Abstract
Introduction
Roller massage (RM) has been reported to reduce pain associated with exercise-induced muscle soreness and increase range of motion without force or activation impairments. The objective was to examine RM effects on evoked pain and contractile properties.
Methods
Twelve men received three sets of 30-s RM at a perceived discomfort level of 7/10 on a visual analogue scale on the ipsilateral (IPSI-R) stimulated plantar flexors (PF), contralateral PF (CONTRA-R), Sham (light rolling on stimulated PF), or Control. At pre-test, post-test, and 5-min post-test, they received evoked maximal twitch, tetanus, and 70% maximal tetanic stimulation, and performed a maximal voluntary isometric contraction (MVIC). Data analysis included perceived pain and contractile properties.
Results
The 70% tetanus illustrated significant 9–10% increases in pain perception with Sham and Control at post- and 5-min post-test, respectively (p < 0.01). There was no pain augmentation with IPSI-R and CONTRA-R. There were no main effects or interactions for most contractile properties. However, MVIC force developed in the first 200 ms showed 9.5% (p = 0.1) and 19.1% (p = 0.03) decreases with IPSI-R at post-test and 5-min post-test.
Conclusion
Data suggest that RM-induced neural inhibition decreased MVIC F200 and nullified the testing-induced increase in evoked pain associated with 70% tetanic stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foam rollers and roller massagers (RM) are popular devices used in competitive, recreational, and rehabilitation training. Prior research has demonstrated that foam rollers and RM have increased joint range of motion (MacDonald et al. 2013; Sullivan et al. 2013; Halperin et al. 2014; Bradbury-Squires et al. 2015), either increased (Halperin et al. 2014) or provided no change (MacDonald et al. 2013; Sullivan et al. 2013) in subsequent force, improved neuromuscular efficiency (Bradbury-Squires et al. 2015), and reduced pain associated with muscle tender points (Aboodarda et al. 2015) and exercise-induced muscle damage (MacDonald et al. 2014; Pearcey et al. 2015).
Potential analgesic effects of massage-like mechanical pressure on muscle pain pressure threshold (PPT) have been attributed to neurological (e.g., mediation of pain-modulatory system) (Mense 2000; Vaughan 2014; Han and Harrison 1997; Lund et al. 2002; Kutner et al. 2008), physiological (e.g., increase blood circulation) (Weerapong et al. 2005; Okamoto et al. 2014), and mechanical parameters (e.g., release of myofascial restrictions with connective tissue and blood vessels, and thixotropic responses) (Barnes 1997; Schleip 2003a, b). Vaughan (2014) demonstrated that 3 min of foam rollers over the iliotibial band resulted in a significant increase in PPT (decreased pain perception) immediately post-treatment. Both MacDonald et al. (2014) and Pearcey et al. (2015) reported that foam rollers reduced pain perception during the 3 days of recovery from exercise-induced delayed onset muscle soreness (DOMS). Aboodarda et al. (2015) highlighted the contribution of a central pain-modulatory system in enhancement of the PPT recorded from plantar flexor muscle tender spots following RM. Since pain perception was alleviated following application of RM on both ipsilateral (muscles containing tender spots) and contralateral plantar flexors (without even touching the tender spots in the testing leg), Aboodarda et al. (2015) concluded that the neural component for this type of pain modulation would play a more important role than peripheral components. While these studies examined the effects of foam rollers or RM on chronic (i.e., myofascial tender spots) and prolonged (i.e., DOMS) pain, it is unknown if RM would have an effect on acute, short-term noxious stimuli (i.e., evoked stimulation).
Mechanical pressure on a muscle can affect voluntary activation (Lund et al. 2002, Morelli et al. 1999, Pierrot-Deseilligny and Burke 2005, Schleip 2003a, b). For example, H-reflex amplitude was depressed during and after a short bout of massage (Behm et al. 2013; Morelli et al. 1999). However, there is a little research examining the effect of RM on evoked contractile properties. MacDonald et al. (2013) reported no significant effects of an acute bout of foam rollers on any voluntary or evoked contractile force-related properties. They explained that the lack of change with knee extension force output, evoked tetanic force, rate of twitch development and voluntary activation could be attributed to the 2-min time lag between foam rollers and neuromuscular evaluation. In a subsequent experiment, MacDonald et al. (2014) examined the influence of foam rollers for 72 h after exercise-induced muscle damage reporting a decreased electromechanical delay (EMD) but deficits in twitch and tetanic force-related variables. The conflicting findings regarding the effect of FR on evoked contractile properties may be related to the examination of an acute bout of foam rolling (MacDonald et al. 2013) versus foam roller effects on exercise-induced muscle damage (MacDonald et al. 2014). Furthermore, in both Macdonald and colleague studies, subjects placed most or all of their body mass on the foam roller, whereas during RM, less mechanical pressure is applied to the muscle. Hence further research is required to examine the effect of RM on voluntary and evoked contractile properties.
Therefore, the aims of this study were to determine the immediate effects of RM on pain perception and evoked contractile properties of the plantar flexor muscles with evoked twitch and tetanic nerve stimulation.
Methods
Participants
Based on prior related studies (MacDonald et al. 2013, 2014; Pearcey et al. 2015; Aboodarda et al. 2015), a statistical power analysis indicated that a minimum of 12 participants would be needed to attain an alpha of 0.05 with a power of 0.8. Due to the pain and discomfort associated with maximal tetanic stimulation, recruiting 12 participants was challenging and we could not exceed the minimum necessary number of participants (Table 1). Inclusion factors for participants included no prior musculoskeletal or visceral pain, experience with receiving evoked stimulation, and currently resistance training for at least 1 year (minimum 2–3 times per week). Whereas one participant resistance trained for competition (power lifting), all others trained for health and fitness concerns. Participant exclusion criteria included: the presence of widespread visceral or musculoskeletal pain and/or other symptom concomitant with myofascial pain, taking pain relief medications and any neurophysiological and metabolic disease, or general health problem that would impair sensory input. The institution’s Human Research Ethics Board approved the study (#15.240).
Research design
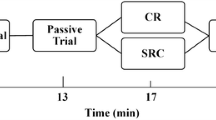
In a single group, randomized, crossover study, the acute effect of RM interventions on pain perception (i.e., change in pain visual analogue scale (VAS) from pre- to post-intervention) and voluntary and evoked contractile properties were investigated (Fig. 1). Each participant attended the laboratory for four experimental sessions. These sessions included (1) heavy RM on the stimulated calf (Ipsilateral Rolling: IPSI-R), (2) heavy RM on the contralateral calf (Contralateral Rolling: CONTRA-R), and (3) light stroking of the skin with RM on the stimulated calf (Sham) and Control (no RM). The participants were seated with hips and knees at 90°. The dominant lower limb, as identified by the leg used to kick a ball, was secured with the ankle fixed in an isometric boot apparatus equipped with strain gauges (Omega Engineering Inc. LCCA 250, Don Mills, ON, Canada) (Behm et al. 2002b).
Evoked contractile properties The anode (8 × 4-cm carbon rubber electrode: Diamond Athletics, Winnipeg, MB, Canada) was placed over the gastrocnemius-soleus intersection (Behm and St-Pierre 1997a, b, 1998). The gastrocnemius-soleus intersection point was identified by the operator’s visual/manual inspection. The cathode (8 × 4 cm carbon rubber electrode) was placed in the popliteal space over the tibial nerve to obtain the highest twitch torque (Behm and St-Pierre 1997b, 1998). Both electrodes were secured with an elastic bandage (low compression). Electrodes were connected to a high-voltage constant-current stimulator (Stimulator Model DS7AH; Digitimer, Welwyn Garden City, Hertfordshire, UK). The amperage (10–1000 mA) and voltage (100–200 V) of a single square-wave pulse lasting 200 µs were progressively increased until the maximum twitch torque and M-wave amplitude were achieved. The corresponding current intensity was recorded, so that it was replicated post-intervention.
For tetanic stimulation at 50 Hz, the voltage was initially reduced to 100 V and the amperage decreased to 10 mA and then sequentially increased. The duration of the electrical stimulation from the stimulator for the tetanic contraction was 400 ms. Amperage and voltage for the tetanus were increased until the participant’s maximal tolerable level of discomfort was achieved. The current was noted to replicate it post-test. The maximum stimulation current was then reduced by 30% and a submaximal tetanic stimulation was also evoked with the same procedure for perceived pain. Submaximal tetanic stimulation was included to provide a spectrum of pain/discomfort ranging from mild, moderate to extreme. Stimulation current parameters for twitch and tetanic stimulation were established during the familiarization session. During the experimental sessions, a single twitch, maximal, and submaximal (70%) tetanic stimulation were elicited at pre-, post-, and 5-min post-intervention, respectively.
Perceived pain With each stimulation, the participant reported the perceived pain on a VAS (Duncan et al. 1989; Price et al. 1983). The VAS was a horizontal line with anchors at the ends indicating no pain (0 cm) and intolerable pain (10 cm). There were no numbers along the line and the score was calculated by measuring the distance in centimeters from zero to the position indicated by the participant.
Voluntary contractile properties Voluntary contractile properties were derived from maximal voluntary isometric contractions (MVIC). A minimum of two MVICs were performed after the evoked contractions, with a third contraction if the first two contractions had a greater than 5% deviation in torque. Two-minute rest periods were allotted between each pre-test MVIC. Participants were informed to contract as hard and as fast as possible (explosively). Since twitch properties could be affected by prior contractions, they were always performed first followed by the maximal and 70% maximal tetanic contractions and, finally, the MVIC.
Intervention After completion of pre-intervention measurements, participants were randomly assigned to one of three intervention conditions, including (1) heavy RM on the stimulated calf (Ipsilateral Rolling: IPSI-R), (2) heavy RM on the contralateral calf (Contralateral Rolling: CONTRA-R), and (3) light stroking of the skin with RM on the stimulated calf (Sham). The original version of the experiment used the Sham condition as the control condition; however, our recent study (Aboodarda et al. 2015) demonstrated that even light rolling could activate cutaneous receptors resulting in greater pain sensitivity. Thus, participants attended another testing session to undertake a Control condition without RM to determine if the increased pain perception was due to light RM or the testing procedure. Unfortunately, due to technical difficulties, evoked contractile properties could not be measured in the Control session. During the Control condition and following the pre-test, participants sat and relaxed for a similar intervention duration as the other three conditions.
The three massage interventions (IPSI-R, CONTRA-R, Sham) involved three repetitions of 30-s RM with 30-s rest between repetitions. The RM was employed with a Theraband® RM (Hygienic Corporation, Akron, OH, USA). The RM consisted of a hard rubber material (24 cm in length and 14-cm circumference) with low amplitude, longitudinal grooves surrounding a plastic cylinder (Halperin et al. 2014; Sullivan et al. 2013). The participants were instructed to provide feedback on the level of perceived pain during the heavy rolling and the intensity of massage would be adjusted accordingly to ensure 7/10 on the VAS which was maintained. The RM was moved proximal to distal at a slow pace (2-s up and 2-s down) over the muscle bellies of the medial and lateral heads of gastrocnemius, while the participant lays prone on a padded mat with knee extended. Participants in the Sham condition received very light pain-free cutaneous strokes of rolling massage with the same pace of rolling as performed for the IPSI-R and CONTRA-R groups.
Post-testing Thirty seconds (time needed to move from RM to boot apparatus) and 5 min after completion of the RM interventions, a twitch and two tetanic contractions (maximal tolerance and 30% less current) using the same parameters used in the pre-test were elicited. Perceived pain VAS values were obtained immediately after the evoked stimulations. A MVIC was performed within 30 s following the tetanic contractions. Parameters for analysis of evoked contractile properties included peak twitch torque, time to peak twitch torque, twitch 1/2 relaxation time, tetanic torque, and tetanic torque produced in the first 200 ms (F200). Voluntary contractile property parameters include MVIC peak torque and MVIC F200. In addition, twitch/MVIC and tetanus/MVIC torque ratios were calculated to provide further insight upon the relative prevalence of central versus peripheral responses to the evoked stimulation. Figures 2, 3, 4 provide illustrations of how the variables were analyzed and examples of actual mechanical recordings.
a Figure illustrates the analysis of twitch contractile property measures. PT peak torque, TPT time to peak torque. b Figure illustrates examples from a single subject of actual mechanical twitch recordings. Sham: ipsilateral rolling at 1/10 on VAS pain scale; IPSI-R: ipsilateral rolling at 7/10 on VAS pain scale; CONTRA-R: contralateral rolling at 7/10 on VAS pain scale
a Figure illustrates the analysis of tetanic contractile property measures. PT peak torque, F200 force produced in the first 200 ms. b Figure illustrates examples from a single subject of actual mechanical maximal tetanic (maximal tolerable intensity tetanic stimulation) recordings. Sham: ipsilateral rolling at 1/10 on VAS pain scale; IPSI-R: ipsilateral rolling at 7/10 on VAS pain scale; CONTRA-R: contralateral rolling at 7/10 on VAS pain scale. c Figure illustrates examples from a single subject of actual mechanical 70% of maximal tetanic recordings. Sham: ipsilateral rolling at 1/10 on VAS pain scale IPSI-R: ipsilateral rolling at 7/10 on VAS pain scale, CONTRA-R: contralateral rolling at 7/10 on VAS pain scale
a Figure illustrates the analysis of maximal voluntary isometric contraction (MVIC) contractile property measures. PT peak torque, F200 force produced in the first 200 ms. b Figure illustrates examples from a single subject of actual mechanical maximal voluntary isometric contraction (MVIC) recordings. Sham: ipsilateral rolling at 1/10 on VAS pain scale; IPSI-R: ipsilateral rolling at 7/10 on VAS pain scale; CONTRA-R: contralateral rolling at 7/10 on VAS pain scale
Statistical analysis
Statistical analyses were computed using the SPSS software (Version 16.0, SPSS, Inc, Chicago, IL, USA). The assumption of sphericity (Mauchley test) and normality (Shapiro–Wilk test) was tested for all dependent variables. If the assumption of sphericity was violated, the corrected value for non-sphericity with Greenhouse-Geisser epsilon was reported. To determine effect of the four interventions (IPSI-R, CONTRA-R, Sham and Control) on pain perception at pre-test, 30-s and 5-min post-intervention a two-way repeated measures ANOVA (4 × 3) with Bonferroni post hoc test used to identify specific main effects. Since evoked contractile properties could not be collected for the control condition, a two-way repeated measure ANOVA (3 × 3) was utilized. If significant interactions’ effect was revealed, paired sample t tests were applied. In addition, Cohen’s d effects sizes (ES, Cohen 1988) were also calculated to determine the magnitude of the differences between interventions and time. The following criteria were used: ES < 0.2 was classified as trivial, ES = 0.2–0.49 was considered as “small” effect size; ES = 0.5–0.79 represented a “medium” effect size; and ES > 0.8 represented a “large” effect size. The intra-class correlation coefficient was calculated for all variables recorded at pre-test within the three testing sessions (IPSI-R, CONTRA-R, Sham). Significance was defined as p < 0.05.
Results
Reliability Intra-class correlation coefficients were high for force-related measures but fair to moderate for pain perception (Table 2). ANOVAs revealed no significant differences between any of the pre-test measures (force or pain).
Pain perception The interventions provided a significantly [F(2,22) = 182.38, p < 0.0001] wide spectrum of pain perception with the 70% tetanic stimulation (mean VAS score of 6.1 ± 1.3) providing a 28.2% (ES: 2.4) reduction in VAS pain scores from the maximum tetanic stimulation (mean VAS score of 8.5 ± 0.7). Mean VAS scores with evoked twitches (mean VAS score of 1.05 ± 0.6) were 87.6% (ES: 11.4) lower than maximum tetanus.
There were no main effects or interactions with the pain perception associated with maximal tetanic stimulation. A significant condition × time interaction [F(6,54) = 2.913, p = 0.016] for pain perception associated with the 70% tetanic stimulation illustrated small magnitude 9.3% (p = 0.002; ES: 0.43) and 9.5% (p = 0.003; ES: 0.43) increases in pain perception with the Sham condition at post- and 5-min post-test, respectively (Fig. 5). Similarly, the control condition exhibited significant 10.8% (p = 0.012; ES: 0.47) and 10.5% (p = 0.04; ES: 0.54) small-to-moderate magnitude increases in pain perception at post- and 5-min post-test, respectively. These results were in contrast to the lack of pain augmentation with the IPSI-R and CONTRA-R post-tests for the 70% maximal tetanic stimulation (Fig. 5).
Figure illustrates mean ± standard deviation changes in pain perception (visual analogue scale) of the three interventions over time with the 70% maximum tetanic stimulation. Asterisk represents significant increases for the control (CON) and SHAM interventions compared to pre-test. Ipsilateral: IPSI-R condition. Contralateral: CONTRA-R condition
Furthermore, a main effect for time was revealed for pain perception with evoked twitches [F(2,54) = 7.373, p = 0.005] with large magnitude 38.5% (p = 0.012; ES: 0.84) and moderate magnitude 26.4% (p = 0.09; ES: 0.57) and increases in pain perception at post-test and 5-min post-test compared with pre-test (Fig. 6).
Evoked contractile properties There were no significant main condition or interaction effects for maximal and 70% of maximal tetanic F200. A significant interaction [F(4,40) = 2.432, p = 0.05] was revealed for MVIC F200 with 9.5% (p = 0.1; ES: 0.37) and 19.1% (p = 0.03; ES: 0.73) decreases with the IPSI-R condition at post-test and 5-min post-test compared with pre-test (Fig. 7).
There were no significant main effects or interactions for MVIC and tetanic peak force, time to peak twitch force, twitch ½ relaxation time, maximal tetanic pain perception, twitch/MVIC, and tetanus/MVC force ratios.
Discussion
The most important findings in this study were that three repetitions of 30 s of RM, whether with IPSI-R or CONTRA-R diminished the increase in pain perception experienced with 70% maximal tetanic stimulation during the Sham and Control conditions. Secondly, MVIC F200 was decreased following IPSI-R.
The testing procedure involving 70% of maximal tetanic stimulation increased pain sensitivity pre- to post-testing with the Sham (9.3–9.5%) and Control (10.5–10.8%) conditions. The application of RM to the tested (IPSI-R) or contralateral (CONTRA-R) limb counteracted the increased sensitivity. This is the first study to examine a range of pain intensity (mild, moderate, and intense) with acute, short duration (400 ms or less), and evoked stimulation. The findings of a pain suppression effect are in accordance with prior research that demonstrated foam roller reduced pain perception during the 2–3 days of recovery from DOMS (MacDonald et al. 2014; Pearcey et al. 2015). Vaughan (2014) demonstrated that 3 min of foam rolling resulted in a decrease in pain perception immediately following treatment. Similarly, Aboodarda et al. (2015) reported a diminished PPT of muscle tender spots when RM was applied either to the affected or contralateral plantar flexors. However, these studies examined either chronic pain (iliotibial band or muscle tender spots) or prolonged pain from muscle damage-induced inflammation (DOMS).
While MacDonald et al. (2014) and Pearcey et al. (2015) speculated that foam roller might reduce pain perception via restoration of soft tissue extensibility; they also suggested the possibility of a central pain-modulatory system. The pain inhibition with the IPSI-R could be attributed to alteration of the response of free nerve endings (i.e., pain receptors) (Schleip 2003a, b), or alterations in blood flow, blood pressure, skin temperature, and galvanic skin responses, which are indications of a lower level of sympathetic stimulation (Weerapong et al. 2005). Previous massage-related research has demonstrated the analgesic effects of intense tissue massage on central pain-modulatory systems (Mense 2000; Vaughan 2014; Han and Harrison 1997). Decreased pain sensitivity with the CONTRA-R supports previous investigations that indicate that neurophysiological responses evoked by noxious stimuli may produce a generalized non-segmental inhibition of pain perception (Aboodarda et al. 2015, Sigurdsson and Maixner 1994).
Sigurdsson and Maixner (1994) state that the pain relieving effects of noxious counterirritants may produce secondary hyperalgesia. The central pain-modulatory systems may be linked to the gate control theory of pain (Moayedi and Davis 2013; Melzack and Wall 1965), parasympathetic nervous system alterations or diffuse noxious inhibitory control (Mense 2000). With the gate control theory of pain, direct activation of percutaneous mechanoreceptor and proprioceptor nerve fibers can alter the transmission of ascending nociceptors via small diameter Aδ fibers to the periaqueductal grey nucleus (Moayedi and Davis 2013). An endogenous analgesia can result from descending signals to the opioid receptors, which would inhibit pain with serotonergic and noradrenergic neurons (Pud et al. 2009). Massage has also been shown to stimulate parasympathetic activation; characterized by changes in serotonin, cortisol, endorphin, and oxytocin contributing to a decrease pain perception (Weerapong et al. 2005). Diffuse noxious inhibitory control is activated by nociceptive stimuli (i.e., mechanical pressure) from a non-local tissue. The non-local receptor activity is transmitted to multi-receptive, wide dynamic range convergent neurons in the cortical subnucleus reticularis dorsalis where it inhibits pain transmission monoaminergically (Mense 2000; Sigurdsson and Maixner 1994; Pud et al. 2009), reducing pain perception not only locally but also at distant sites (Sigurdsson and Maixner 1994; Pud et al. 2009). Since the pain was delivered exogenously for 400 ms or less, the pain did not originate from smooth muscle strain and thus a global parasympathetic response would probably have not provided a substantial contribution to the pain suppression. Whereas IPSI-R pain suppression might be partially attributed to gate control theory mechanisms, CONTRA-R pain modulation would be more likely attributed to diffuse noxious inhibitory control.
The RM-induced pain inhibition was evident for the 70% tetanus but not maximal tetanic stimulation. Falkensteiner et al. (2011) reported that massage reduced pain in oncology patients in 4/5 studies and was most effective with strong pain perceptions. However, in this study, the maximal tetanic stimulation was deemed at the limit of tolerance and the RM did not have an appreciable effect on this extreme level and short duration (400 ms) of discomfort. Although, there was an overall increase for twitch pain perception (main effect for time), there was no indication of RM inhibition for this lower pain threshold. Hence, RM whether with IPSI-R or CONTRA-R was only effective in the mid-range of evoked pain sensitivity.
A number of foam roller, RM, and massage studies have reported no detrimental effects on muscle performance (Sullivan et al. 2013; Halperin et al. 2014; MacDonald et al. 2013; Zainuddin et al. 2005). Similarly, this study demonstrated no deficits for MVIC, twitch, and tetanic force, twitch and tetanic F200, twitch ½ relaxation time, twitch/MVIC, and tetanus/MVC force ratios. However, MVIC F200 was decreased following IPSI-R.
Since the impairments were only evident for MVIC F200 and not for time to peak twitch torque or tetanic F200, a central neural component must have played a role. Neural factors seem to play a greater role at the onset of a rapid voluntary contraction; whereas for longer duration contractions, the voluntary rate of force development becomes more strongly influenced by the speed-related properties of the muscle and MVC force per se (Maffiuletti et al. 2016). The roller would have induced small repetitive passive stretches that could negatively disturb the efficiency of Ia afferent pathway to excite α-motoneurons (Avela et al. 1999). Furthermore, free nerve endings innervated by group II and III afferent fibers are also stretch sensitive and can inhibit force during and after muscle stretch (Cleland and Rymer 1990). The roller intensity at 7/10 on a pain scale was deemed uncomfortable or induced some pain with most subjects. The sensation of pain transmitted through type III and IV afferents can negatively affect muscle force production (Rutherford et al. 1990). Thus, motor neuronal disfacilitation could be mediated by a roller-induced inhibition from Ia, II, III, and IV afferents.
One of the limitations of this study was the inability to monitor evoked contractile property changes with the subsequent Control condition due to the lack of strain gauges with the alternative boot apparatus. As there were no significant changes in contractile properties with the Sham or CONTRA-R conditions or with the previous Control conditions using evoked stimulation from our laboratory (Behm and Sale 1994; Behm and St-Pierre 1997a; Behm et al. 2002a), it can be confidently assumed that the present control condition also did not alter evoked contractile properties post-test. A second limitation was that this boot apparatus had greater padding over the knee than the original boot apparatus. However, there were no statistically significant pre-test pain differences between the conditions for twitch, maximal tetanic, or submaximal tetanic stimulation. The Sham and Control conditions experienced similar increases (9–11%) in pain perception that was in direct contrast to the reduction in pain with IPSI-R and CONTRA-R. A third limitation was the lower reliability scores for pain perception, which was unavoidable as perceived pain may vary considerably from day-to-day. A final limitation was that since the gastrocnemius is not at its optimal length when flexed, it would not have produced its greatest force output during testing. Thus, the reported changes in temporal characteristics might have been more pronounced if tested with an extended knee.
Conclusions
An acute bout of RM (3 sets of 30-s repetitions at 7/10 VAS) diminished the testing-induced increase in pain perception associated with submaximal (70% of maximal) tetanic evoked stimulation. RM did not affect the pain or discomfort associated with nearly intolerable (maximal 50 Hz tetanus) or minor discomforting (maximal twitch) evoked pain. Since the pain was suppressed with both IPSI-R and CONTRA-R, the mechanisms for this pain suppression are likely related to gate control theory and diffuse noxious inhibitory control, respectively. Second, MVIC F200 was decreased following IPSI-R. Since this time-dependent torque decrease was only significant with MVIC and not evoked contractions, the mechanisms should be neural and possibly related to Ia, II, III, and IV (stretch and pain) afferent inhibition. Based on the results of this and prior studies (Aboodarda et al. 2015; MacDonald et al. 2014), roller massage may be recommended as an adjunct to manual massage therapy for muscle pain reduction (below the maximum tolerable limit).
Abbreviations
- ANOVA:
-
Analysis of variance
- CONTRA-R:
-
Contralateral plantar flexors rolling massage
- DOMS:
-
Delayed onset muscle soreness
- ES:
-
Effect size
- F200:
-
Peak force exerted within 200 ms of the maximum voluntary isometric contraction
- IPSI-R:
-
Ipsilateral plantar flexors rolling massage
- MVIC:
-
Maximum voluntary isometric contraction
- PF:
-
Plantar flexors
- PPT:
-
Pain pressure threshold
- RM:
-
Roller massager
- VAS:
-
Visual analogue scale
References
Aboodarda SJ, Spence AJ, Button DC (2015) Pain pressure threshold of a muscle tender spot increases following local and non-local rolling massage. BMC Musculoskeletal Disorders 16:265. doi:10.1186/s12891-015-0729-5
Avela J, Kyrolainen H, Komi PV, Rama D (1999) Reduced reflex sensitivity persists several days after long-lasting stretch-shortening cycle exercise. J Appl Physiol 86(4):1292–1300
Barnes MF (1997) The basic science of myofascial release. Morphologic change in connective tissue. J Body Mov Ther 1:231–238
Behm DG, Sale DG (1994) Voluntary and evoked muscle contractile characteristics in active men and women. Can J Appl Physiol 19(3):253–265
Behm DG, St-Pierre DM (1997a) Effects of fatigue duration and muscle type on voluntary and evoked contractile properties. J Appl Physiol 82(5):1654–1661
Behm DG, St-Pierre DM (1997b) Fatigue characteristics following ankle fractures. Med Sci Sports Exerc 29(9):1115–1123
Behm DG, St-Pierre DMM (1998) Fatigue mechanisms in trained and untrained plantar flexors. J Strength Cond Res 12(3):166–172
Behm DG, Reardon G, Fitzgerald J, Drinkwater E (2002a) The effect of 5, 10, and 20 repetition maximums on the recovery of voluntary and evoked contractile properties. J Strength Cond Res 16(2):209–218
Behm DG, Whittle J, Button D, Power K (2002b) Intermuscle differences in activation. Muscle Nerve 25(2):236–243
Behm DG, Peach A, Maddigan M, Aboodarda SJ, DiSanto MC, Button DC, Maffiuletti NA (2013) Massage and stretching reduce spinal reflex excitability without affecting twitch contractile properties. J Electromyogr Kinesiol 23(5):1215–1221. doi:10.1016/j.jelekin.2013.05.002
Bradbury-Squires DJ, Noftall JC, Sullivan KM, Behm DG, Power KE, Button DC (2015) Roller-massager application to the quadriceps and knee-joint range of motion and neuromuscular efficiency during a lunge. J Athl Train 50(2):133–140. doi:10.4085/1062-6050-49.5.03
Cleland CL, Rymer W (1990) Neural mechanisms underlying the clasp-knife reflex in the cat I Characteristics of the reflex. J Neurophysiol 64(4):1303–1318
Cohen J (1988) Statistical power analysis for the behavioural sciences. L. Erbraum Associates, Hillside N.J. pp 12–65
Duncan GH, Bushnell MC, Lavigne GJ (1989) Comparison of verbal and visual analogue scales for measuring the intensity and unpleasantness of experimental pain. Pain 37:295–303
Edman KA, Josephson RK (2007) Determinants of force rise time during isometric contraction of frog muscle fibres. J Physiol 580(Pt.3):1007–1019. doi:10.1113/jphysiol.2006.119982
Falkensteiner M, Mantovan F, Muller I, Them C (2011) The use of massage therapy for reducing pain, anxiety, and depression in oncological palliative care patients: a narrative review of the literature. ISRN Nurs 2011:929868. doi:10.5402/2011/929868
Halperin I, Aboodarda SJ, Button DC, Andersen LL, Behm DG (2014) Roller massager improves range of motion of plantar flexor muscles without subsequent decreases in force parameters. Int J Sports Phys Ther 9(1):92–102
Han SC, Harrison P (1997) Myofascial pain syndrome and trigger-point management. Reg Anesth 22(1):89–101
Kutner JS, Smith MC, Corbin L, Hemphill L, Benton K, Mellis BK, Beaty B, Felton S, Yamashita TE, Bryant LL, Fairclough DL (2008) Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: a randomized trial. Ann Intern Med 149(6):369–379
Lund I, Ge Y, Yu LC, Uvnas-Moberg K, Wang J, Yu C, Kurosawa M, Agren G, Rosen A, Lekman M, Lundeberg T (2002) Repeated massage-like stimulation induces long-term effects on nociception: contribution of oxytocinergic mechanisms. Eur J Neurosci 16(2):330–338
MacDonald GZ, Penney MD, Mullaley ME, Cuconato AL, Drake CD, Behm DG, Button DC (2013) An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res 27(3):812–821. doi:10.1519/JSC.0b013e31825c2bc1
MacDonald GZ, Button DC, Drinkwater EJ, Behm DG (2014) Foam rolling as a recovery tool after an intense bout of physical activity. Med Sci Sports Exerc 46(1):131–142. doi:10.1249/MSS.0b013e3182a123db
Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J (2016) Rate of force development: physiological and methodological considerations. Eur J Appl Physiol. doi:10.1007/s00421-016-3346-6
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150(3699):971–979
Mense S (2000) Neurobiological concepts of fibromyalgia–the possible role of descending spinal tracts. Scand J Rheumatol Suppl 113:24–29
Moayedi M, Davis KD (2013) Theories of pain: from specificity to gate control. J Neurophysiol 109(1):5–12. doi:10.1152/jn.00457.2012
Morelli MC, Chapman C, Sullivan S (1999) Do cutaneous receptors contribute to changes in the amplitude of the H-reflex during massage? Electromyogr Clinic Neurophysiol 39:441–447
Okamoto T, Masuhara M, Ikuta K (2014) Acute effects of self-myofascial release using a foam roller on arterial function. J Strength Cond Res 28(1):69–73. doi:10.1519/JSC.0b013e31829480f5
Pearcey GE, Bradbury-Squires DJ, Kawamoto JE, Drinkwater EJ, Behm DG, Button DC (2015) Foam rolling for delayed-onset muscle soreness and recovery of dynamic performance measures. J Athl Train 50(1):5–13. doi:10.4085/1062-6050-50.1.01
Pierrot-Deseilligny E, Burke D (2005) The circuitry of the human spinal cord: its role in motor control and movement disorders Cambridge: Cambridge University Press Price pp 385–411
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as a ratio scale measures for chronic and experimental pain. Pain 17:45–56
Pud D, Granovsky Y, Yarnitsky D (2009) The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 144(1–2):16–19. doi:10.1016/j.pain.2009.02.015
Rutherford OM, Jones DA, Round JM (1990) Long-Lasting unilateral muscle wasting and weakness following injury and immobilisation. Scand J Rehabil Med 22:33–37
Schleip R (2003a) Fascial plasticity- a new neurobiological explanation: Part 2. J Bodywork Movement Therapies 7(2):104–116
Schleip R (2003b) Fascial plasticity- a new neurobiological explanation: Part I. J Bodywork Movement Therapies 7(1):11–19
Sigurdsson A, Maixner W (1994) Effects of experimental and clinical noxious counterirritants on pain perception. Pain 57(3):265–275
Sullivan KM, Silvey DB, Button DC, Behm DG (2013) Roller-massager application to the hamstrings increases sit-and-reach range of motion within five to ten seconds without performance impairments. Int J Sports Phys Ther 8(3):228–236
Vaughan BMP (2014) Immediate changes in pressure pain threshold in the iliotibial band using a myofascial (foam) roller. Int J Ther Rehab 21(12):569–574. doi:http://dx.doi.org/10.12968/ijtr.2014.21.12.569
Weerapong P, Hume PA, Kolt GS (2005) The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med 35(3):235–256
Zainuddin Z, Newton M, Sacco P, Nosaka K (2005) Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. J Athl Train 40(3):174–180
Acknowledgements
The Natural Science and Engineering Research Council of Canada (NSERC) and a MITACS Accelerate grant financially supported this study. We would like to acknowledge the contributions of Dr. Thamir Alkanani for his organization and preparation of the laboratory and equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest of the authors with the information contained within the manuscript.
Additional information
Communicated by Olivier Seynnes.
Rights and permissions
About this article
Cite this article
Cavanaugh, M.T., Döweling, A., Young, J.D. et al. An acute session of roller massage prolongs voluntary torque development and diminishes evoked pain. Eur J Appl Physiol 117, 109–117 (2017). https://doi.org/10.1007/s00421-016-3503-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3503-y