuromuscular evaluation and the blood sample collection were performed before starting the test protocol (PRE) and after (POST). Moreover, blood samples were also collected 24 h after the training session (+ 24 h).
After each exercise of the resistance training session, subjects were asked to rate their perceived exertion (RPE) using the 0- to 10-point Borg scale (Borg 1982). Moreover, two visual analogue scales were also used to assess both muscle soreness (VAS) and feelings of perceived recovery (PRS) experienced by the subjects immediately after the neuromuscular assessment performed after completing the whole session (Phillips et al. 2003). Pain score was assessed using a 100-mm VAS with numerical markers ranging from 0 to 10 marked at each 10-mm interval. A score of 0 indicated no pain whatsoever. A score of 10 indicated extreme pain. Subjects made a single vertical mark on the scale that corresponded to their soreness level. For the PRS, a 0 indicated “not at all recovered” and 10 represented “very well recovered”. Standardized instructions explaining how to interpret the VAS and PRS scales and the numerical and verbal anchors contained within them were given and read by subjects.
1RM
Eight exercises were performed bilaterally (seated row, chest press, knee extension, knee flexion, leg press, and calf raise). Initially, each participant performed a warm-up with a series of 15 repetitions at 40–60% of the maximum perceived strength with a 1-min interval between each set. After the 1-min interval, a second series was performed with three-to-six repetitions at 60–80% of the maximum perceive strength. After a 1-min rest, five tests of strength were attempted, and the load was adjusted before each new attempt. The recovery time between attempts was standardized at 3–5 min with an interval of 10 min for recovery between different exercises. The test was interrupted when the individual failed to properly execute the movement and the maximum load considered was the load moved in the last successful attempt (Paulsen et al. 2014). ICCs for 1RM testing are specific to the exercise and ranged between 0.64 and 0.99 for leg press, chest press, and leg extension.
Resistance exercise
Participants completed 3 sets of 8 repetitions at 80% 1RM with 2-min intervals between each series and a 1-min interval between each exercise. The program was designed to target all major muscle groups and thus to enhance exercise stimuli (Paulsen et al. 2014). The total volume of the exercise was calculated by multiplying the total load by the number of sets and repetitions completed in the 8 exercises (load × sets × repetitions).
Experimental protocol
Knee extensors force of the right limb was recorded using a Biodex dynamometer (Biodex, System 3, Medical Systems, USA). The Biodex was calibrated to allow the knee to be set at 90° flexion, with a range of motion limited to 90° (30° to 120°; 0° full extension). Participants were seated comfortably and strapped onto a rigid chair to prevent hip joint movement during knee extensions (Remaud 2005). The rotational axis of the dynamometer was aligned to the lateral femoral epicondyle, and the lower extremity was strapped to the machine lever arm 2 cm above the lateral malleolus. Individual subject positioning on the chair was documented and controlled throughout the study with participants asked to keep their arms across the chest at all times during contractions.
Participants commenced trials with a specific isokinetic warm-up (3 sets x10 repetitions at 210°/s) with 30-s rest periods. MVIC for knee extension was assessed at 60° knee extension. Three trials were performed with a 2-min rest in between to prevent from cumulative fatigue. The attempt that contained the highest peak torque value was defined as MVIC and used for subsequent analyses.
Velocities of 30°/s, 90°/s, 180°/s, and 240°/s were then selected in random order and subjects were requested to extend the knee “as hard as possible”. A set of three maximal concentric knee extensions was performed at each velocity. The range of motion was 90 degrees starting from 30° to 120° and between each set a 5-min rest was allowed. The ICC for this isokinetic protocol in our laboratory ranged from 0.87 to 0.98 using the Biodex isokinetic dynamometer.
An isometric fatiguing test (IFT) corresponding to 50% of knee extensor MVIC was also performed. Participants were required to exert a constant force for 30 s by following a visual feedback. Standardized verbal instructions and encouragement were given to each of the participants for all trials.
Surface EMG
Surface EMG was recorded during the experimental protocol from the rectus femoris (RF), and the long head of the biceps femoris (BF) muscles, as representative of the neural activation of knee extension and knee flexion muscle groups, respectively (De Vito et al. 2003). Low impedance at the skin–electrode interface was obtained by standardized-skin preparation procedures (shave, abrasion, and cleansing with alcohol wipes)(Hermens et al. 2000). The electrode locations were recorded during the first visit. To ensure the same electrode placement throughout the two experimental sessions, individual maps were made on transparent plastic by marking the position of permanent skin blemishes with respect to the electrodes. Then, two Ag/AgCl bipolar electrodes (Blue Sensor N-00-S, Ambu Medicotest A/S, Ølstykke, Denmark) were positioned with 20-mm interelectrode distance according to the SENIAM guidelines (Hermens et al. 2000). A reference electrode was attached to the tibial tuberosity of the proximal tibia. The electrodes were left on the participant’s leg for the second test session to avoid the low reliability of repeated electrode placement (Rainoldi et al. 1999). Surface EMG signals were synchronized with mechanical signals that were all sampled at 2048 Hz, amplified with a gain of 1000 and stored on a personal computer using a 16-bit A/D converter data acquisition system (Biopac System, Inc., Goleta, CA, USA).
Data processing and variable extraction
The torque signal was off-line low-pass filtered by a digital fourth-order zero-lag Butterworth filter (cut-off frequency 15 Hz). In each condition, the trial with the highest peak torque was considered for later analysis. For the MVIC task, data were calculated on a 1-s window centered around peak force (Bazzucchi et al. 2011).
For the isokinetic contractions, maximal torque values were calculated on a 1-s window centered on peak torque during the constant iso-velocity period for each velocity (MVC30, MVC90, MVC180 and MVC240), to avoid the transient phases of the contractions (initial and final non-isokinetic activity). Isokinetic maximal torque values were expressed as percentage of the MVIC value obtained during the PRE-session (Bazzucchi et al. 2009).
The sEMG signals were online band-pass–filtered (1–500 Hz) by the hardware (Biopac Systems) using a fourth-order Butterworth filter and off-line high-pass filtered (fourth-order Butterworth filter, cut-off frequency 20 Hz). EMG parameters were determined from the MVIC trial. In the MVIC with the highest torque value, the time point which had the maximum EMG amplitude was detected, then a 1-s window consisting of 0.5 s before and after that time point was selected and analyzed to quantify the root-mean-square (RMS) and median frequency (MDF) EMG values.
A coactivation index (CI) (i.e., the ratio between RMS from antagonist and agonist muscles) was calculated in IFT, as previously described (Bazzucchi et al. 2008). Briefly, the RMS of agonist muscles during knee extension (rectus femoris, RF) and knee flexion (biceps femoris, BF) were evaluated. The average RMS value of RF muscle and BF muscle recorded during IFT (RFRMSf, BFRMSf) was reported as a percentage of the RMS amplitude during its MVIC when acting as an agonist and used to express the level of antagonist coactivation during IFT:
MDF during the IFT was calculated using the Fast Fourier Transform over non-overlapped 1-s windows and expressed as the average of the 30 values. Mean MDF values were normalized to the values generated at MVICPRE.
Rate of torque development
The isometric rate of torque development (RTD, Nm/s) was defined as the slope of the torque–time curve (i.e., ΔTorque/ΔTime) in incrementing time periods of 0–200 ms from the onset of contraction (Andersen and Aagaard 2006). ICC with 95% confident intervals of the RTD measurement for our laboratory ranged from 0.82 to 0.94 (Wu et al. 2016). RTD was calculated using the average slope of the filtered torque profile from 0 to 200 ms (RTD200). Based on the methods previously described by Aagaard and colleagues, the onset of muscle contraction was defined as the point at which the torque curve exceeded the baseline level by 3 N m during MVIC of knee extension (Aagaard et al. 2002). Baseline torque was computed by taking the average reading over 0.5 s, starting 1 s before the onset of muscle contraction.
Neuromuscular efficiency
NME was defined as the ratio between MVIC and the average sEMG value of the same 250 ms epoch centered around peak torque and measured in Nm mV−1. As previously described, NME estimates the amount of torque produced per unit of EMG amplitude, with higher ratios being indicative of greater efficiency (Rainoldi et al. 2008).
Blood sample collections
Venous blood samples (9 mL per blood sample) were collected by a standard venipuncture technique of the antecubital vein into glass tubes on days 8 and 15; once before the testing protocol, and the second on completion of the re-test. A third blood sample was collected the following morning, 24 h after the first and thus beginning the 1-week wash-out period. Blood samples were maintained at + 4 °C and the plasma was immediately separated (3000 rpm × 10 min, + 4 °C) and stored at − 80 °C until biochemical assays. During the sample collection, a single blood sample for each experimental point was withdrawn. All analyses performed on these samples were performed twice in triplicate. Inter- and intra-assays were performed to assess precision within and between assays (data not shown). The markers of stress oxidative and muscle damage tested for the purpose of this study are blood-reduced (GSH) and oxidized (GSSG) glutathione, and creatine kinase (CK). Acute tissue injury marker CK and plasma measures of oxidized gluthanione (GSSG) have been previously reported to be elevated after intensive exercise (Bloomer et al. 2005; O’Fallon et al. 2012).
Biochemical analysis
All chemical reagents, unless specified otherwise, were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA).
Creatine kinase (CK)
Plasma CK activity was determined spectrophotometrically, using a commercial test kit (Greiner Diagnostic GmbH, Bahlingen-Gremany) as previously described (Ceci et al. 2014). Briefly, 50 µl plasma were incubated in Hexokinase-Glucose 6 Phosphate-G6P Dehydrogenase buffer for 3 min, and then, NADPH production was followed at 340 nm for further 3 min.
Glutathione homeostasis
Blood-reduced (GSH) and -oxidized (GSSG) glutathione contents were quantified by a DTNB–glutathione reductase recycling assay, as previously described (Ceci et al. 2017). Briefly, 100 µl of blood samples were added to 500 µl of 10 mM HCl. Red blood cells were lysed by freezing and thawing three times; after centrifugation at 10,000g for 5 min at 4 °C, the supernatant solution was deproteinized with 5% 5-sulfosalicylic acid. After centrifugation, the deproteinized supernatant solution was utilized for the assay. GSSG was selectively measured in samples where reduced GSH was masked by pretreatment with 2-vinylpyridine (2%). 20 µl of sample were added to the reaction buffer [700 µl NADPH (0.3 mM), 100 µl DTNB (6 mM), and 190 µl H2O]. The reaction was started by adding 2.66 U/ml glutathione reductase, and followed at 412 nm by the TNB stechiometrical formation. Sample ΔOD/min412 were compared to those obtained using glutathione standards.
Statistical analysis
All data are reported as mean ± SD. A two-way repeated-measures ANOVA [treatment (two levels: Q and PLA) vs velocity (five levels; 0°/s; 30°/s; 90°/s; 180/s; 240°/s) was applied to torque and RMS values. A two-way repeated-measures ANOVA [treatment (two levels: Q and PLA) vs time (three levels: PRE, POST and 24 h)] was applied to biochemistry data. Where Maulchy test of sphericity was significant, the Greenhouse–Geisser adjustment was used. In case of a significant interaction, a one-way repeated-measures ANOVA was used to test the simple main effects of treatment at different time points, with subsequent follow-up Bonferroni test performed in case of a significant simple main effect. Differences between Q and PLA treatments in torque decline recorded during MVIC after the resistance exercise (ΔMVIC), in RMS, MDF, RTD, NME, CI, and MDF calculated during IFTin POST, RPE, and session total volume were determined using t test for independent samples. The statistical analyses were performed in IBM SPSS Statistics 20 (SPSS Inc, Chicago, IL, USA). The overall acceptable significance level of difference for all statistical tests was set at p ≤ 0.05. The sample size was calculated using the G POWER software (version 3.1) (F tests: repeated measures; within factors, analysis a priori: INPUT: Effect size f = 0.25; number of groups = 1; number of measurements = 6) to determine that a minimum sample size of 8–10 subjects would provide a statistical power of over 0.80 (α = 0.05, ß = 0.20) in the strength performance variables.
Results
Torque and EMG data
Torque–velocity relationship
There was a significant main effect for treatment (p = 0.009) in knee extension torque exerted by the subjects at the different angular velocities. However, no significant treatment x velocity interaction was observed (p = 0.633) (Fig. 2).
There were no differences between treatments (main effect, p = 0.688) in the neuromuscular activity (RMS) of RF, recorded during isokinetic contractions, nor treatment × velocity interaction (p = 0.584).
MVIC
After the resistance exercise, a significant greater loss of strength in PLA than Q treatment was observed in knee extension (p < 0.05). Table 1 shows the significant percent decline in isometric knee extension in Q and PLA. No significant changes in RMS and MDF values (%PRE) were observed during MVIC in any of treatments for any muscle (Table 1). After resistance exercise, the RTD200 increased (+ 10.6%) in Q, whereas decreased (-8.3%) in PLA and these values were significantly different (p < 0.05). There were significant differences between treatments in knee extensors maximal RTD200 in POST expressed as percentage of PRE resistance exercise (Q vs PLA, P = 0.006; Table 1).
In POST, the NME of rectus femoris muscle recorded in MVIC was 28.2% greater than PRE in Q, whereas it was 10.9% lower than PRE in PLA (p < 0.05). In addition, when comparing the two conditions, NME was significantly higher in Q than PLA in POST-resistance exercise (43.62%; p = 0.038; Table 1).
Isometric fatiguing task (IFT)
The average MDF of rectus femoris recorded during isometric fatiguing task was higher in POST (MDFPOST; expressed in % of MDF recorded of PRE resistance exercise) when the subjects ingested Q compared to PLA (p < 0.001) (Table 1).
Coactivation index values for knee extension showed different levels of coactivation between the two treatments after the resistance exercise. In particular, the BF muscle antagonist intervention was higher in PLA compared to Q during the isometric knee extension in POST (p = 0.014) (Table 1).
Performance measurements
The performance parameters are shown in Table 2. Pain measures assessed with the VAS showed that participants level of muscle soreness was the same following the resistance exercise for both treatments. In POST, subjects’ feeling of perceived recovery assessed with the PRS was not different between treatments (p > 0.05). Session total volume was higher in number of repetitions for Q compared with PLA (p = 0.027) with a global session RPE not different between treatments (p > 0.05).
Biochemical results
There was no significant treatment by time interactions or treatment main effects for CK, GSSG, or GSH–GSSG ratio. However, a significant main effect of time was evident for all of these variables. CK (expressed as a percentage of CKPRE) was significantly increased after 24 h (p < 0.016) compared to PRE-exercise in both conditions. GSSG levels were higher in POST compared to PRE. GSH-GSSG ratio was lower POST-exercise compared to PRE in PLA, whereas GSSG levels were greater POST-exercise in both conditions (p < 0.01). No significant differences were found in plasma CK, GSH–GSSG ratio, and GSSG levels after the resistance exercise session between treatments (Table 3).
Discussion
The aim of this study was to assess the impact of an acute dose of the Q supplement consumed before a resistance training session on neuromuscular, biochemical, and perceived fatigue parameters of muscle function in young healthy males. We hypothesized that an acute dose of Q (1 g/day) would have a beneficial effect on resistance exercise-induced neuromuscular efficiency impairment (Davis et al. 2009).
The main findings of the present study showed that: (a) there was a lower reduction of the knee extensors muscles MVIC in POST accompanied by an enhancement of NME and RTD200; (b) the torque–velocity relationship (described by values recorded during the isokinetic test) was preserved after a single dose of Q; (c) MDF and CI recorded during the isometric fatiguing test were different between Q and PLA conditions; (d) the total volume of the resistance exercise session was higher in Q with a comparable RPE of PLA.
The present investigation showed that the EMG amplitude was lower when participants assumed Q compared to PLA and that this was associated with a higher MVIC, suggesting that Q may affect the neuromuscular efficiency defined as the motor unit activity required to produce a given amount of contractile force. Our results in young men are similar to those of Connolly and colleagues who reported a 22% strength loss after a bout of eccentric elbow flexion contractions with placebo compared to the 4% obtained after the consumption of cherry juice, a natural source of quercetin (Connolly 2006). Conversely, our findings conflict with those of the only two acute studies which we found in the literature, since they reported that an acute dose of Q was not able to affect parameters of endurance performance (Cheuvront et al. 2009; Konrad et al. 2011). Most likely, the aerobic type of exercise may be partly the reason of the different results found in our study. In addition, the fact that Q was mixed with other flavonoids and the timing of Q supplementation used in these studies (15′ before exercise) may have moderated the effects of Q. In fact, it has been shown that the optimal Q peak plasma level is reached 3–9 h after the assumption (Kale et al. 2010) and that Q may be more effective of on resistance performance than on endurance exercises (Clifford et al. 2016). In addition, it is possible that heat-stress conditions elicited by endurance exercise may have negated the potentially ergogenic efficacy of Q on muscle performance (Cheuvront et al. 2009).
The mechanism by which Q could enhance the muscle activation is believed to be related to an increased neuromuscular modulation at a central-level (Alexander 2006; Cheuvront et al. 2009). In fact, Q has been shown to have a caffeine-like psychostimulant effect characterized by a high affinity for adenosine receptors in the brain. Q easily crosses the blood–brain barrier and counteracts most of the inhibitory effects of adenosine on neuro-excitability and neurotransmitter release/activity. Then, the ergogenic effect of Q ingestion might occur through stimulation of the central nervous system (CNS). In the present study, the effects of Q on EMG activity are represented by an increased neuromuscular efficiency during the knee extensors’ MVIC, a decreased antagonistic muscle contraction and an increased MDF, and during the fatiguing test. This last result may suggest an increase of the central drive in Q condition. However, this general conclusion must be taken with some caution for the frequency analysis limits (Farina 2004). To what concerns the significant reduced coactivation levels in Q with respect to PLA, this could be the result of an alteration in the agonist–antagonist balance control. In fact, it is likely that the muscle activation during a sustained contraction may decrease with fatigue and the inability to activate agonist muscles at a central level could induce a decrease in torque production. Therefore, in the attempt to maintain the prefixed target of knee extension torque and to compensate for the agonist muscles fatigue, there was an increase in the EMG activity of the antagonist muscle. Findings from the present study suggested that a good agonist–antagonist activation was preserved with Q supplementation with respect to PLA. Moreover, in Q condition both the RTD and NME were preserved in knee extension MVIC and the MDF was increased by 5% when compared to PRE-exercise. The reduced loss of strength recorded after the resistance exercise with Q may be the result of a greater neuromuscular efficiency.
The effects of Q on fatigue may in part be due to its lipophilic properties, which allows to cross the blood–brain barrier and influences adenosine receptors in the brain. Studies examining the effects of Q on physical performance suggested that the potential mechanism of action resides in blocking-specific adenosine receptors in the brain (Alexander 2006; Davis et al. 2009). It is reasonable to assume that the increase in physical activity with Q may in part be due to reduced perception of effort or facilitation of motor unit recruitment and may potentially explain the findings observed in this study.
RPE and total volume data represented an interesting and unexpected finding supporting the beneficial effects of Q to improve performance during and after a resistance exercise. In fact, we observed that the increased volume with Q was associated with a similar global session RPE between Q and PLA sessions, which indicates that the participants perceived the same effort, although they were able to exert higher volume in Q condition. This could be first ascribed to some reported effects of Q on the central nervous system (Alexander 2006). Indeed, Q can affect either the motor cortex or the generation of the effort sensation through the corollary discharge, inducing a positive effect on the general perception of effort (MacRae and Mefferd 2006).
To what concerns the peripheral level, Q has been shown to affect the Ca2+ release from the sarcoplasmic reticulum (Kim et al. 1983), which could be increased in a caffeine-like fashion as a result of the Q inhibitory effect on the Ca2+-ATPase as well as its stimulatory effect on Ca2+ release channels (Lee et al. 2002). The increased sensitivity of myofibrils to Ca2+ could positively affect muscle strength, as already demonstrated after caffeine supplementation (Bazzucchi et al. 2011).
In the present study, blood biomarkers were not affected by the acute administration of Q. It may be that the amount of muscle mass experiencing a muscle damage was too small, or that inflammation did not occur under these experimental conditions. Markers of muscle damage and oxidative stress (GSSG and CK levels) were increased immediately and 24-h POST-exercise, but this was not significantly different between the treatments.
Limitations
Despite the significant findings obtained in the present investigation, the small sample size adopted may deserve consideration. In fact, the sample size has been determined based on the effect size of the strength performance variables, but other variables showed a higher level of variability (such as blood markers). Thus, future studies using larger sample sizes are warranted. In addition, our sampling time was focused on the 24-h post-exercise; however, it is possible that further modifications in our measured variables may have been present later in the recovery phase.
The analysis of indirect indices of muscle damage may also represent a limitation. Moreover, our mainly concentric-based exercise regime may have failed to elicit pronounced muscle damage, which in turn has not been detected by the indirect markers. In addition, it should be noted that we did not measure inflammatory markers in our subjects. It is possible that inflammation could have been present or that other blood biomarkers may have experienced a different response. Future studies may be designed to focus on the eccentric phases of resistance exercises to elicit greater muscle damage.
Conversely, this represented the first study investigating simultaneously the EMG activity and the performance parameters (strength, RPE, and total volume) after an acute Q ingestion during maximal isometric and isokinetic contractions. The present findings extend the knowledge on the potential positive effects of Q ingestion, with the implications that this could have for sport performance. In particular, our findings showed that Q consumption before a resistance exercise session may be a valuable nutritional strategy for an increase in the total volume of a resistance training.
Conclusions
In this study, we used a single dose of Q (1 g) to attenuate symptoms of neuromuscular fatigue consequent to a resistance training session. Collectively, the results indicate that the consumption of a Q supplement consumed 3 h prior to a bout of resistance exercise may represent a strategy to enhance the muscular performance. In fact, participants were able to significantly increase their exercise performance compared with the placebo condition and this increased total volume was accompanied by a similar RPE.
Abbreviations
- ANOVA:
-
Analysis of variance
- BF:
-
Biceps femoris
- BMI:
-
Body mass index
- CI:
-
Coactivation index
- CK:
-
Creatine kinase
- EMG:
-
Electromyography
- GSH:
-
Glutathione status
- GSSG:
-
Oxidized gluthanione
- ICC:
-
Intraclass correlation coefficients
- IFT:
-
Isometric fatiguing test
- MDF:
-
Median frequency
- MVIC:
-
Maximal voluntary isometric contraction
- NME:
-
Neuromuscular efficiency
- PLA:
-
Placebo
- PRS:
-
Perceived recovery scale
- Q:
-
Quercetin
- RF:
-
Rectus femoris
- RMS:
-
Root mean square
- RPE:
-
Rate of perceived exertion
- RTD:
-
Rate of torque development
- VAS:
-
Visual analogue scale
- 1RM:
-
One repetition maximum
References
Aagaard P, Simonsen EB, Andersen JL et al (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93:1318–1326. https://doi.org/10.1152/japplphysiol.00283.2002
Ackerman J, Clifford T, McNaughton LR, Bentley DJ (2014) The effect of an acute antioxidant supplementation compared with placebo on performance and hormonal response during a high volume resistance training session. J Int Soc Sports Nutr 11:10. https://doi.org/10.1186/1550-2783-11-10
Alexander SPH (2006) Flavonoids as antagonists at A1 adenosine receptors. Phyther Res 20:1009–1012. https://doi.org/10.1002/ptr.1975
Andersen LL, Aagaard P (2006) Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 96:46–52. https://doi.org/10.1007/s00421-005-0070-z
Bazzucchi I, Riccio ME, Felici F (2008) Tennis players show a lower coactivation of the elbow antagonist muscles during isokinetic exercises. J Electromyogr Kinesiol 18:752–759
Bazzucchi I, Felici F, Sacchetti M (2009) Effect of short-term creatine supplementation on neuromuscular function. Med Sci Sport Exerc. https://doi.org/10.1249/MSS.0b013e3181a2c05c
Bazzucchi I, Felici F, Montini M et al (2011) Caffeine improves neuromuscular function during maximal dynamic exercise. Muscle Nerve 43:839–844
Bazzucchi I, Patrizio F, Felici F et al (2016) CHO mouth rinsing improves neuromuscular performance during isokinetic fatiguing exercise. Int J Sports Physiol Perform ahead of p:1–23. https://doi.org/10.1123/ijspp.2016-0583
Bloomer RJ, Goldfarb AH, Wideman L et al (2005) Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J Strength Cond Res 19:276. https://doi.org/10.1519/14823.1
Boots AW, Haenen GRMM., Bast A (2008) Health effects of quercetin: From antioxidant to nutraceutical. Eur J Pharmacol 585:325–337. https://doi.org/10.1016/j.ejphar.2008.03.008
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381. https://doi.org/10.1249/00005768-198205000-00012
Braakhuis AJ, Hopkins WG (2015) Impact of dietary antioxidants on sport performance: a review. Sport Med 45:939–955. https://doi.org/10.1007/s40279-015-0323-x
Ceci R, Duranti G, Sgrò P et al (2014) Effects of tadalafil administration on plasma markers of exercise-induced muscle damage, IL6 and antioxidant status capacity. Eur J Appl Physiol 115:531–539. https://doi.org/10.1007/s00421-014-3040-5
Ceci R, Duranti G, Leonetti A et al (2017) Adaptive responses of heart and skeletal muscle to spermine oxidase overexpression: evaluation of a new transgenic mouse model. Free Radic Biol Med 103:216–225. https://doi.org/10.1016/j.freeradbiomed.2016.12.040
Cheuvront SN, Ely BR, Kenefick RW et al (2009) No effect of nutritional adenosine receptor antagonists on exercise performance in the heat. Am J Physiol Regul Integr Comp Physiol 65:R394–R401. https://doi.org/10.1152/ajpregu.90812.2008
Chun OK, Chung SJ, Song WO (2007) Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr 137:1244–1252 pii]
Clifford T, Bell O, West DJ et al (2016) The effects of beetroot juice supplementation on indices of muscle damage following eccentric exercise. Eur J Appl Physiol 116:353–362. https://doi.org/10.1007/s00421-015-3290-x
Connolly DAJ (2006) Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage * Commentary 1 * Commentary 2. Br J Sports Med 40:679–683. https://doi.org/10.1136/bjsm.2005.025429
Davis JM, Murphy EA, Carmichael MD (2009) Effects of the dietary flavonoid quercetin upon performance and health. Curr Sports Med Rep 8:206–213. https://doi.org/10.1249/JSR.0b013e3181ae8959
Davison G, Callister R, Williamson G et al (2012) The effect of acute pre-exercise dark chocolate consumption on plasma antioxidant status, oxidative stress and immunoendocrine responses to prolonged exercise. Eur J Nutr 51:69–79. https://doi.org/10.1007/s00394-011-0193-4
De Vito G, McHugh D, Macaluso A, Riches PE (2003) Is the coactivation of biceps femoris during isometric knee extension affected by adiposity in healthy young humans? J Electromyogr Kinesiol 13:425–431. https://doi.org/10.1016/S1050-6411(03)00061-0
Farina D (2004) The extraction of neural strategies from the surface EMG. J Appl Physiol 96:1486–1495. https://doi.org/10.1152/japplphysiol.01070.2003
García-Alonso J, Ros G, Vidal-Guevara ML, Periago MJ (2006) Acute intake of phenolic-rich juice improves antioxidant status in healthy subjects. Nutr Res 26:330–339. https://doi.org/10.1016/j.nutres.2006.06.004
Goldfarb AH, Bloomer RJ, Mckenzie MJ (2005) Combined antioxidant treatment effects on blood oxidative stress after eccentric exercise. Med Sci Sports Exerc 37:234–239. https://doi.org/10.1249/01.MSS.0000152887.87785.BE
Goldfarb AH, Garten RS, Cho C et al (2011) Effects of a fruit/berry/vegetable supplement on muscle function and oxidative stress. Med Sci Sports Exerc 43:501–508. https://doi.org/10.1249/MSS.0b013e3181f1ef48
Harwood M, Danielewska-Nikiel B, Borzelleca JF et al (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 45:2179–2205. https://doi.org/10.1016/j.fct.2007.05.015
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374. https://doi.org/10.1016/S1050-6411(00)00027-4
Hollman PCH, Gaag MVD, Mengelers MJB et al (1996) Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med 21:703–707. https://doi.org/10.1016/0891-5849(96)00129-3
Kale A, Gawande S, Kotwal S et al (2010) Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract. Phyther Res. https://doi.org/10.1002/ptr.2899
Kaushik D, O’Fallon K, Clarkson PM et al (2012) Comparison of quercetin pharmacokinetics following oral supplementation in humans. J Food Sci doi. https://doi.org/10.1111/j.1750-3841.2012.02934.x
Kim DH, Ohnishi ST, Ikemoto N (1983) Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J Biol Chem 258:9662–9668
Konrad M, Nieman DC, Henson DA et al (2011) The acute effect of ingesting a quercetin-based supplement on exercise-induced inflammation and immune changes in runners. Int J Sport Nutr Exerc Metab 21:338–346. https://doi.org/10.1249/01.MSS.0000402819.42267.6d
Larson A, Witman MAH, Guo Y et al (2012) Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: Nitric oxide. Nutr Res 32:557–564. https://doi.org/10.1016/j.nutres.2012.06.018
Lee EH, Meissner G, Kim DH (2002) Effects of quercetin on single Ca2+ release channel behavior of skeletal muscle. Biophys J 82:1266–1277. https://doi.org/10.1016/S0006-3495(02)75483-0
MacRae HSH, Mefferd KM (2006) Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int J Sport Nutr Exerc Metab 16:405–419
O’Fallon KS, Kaushik D, Michniak-Kohn B et al (2012) Effects of quercetin supplementation on markers of muscle damage and inflammation after eccentric exercise. Int J Sport Nutr Exerc Metab 22:430–437
Paulsen G, Hamarsland H, Cumming KT et al (2014) Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J Physiol 592:5391–5408. https://doi.org/10.1113/jphysiol.2014.279950
Phillips T, Childs AC, Dreon DM et al (2003) A dietary supplement attenuates IL-6 and CRP after eccentric exercise in untrained males. Med Sci Sports Exerc 35:2032–2037. https://doi.org/10.1249/01.MSS.0000099112.32342.10
Powers SK, Nelson WB, Hudson MB (2011) Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med 51:942–950. https://doi.org/10.1016/j.freeradbiomed.2010.12.009
Rainoldi A, Galardi G, Maderna L et al (1999) Repeatability of surface EMG variables during voluntary isometric contractions of the biceps brachii muscle. J Electromyogr Kinesiol 9:105–119. https://doi.org/10.1016/S1050-6411(98)00042-X
Rainoldi A, Gazzoni M, Casale R (2008) Surface EMG signal alterations in carpal tunnel syndrome: a pilot study. Eur J Appl Physiol 103:233–242. https://doi.org/10.1007/s00421-008-0694-x
Remaud A (2005) A methodologic approach for the neuromuscular system
Schoenfeld BJ, Ratamess NA, Peterson MD et al (2014) Effects of different volume-equated resistance training loading strategies on muscular adaptations in well-trained men. J Strength Cond Res 28:2909–2918. https://doi.org/10.1519/JSC.0000000000000480
Sureda A, Tejada S, Bibiloni M et al (2014) Polyphenols: well beyond the antioxidant capacity: polyphenol supplementation and exercise-induced oxidative stress and inflammation. Curr Pharm Biotechnol 15:373–379. https://doi.org/10.2174/1389201015666140813123843
Urso ML, Clarkson PM (2003) Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189:41–54. https://doi.org/10.1016/S0300-483X(03)00151-3
USDA NASS (2009) 2007 Census of Agriculture. 1:p 739
Wu R, Delahunt E, Ditroilo M et al (2016) Effects of age and sex on neuromuscular-mechanical determinants of muscle strength. Age (Omaha). https://doi.org/10.1007/s11357-016-9921-2
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Patrizio, F., Ditroilo, M., Felici, F. et al. The acute effect of Quercetin on muscle performance following a single resistance training session. Eur J Appl Physiol 118, 1021–1031 (2018). https://doi.org/10.1007/s00421-018-3834-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3834-y