Abstract
Introduction
Inorganic nitrate ingestion has been posited to affect arterial blood pressure and vascular function.
Purpose
We sought to determine the acute effect of a red spinach extract (RSE) high in inorganic nitrate on vascular reactivity 1-h after ingestion in peripheral conduit and resistance arteries.
Methods
Fifteen (n = 15; males 8, females 7) apparently healthy subjects (aged 23.1 ± 3.3 years; BMI 27.2 ± 3.7 kg/m2) participated in this crossover design, double-blinded study. Subjects reported to the lab ≥2-h post-prandial and consumed RSE (1000 mg dose; ~90 mg nitrate) or placebo (PBO). Venipuncture was performed on three occasions: baseline, 30-min post-ingestion and between 65 to 75-min post-ingestion. Baseline vascular measurements [i.e., calf venous occlusion plethysmography, brachial artery flow-mediated dilation (FMD)], 30-min of continuous blood pressure (BP) and heart rate (HR) analysis, and follow-up vascular measurements beginning at 40-min post-ingestion were also performed.
Results
Humoral nitrate following RSE ingestion was significantly higher at 30- (+54 %; P = 0.039) and 65 to 75-min post-ingestion compared to baseline (+255 %, P < 0.001) and PBO at the same time points (P < 0.05). No significant changes in BP or HR occurred in either condition. Peak reactive hyperemia (RH) calf blood flow increased significantly (+13.7 %; P = 0.016) following RSE ingestion, whereas it decreased (−14.0 %; P = 0.008) following PBO ingestion. No significant differential FMD responses were detected (P > 0.05), though RH was decreased following the baseline measure in both conditions.
Conclusions
RSE significantly increased plasma nitrate 30-min post-ingestion, but acute microvascular (i.e., resistance vasculature) reactivity increases were isolated to the lower limb and no appreciable change in brachial artery FMD was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelium derived nitric oxide (NO), a well-known molecule involved in vascular homeostasis, is conventionally thought to be derived from endothelial NO synthase (eNOS) activity utilizing l-arginine as a substrate (Dejam et al. 2004; Lundberg et al. 2008). However, the bioconversion of exogenous nitrate to NO (via the nitrate-nitrite-NO pathway) in vascular physiology can be appreciated from investigations of dietary nitrate supplementation (Benjamin et al. 1994; Cosby et al. 2003; Lundberg et al. 1994; Zweier et al. 1995). Indeed, inorganic nitrate can be converted to nitrite enzymatically in the saliva and gastrointestinal tract and nitrite can then be further reduced to NO (e.g., hemoglobin, xanthine oxidoreductase, and polyphenol pathways). Furthermore, evidence suggests that NOS itself can utilize nitrate/nitrite as a substrate for production of NO (Cao et al. 2009; Gautier et al. 2006; Vanin et al. 2007). While the effects of ultimate conversion of nitrate to NO have obvious implications for the vasculature, nitrite has also been shown to have vasodilatory properties and may compliment NO actions.
The acute effects of inorganic nitrate ingestion on flow-mediated dilation (FMD), a bioassay of NO bioavailability and endothelial function, have been conflicting (Hobbs et al. 2013). However, the source of inorganic nitrate, the dose, and the timing of FMD measurements is inconsistent between investigations. Indeed, reported nitrate doses range from as little as 3 mmol to 23 mmol while FMD measurement timing ranged from 1.5- to 3-h post-ingestion (Hobbs et al. 2013). Moreover, the variability of the vehicle for nitrate delivery is important to consider given that supplements derived from plant sources may contain phytochemicals that directly or indirectly affect outcomes (e.g., NO production and/or blood flow). Another method employed to assess vasodilator function is the change in limb blood flow during reactive hyperemia (RH) measured by venous occlusion plethysmography (VOP). Specific to VOP, vasodilation during RH correlates highly with acetylcholine-mediated dilation and serves as a good noninvasive measure of microvascular vasodilator function (Higashi et al. 2001; Meredith et al. 1996). However, to our knowledge, the acute effect of inorganic nitrate ingestion on variables associated with VOP assessment of microvascular vasodilator function has not been characterized.
While FMD and VOP both provide surrogates of vascular reactivity, FMD measures vascular reactivity to a RH stimulus in the muscular conduit arteries (e.g., brachial artery), whereas VOP does so in the peripheral resistance vasculature (i.e., arterioles and capillaries). Importantly, both VOP and FMD methods include the induction of tissue hypoxia (via blood flow occlusion) to potentiate the RH stimulus at the site of vascular reactivity measurement. However, occlusion cuff placement is upstream (i.e., thigh) with VOP and downstream (i.e., forearm) with FMD protocols. Thus, hypoxia is induced in the tissues at the site of measurement for VOP, but likely to a much lesser degree, if at all, with FMD. Indeed, FMD guidelines generally recommend distal placement of the blood flow occlusion cuff to minimize the influence of hypoxia and/or myogenic responses at the site of measurement (Betik et al. 2004; Harris et al. 2010). Given that hypoxia is known to be a primary signal for mediating the conversion of nitrite to NO (Lundberg et al. 2008; Jansson et al. 2008), it stands to reason that VOP, where hypoxia is produced at the site of measurement, may demonstrate a markedly different response than that observed with FMD.
Recent evidence suggests that muscle groups composed of predominantly type II muscle fibers are more responsive to nitrate supplementation (Ferguson et al. 2015; Jones et al. 2016). This may be due, in part, to lower resting PO2 levels in fast-twitch muscle fibers compared to slow twitch fibers. It follows that these fiber types may be more susceptible to hypoxia and subsequent reduction of nitrite to NO (Behnke et al. 2003; Ferguson et al. 2015). While fiber type composition varies markedly from muscle to muscle, the gastrocnemius (the primary muscle at the site of measurement for VOP) is known to have a relatively high proportion of fast-twitch fibers (Johnson et al. 1973). Thus, in the context of this protocol, the vasculature of the gastrocnemius may be more responsive to nitrate ingestion due to regional differences in fiber type composition. Moreover, the absolute proportion of tissue from which blood flow is occluded is markedly higher with VOP compared to FMD due to the proximal occlusion cuff placement.
In this investigation, we sought to determine the acute effect of a red spinach extract (RSE) high in nitrate (1000 mg Amaranthus tricolor; ≥9 % nitrate) on vascular reactivity. Given the recent evidence that plasma nitrate and nitrite concentrations peak only 30- to 60-min following ingestion of this RSE (Subramanian and Gupta 2016), we chose to assess vascular reactivity at baseline and between 40- to 65-min post-ingestion with FMD and VOP. Moreover, given the evidence suggesting a significant acute reduction in blood pressure (BP) (Hobbs et al. 2013) with inorganic nitrate ingestion, we evaluated changes in peripheral and central BP between measures of vascular reactivity to better characterize any potential effect of BP and/or sympathetic nervous system activity on the observed responses. We hypothesized that inorganic nitrate ingestion would acutely increase both FMD and VOP responses, but that the effect would be greater with VOP measurements due to RH induction methods (i.e., occlusion site).
Methods
Subjects
Fifteen (n = 15; males 8, females 7) apparently healthy subjects (aged 23.1 ± 3.3 years BMI 27.2 ± 3.7 kg/m2) were recruited from the local community through advertisement to participate in this crossover design, double-blinded study. All subjects reported for two separate visits, separated by ≥72-h, and were instructed to abstain from exercise and alcohol for 24-h and from caffeine for 12-h. Subjects were also instructed to adhere to a low nitrate diet for ≥48-h and to abstain from mouthwash due to its potential to inhibit bioactivation of nitrate (Petersson et al. 2009) for 24-h prior to visits. Finally, subjects were instructed to replicate their 24-h diet prior to laboratory visits. All subjects reported to the laboratory ≥2-h post-prandial. To control for any potential diurnal variation, all subjects reported for the study at the same time of the day. All data were collected in the same indoor location in a temperature controlled room between early August 2015 and early October 2015.
Study design
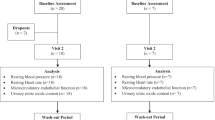
Time and events associated with the protocol utilized herein are illustrated in Fig. 1. Each visit was comprised of baseline vascular measurements, ingestion of RSE or placebo (PBO), 30 min of continuous BP and heart rate (HR) analysis and follow-up vascular measurements. Vascular measurements included brachial artery BP, radial artery pulse wave analysis (PWA), assessment of limb blood flow and peripheral resistance artery reactivity in the calf via VOP, and assessment of FMD and RH in an upper limb peripheral conduit artery (i.e., brachial) using ultrasonography. All procedures described herein were approved by the Auburn University Institutional Review Board and conformed to the standards set by the latest revision of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to their voluntary participation in the study.
Time and events for study protocol. Following height and weight measurements, subjects rested supine in quiet, temperature controlled room for 10-min prior to assessment of peripheral arterial blood pressure (BP) via automated sphygmomanometry in both arms. Thereafter, mean peripheral BP values were used for the calibration and measurement of pulse pressure waveforms characteristics at the radial artery (PWA). Following PWA, conduit vessel reactivity was assessed via flow-mediated dilation (FMD) at the brachial artery and resistance artery reactivity was assessed via venous occlusion plethysmography (VOP) in the calf. The order of the first VOP and FMD procedures was randomized for each subject, but the order was repeated for all additional VOP and FMD procedures thereafter within-subject. Following FMD and VOP procedures, subjects ingested red spinach extract (RSE) or placebo (PBO). Heart rate (HR) and BP were continuously monitored via finger photoplethysmography for 30-min following RSE/PBO ingestion. At exactly 30-min post-RSE/PBO ingestion, a second venipuncture was performed followed by 10-min of supine rest. At 40-min post-RSE/PBO ingestion, peripheral BP, PWA and FMD/VOP procedures were repeated as described above. At the completion of the FMD/VOP procedure (whichever occurred last), a final (3rd) venipuncture was performed. Due to slight variability in the procedural time this last venipuncture occurred, approximately 65–75-min post-ingestion of RSE/PBO. Given the crossover design of this study, this protocol was repeated in exactly the same manner with whichever supplement was not given during the first visit being given during the second vist
Procedures
Upon reporting to the laboratory, adherence with dietary, physical activity, and mouthwash guidelines was verbally confirmed for each subject. Participants were then evaluated for height and weight (digital scale with height rod). HR, brachial systolic (PSBP) and diastolic (PDBP) BP measurements were made in triplicate at the brachial artery of the left and right arms using an automated oscillometric device (OMRON BP785, Omron Corporation, Kyoto, Japan). Initial bilateral brachial artery BP measures were employed to assure no significant between arm difference (defined as ≥10 mmHg for PSBP or PDBP), as this may suggest bilateral variability that would confound the results (Martin et al. 2015b). After confirming the absence of a bilateral difference in brachial artery BP, the average of the triplicate measurements at the left arm only was recorded for characterization of peripheral brachial artery BP.
Blood collection and humoral nitrate/nitrite concentrations
Venipuncture was performed on three occasions; at baseline, 30-min post-RSE or PBO ingestion, and at one instance between 65- and 75-min post-RSE and PBO ingestion. ~4 mL of venous blood was collected from the antecubital space in tubes containing EDTA and immediately centrifuged at 4000g at 4 °C for 5-min. Plasma was then separated in cryotubes and immediately placed in a −80 °C freezer. Plasma samples were frozen for no more than 3 months. Batch processing for assessment of total plasma nitrate and nitrite (NO x ) and nitrite only were performed by commercial assay (Cayman, Ann Arbor, MI USA; kit #780001 and 780051, respectively) to estimate circulating concentrations. Per manufacturer instructions, plasma was subjected to ultracentrifugation using 30 kDa filtration tubes at 10,000g at 4 °C for 5 min with the resulting filtrate used for assays.
Pulse wave analysis (PWA)
Following venipuncture at baseline and 30-min following supplementation with RSE or PBO, subjects rested quietly in a supine position for 10-min. Thereafter, assessment of arterial wave reflection characteristics was performed noninvasively using the SphygmoCor CPVH Pulse Wave Velocity system and Research Version 9.0 software (AtCor Medical, Sydney, Australia). The SphygmoCor system utilizes a Medical Electronics Module Model EM3, an AtCor Medical/Millar tipped pressure tonometer (Millar Instruments, Houston, TX, USA) and a validated generalized mathematical transfer function to synthesize a central aortic pressure waveform (Sharman et al. 2006). The generalized transfer function has been validated using both intra-arterially and noninvasively obtained radial pressure waves (Pauca et al. 2001).
Consecutive measurements were performed at the radial artery in the left arm and the average of the first three high quality recordings per subject were captured for analysis. Pulse pressure waveforms were calibrated to PSBP and PDBP. Although the use of PSBP and PDBP to calibrate radial pressure waveforms omits a possible brachial-to-radial pressure amplification and may lead to underestimation of the central pressure (Bazaral et al. 1990; Mahieu et al. 2010; Picone et al. 2014; Verbeke et al. 2005), PSBP and PDBP were used for radial artery waveform calibration, as recommended by the manufacturer. This was done in consideration of the fact that automated oscillometric blood pressure monitors are not required to verify and validate mean arterial pressure (MAP) (White et al. 1993) and the range of error in calibration was shown to be reduced when utilizing PSBP and PDBP rather than MAP and PDBP (Shih et al. 2011).
Optimal recording of the pressure wave was obtained when the hold down force of the transducer on the artery was such that the resulting waveform had a stable baseline for at least ten cardiac cycles and resulted in a quality index of ≥80 % (derived from an algorithm including average pulse height variation, diastolic variation, and maximum rate of rise of the peripheral waveform). This technique has been shown to be highly reproducible (Wilkinson et al. 1998), and in our hands, reproducibility in young, healthy subjects has previously been reported with a mean coefficient of variation of 2.8, 3.0, 0.5 and 0.3 % for aortic augmentation index (AIx), time to reflection of the reflected pressure wave (T1r), central systolic and diastolic BP, respectively, for repeated measures at the left radial artery calibrated to brachial artery BP in the same arm (Martin et al. 2015b).
A typical aortic pressure waveform synthesized from radial pulse pressure using applanation tonometry and the generalized transfer function and descriptions of pulse wave analysis (PWA) parameters have been described in detail previously (Martin et al. 2010). Specifically, the following PWA parameters, related to the amplification and temporal characteristics of the reflecting wave, were used as dependent variables in this study: central aortic SBP (CSBP), central aortic DBP (CDBP), central mean arterial pressure (CMAP), central pulse pressure (CPP), augmented central pressure (AgBP), AgBP normalized to a heart rate of 75 bpm (AgBP@75), AIx, AIx normalized to an HR of 75 bpm (AIx@75) and systolic duration of the reflected pressure wave (∆Tr). MAP was obtained from an integration of the waveform. AIx, expressed as a percentage, characterizes augmentation of central pressures and is defined as reflected wave amplitude (AgBP) divided by CPP (Wilkinson et al. 2000; Murgo et al. 1981).
Venous occlusion plethysmography and flow-mediated dilation
Following PWA assessment, VOP and FMD measures were performed. Measurements were performed in random order for each subject at the first visit, but this order was repeated for the second visit for each subject.
Calf blood flow (CBF) at rest and during reactive hyperemia (RH) was determined independently by VOP (EC-6, D.E. Hokanson, Inc., Bellevue, WA, USA) using calibrated indium-gallium strain gauges, as previously described (Martin et al. 2015a). Briefly, strain gauges were applied to the widest part of the left calf after limb circumference measurement with the legs elevated slightly above heart level. An ankle cuff was inflated to 200 mmHg of constant pressure to occlude ankle circulation during CBF measurements. To measure CBF, an upper thigh cuff, placed 10 cm above the popliteal fossa, was inflated to 50 mmHg for 7-s each 15-s measurement cycle using a rapid cuff inflator (EC-20; DE Hokanson Inc.) to prevent venous outflow (Hokanson et al. 1975; Patterson and Whelan 1955).
Twelve plethysmograph measurements (over a total of 3 min) were averaged for baseline CBF values. After baseline CBF measurements, the thigh cuff was rapidly inflated to 200 mmHg for 5-min and then rapidly deflated. Following the 5-min of arterial occlusion in the thigh, endothelium-dependent CBF was measured during RH of the calf. Peak CBF (PkCBF) was recorded as the highest blood flow observed during 3-min of RH, and total CBF (TCBF) was recorded as the area under the time-curve after baseline BF was subtracted (Meredith et al. 1996).
The plethysmography output signal was transmitted to the Noninvasive Vascular Program (NIVP3) calibrated software program (DE Hokanson Inc.) and BF was expressed as milliliters (mL) per minute per deciliter (dL) of calf tissue (mL/min/dL tissue).
The FMD technique was used to determine endothelial-dependent reactivity in the right brachial artery using high resolution ultrasound (Logiq S7 R2 Expert; General Electric, Fairfield, CT USA) with a 3–12 MHz multi-frequency linear phase array transducer. The brachial artery was imaged longitudinally with the transducer placed 3–5 cm above the antecubital fossa. Simultaneous measurement of brachial artery diameter and blood velocity was performed using duplex mode imaging (B-mode and Doppler) and video was captured through a digital interface at 30 frames/s with real time analysis (FMD Studio, Pisa, Italy). Resting measurements were captured for one continuous min, RH was produced by inflating a blood pressure cuff placed on the upper forearm, 1–2 cm below the elbow, for 5-min at 200 mmHg, and after cuff release, measurements were made for another 3-min. The transducer was held manually in the same position, with the aid of a mechanical transducer support arm, for the duration of the study.
Vessel diameters were determined frame-by-frame via automatic edge detection software (FMD Studio, Pisa, Italy) measuring the distance between the near and far wall of the intima. Blood velocity was determined via selection of a region of interest around the Doppler waveform and a trace of the velocity–time integral was used to calculate mean velocity for each cardiac cycle. Brachial FMD was calculated as absolute (aFMD, reported in mm) and relative (%FMD, reported in %) peak change in brachial artery diameter in response to the hyperemic stimulus. Because dilation also depends on the resultant RH blood flow stimulus, all measurements of peak %FMD were also normalized (nFMD) to the shear rate area under the curve to the time at which the maximal diameter was observed (100 × %FMD/shear rate AUC−1). Shear rate was calculated as [(4 × time averaged mean velocity)/vessel diameter]. In addition to assessment of NO bioavailability and brachial artery reactivity measures, assessment of the RH response during FMD procedures also allows for evaluation of the microvascular (i.e., arterioles and capillaries) response. Thus, blood flow during RH was calculated from continuous diameter and mean blood velocity measurements during ultrasonography as [Π × (diameter/2)2 × time average mean velocity × 60]. These values were used to calculate peak RH forearm blood flow (PkFBF) and total RH forearm blood flow (TFBF) area under the time-curve using the sum of trapezoids method after baseline blood flow was subtracted.
Supplementation: RSE and placebo
A single oral dose of either 1000 mg RSE (i.e., amaranthus tricolor) powder or PBO (maltodextrin) in gelatin capsules was consumed with bottled water at the corresponding point in the study procedures. The RSE has an herb to extract ratio of 50:1, contains ≥9 % nitrate (by HPLC) and ≥13 % potassium (by ICP-MS). Thus, the ingested dose of nitrate in the RSE was ~90 mg.
Continuous blood pressure measurement
Following RSE and PBO ingestion, participants were required to remain at rest in the supine position. Continuous beat-to-beat arterial BP was measured by finger cuff photoplethysmography using a Finometer PRO (Finapres Medical Systems, Amsterdam, The Netherlands). The Finometer is a noninvasive, automated system for the measurement of arterial BP. The technology utilizes the finger clamp method to record digital artery pulse waveforms. The Finometer noninvasive BP monitor uses the methodology of Penaz to continuously display the arterial pressure waveform and has been described in detail previously (Penaz et al. 1976; Lal et al. 1995; Imholz et al. 1998). Briefly, a finger cuff was placed on the middle phalanx of the middle finger of the right hand and connected to a transducer placed on top of the wrist of the same-side hand resting at heart level. An arm cuff was placed on the same-side upper arm of the participant for brachial BP calibration using the return-to-flow method. Participants remained awake, relaxed, and refrained from speaking or moving during data recording for the duration of data acquisition. The Finometer has been previously validated against invasive hemodynamic measurements in normal healthy participants, unstable intensive care patients, cardiac surgery patients, and noninvasively across a variety of BP ranges (Harms et al. 1999; Jansen et al. 2001; Jellema et al. 1999; Schutte et al. 2004).
Statistical analysis
All data were tested for normal distribution using the Shapiro–Wilk test for normality. An alpha level of P ≤ 0.05 was required for statistical significance. Satterthwaite corrected t tests were performed to compare male and female subject characteristics at baseline. Repeated measures two-way ANOVA was used to evaluate the continuous primary dependent variables associated with this study. When a significant treatment-by-time interaction was observed, within-treatment (e.g., pre-RSE vs. post-RSE) and between-treatment (e.g., post-RSE vs. post-RSE) comparisons were performed using Student’s paired t tests to analyze differences between time points. All statistical analyses were performed using IBM SPSS Statistics 22 for Windows (Chicago, IL, USA). All data are reported as mean ± SD.
Results
Subject characteristics
Subject characteristics are presented in Table 1. There were no significant differences between male and female groups for age, height, weight and BMI. Moreover, sex and body mass were not found to be significant covariates of the statistical model for any variable analyzed.
Change in circulating concentrations of nitrate and nitrite
Treatment means for NO x (total nitrate/nitrite), nitrite and nitrate at baseline and at 30 and 65- to 75-min following RSE and PBO ingestion are presented in Fig. 2. Highly significant main effects of time, treatment, and the time × treatment interaction were observed for plasma NO x (P ≤ 0.001 for all). RSE ingestion significantly increased NO x concentrations at 30 and 65- to 75-min post ingestion (P ≤ 0.01 for both time points) and the concentrations were significantly higher than during the PBO condition at the same time points (P = 0.027 and <0.001 for 30 and 65- to 75-min time points, respectively). No main effect of time (P = 0.116), treatment (P = 0.798) nor an interaction (P = 0.106) was observed for nitrite concentrations.
Plasma concentrations of total nitrate and nitrite (NO x ; a) and nitrite only (b) at baseline and 30- and 65- to 75-min following ingestion of a red spinach extract (RSE; filled circles) and placebo (PBO; open circles). Data are presented as mean absolute values in μM concentrations ± SD. When a significant time × condition interaction was observed, post hoc tests were performed using Student’s paired t tests. *,**Significantly different from baseline within condition (P ≤ 0.05 and 0.01, respectively). †,††Significantly different between condition at the same time point (P ≤ 0.05 and 0.01, respectively)
Blood pressure and wave reflection characteristics
Variables derived from baseline peripheral BP measurements and continuous Finapres evaluation of hemodynamics for 30-min after ingestion of RSE and PBO are presented in Table 2. No significant differences at baseline were found between treatments with respect to HR, PSBP, PDBP and PMAP. Moreover, no main effect of time or treatment, nor an interaction, were observed for HR, PSBP, PDBP, PMAP, CO, and TPR during the 30-min of monitoring following RSE and PBO ingestion (P > 0.05).
Variables derived from PWA at baseline and 30-min following RSE and PBO ingestion are presented in Table 3. No main effect of time or treatment, nor an interaction, was observed for CSBP, CDBP, CMAP, CPP, AgBP, ΔTr and AIx. A significant main effect of time was observed for AgBP@75 and AIx@75 (P = 0.043 and 0.046, respectively). However, within treatment changes from baseline for both AgBP@75 and AIx@75 were not statistically significant in the RSE or PBO condition (P > 0.05).
VOP
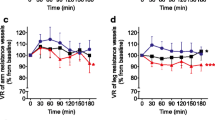
Results from VOP assessment of calf blood flow at rest and during RH before and after RSE and PBO ingestion are presented in Fig. 3. No main effect of time, treatment nor an interaction was observed for RCBF (P > 0.05). For both PkCBF and TCBF, a significant time × treatment interaction was observed (P = 0.046 and 0.043 for PkCBF and TCBF, respectively). PkCBF increased significantly (+13.7 %; P = 0.016) following RSE ingestion, whereas it decreased (−14.0 %; P = 0.008) following PBO ingestion. Moreover, the change from baseline for PkCBF between RSE and PBO conditions was significantly different (Fig. 3d; P = 0.001). TCBF did not change significantly following RSE ingestion (+4.0 %; P = 0.511) though it did significantly decrease with PBO (−20.0 %, P = 0.010). The change from baseline for TCBF between RSE and PBO conditions was significantly different (Fig. 3e; P = 0.043).
Venous occlusion plethysmography measures of limb blood flow in the calf at baseline (PRE) and following ingestion (POST) of a red spinach extract (RSE; filled circles) and placebo (PBO; open circles). a resting calf blood flow (RCBF), b peak reactive hyperemia CBF (PkCBF), c total reactive hyperemia CBF (TCBF), d change from baseline (POST–PRE) in reactive hyperemic peak calf blood flow (ΔPkCBF), and e change from baseline in total reactive hyperemic calf blood flow (ΔTCBF). Data in a–c are presented as mean absolute values in mL/min/dL of tissue ± SD and data in d and e are presented as mean change from baseline (with individual values) in mL/min/dL of tissue ± SD. When a significant time × treatment interaction was observed, post hoc tests were performed using Student’s paired t tests. *Significantly different from baseline (PRE) within RSE condition (P < 0.05). ϕSignificantly different from baseline (PRE) within PBO condition (P < 0.05). †,††Significantly different between condition at the same time point (P ≤ 0.05 and 0.01, respectively)
FMD
Results from brachial artery FMD assessment are presented in Fig. 4. No main effect of time, treatment nor an interaction was observed for baseline diameter, aFMD, %FMD or nFMD (Fig. 4a–d; P > 0.05). A significant main effect of time (P < 0.001) was observed for both peak and total RH blood flow which were, on average for both groups, lower post-RSE/PBO ingestion relative to baseline (Fig. 4e, f). However, no main group effect nor time × group interaction was observed (P > 0.05).
Parameters derived from flow-mediated dilation (FMD) measurement in the brachial artery at baseline (PRE) and following ingestion (POST) of a red spinach extract (RSE; filled circles) and placebo (PBO; open circles). a Resting brachial artery diameter in mm, b brachial artery absolute peak FMD (%FMD, expressed as a percentage), c brachial artery peak relative FMD (%FMD, expressed as a percentage), d brachial artery normalized peak FMD (nFMD), e peak reactive hyperemic forearm blood flow (PkFBF), and f total reactive hyperemic forearm blood flow (TFBF). A main effect of time (P < 0.001) was observed for peak and total hyperemic forearm blood flow and post hoc analysis was performed using Student’s paired t tests. *Significantly different from baseline (PRE) within RSE condition (P < 0.05). ϕSignificantly different from baseline (PRE) within PBO condition (P < 0.05). Data are presented as mean values ± SD
Discussion
The principle findings of this investigation included: (1) plasma nitrate/nitrite (NO x ) concentration was significantly increased relative to baseline at both 30- and 65- to 75-min post-RSE ingestion, (2) PkCBF was significantly higher following RSE ingestion, whereas it was significantly lower following PBO ingestion, (3) FMD associated parameters of brachial artery reactivity were unaffected by RSE and PBO ingestion, and (4) FMD associated reactive hyperemia PkFBF and TFBF values were significantly lower following both RSE and PBO ingestion.
Plasma nitrite concentrations exhibit a delayed peak compared to plasma nitrate in response to inorganic nitrate ingestion (Webb et al. 2008; Lundberg and Govoni 2004). Indeed, Webb et al. demonstrated a similar increase in plasma nitrate approximately 60-min post inorganic nitrate ingestion to that observed herein. However, Webb et al. (2008) also reported that plasma nitrite did not significantly increase relative to pre-ingestion levels until approximately 3-h post-ingestion. In this study, no statistically significant change in plasma nitrite was observed through 65- to 75-min post-RSE ingestion. This may in fact be due to lag time in conversion of nitrate to nitrite and the ultimate appearance of nitrite in the circulation. However, a recent investigation using the same compound as that ingested herein demonstrated marked, significant increases in plasma (~80 %) and salivary (~700 %) nitrite at only 30-min post-consumption (Subramanian and Gupta 2016). While the dosage was twice that used in our investigation, the point remains that bioavailability of nitrite may be realized sooner than previously thought and/or dependent upon the source of inorganic nitrate. Notably, variability with serial measures (15-min intervals) of plasma nitrite in the aforementioned study was quite high with consecutive measures, at times alternating between significant elevations and no difference from baseline (Subramanian and Gupta 2016). Thus, in addition to the source of inorganic nitrate, measurement timing and methods (e.g., plasma vs. saliva) to detect plasma nitrite may have a profound effect on the results. Of critical importance, in this study we utilized a commercial fluorometric assay for plasma nitrite measures and it is likely that alternative measurement timing, assays, or sample sources (i.e., saliva) may have yielded different results. Indeed, salivary levels of nitrate or nitrite have been shown to change more dramatically upon ingestion of inorganic nitrate (Gilchrist et al. 2011; Lundberg and Govoni 2004).
Given the conceptual basis of ingesting inorganic nitrate via supplementation to enhance NO bioavailability and vascular reactivity, particularly with regards to tissues under physiologic hypoxia (Lundberg et al. 2008), the observed significant increase in resistance vascular reactivity in the calf following RSE supplementation seemed to confirm our hypothesis. Indeed, PkCBF, a measure of microvascular dilator function, was significantly increased acutely following RSE ingestion (Fig. 3). Peak RH limb blood flow measured via plethysmography is thought to be determined by both myogenic autoregulation and metabolic factors (e.g., vasodilatory prostaglandins) (Shepherd 2011), but not NO (Engelke et al. 1996; Joyner et al. 2001). Importantly, the role (or lack thereof) of NO in mediating the peak RH response is founded on investigations utilizing NOS inhibitors (e.g., L-NMMA) and the role of non-NOS sources of NO is, to our knowledge, unknown. For example, deoxygenated myoglobin, which is expressed in vascular smooth muscle, and erythrocyte xanthine oxidase activity, have been shown to reduce nitrite to NO and participate in hypoxia mediated vasodilation (Totzeck et al. 2012; Ghosh et al. 2013). Notably, PkCBF significantly decreased following PBO ingestion (Fig. 3) suggesting that RSE not only increased the PkCBF response, but also mitigated the decline observed with repeated plethysmography measures in the PBO condition. We have previously observed that with exposure to repeated bouts of RH in a short period of time that the resultant measures have been moderately decreased in sham/PBO conditions (Martin et al. 2015a). Indeed, a similar pattern to PkCBF responses was noted with TCBF responses herein as there was a significant decrease with the PBO condition, but not the RSE condition (Fig. 3). While the exact mechanism(s) by which interventions may mitigate these declines is unknown, we posit that nitrite and/or NO bioavailability are likely involved.
Due to the PkCBF responses observed with VOP, microvascular reactivity assessment via RH blood flow responses in the arm with ultrasonography (i.e., during FMD) were also of interest. In contrast to the PkCBF findings, we found that the PkFBF and TFBF response were significantly lower following both RSE and PBO ingestion. Specifically, PkFBF was decreased from baseline, on an average, by 17.1 % and TFBF was decreased from baseline, on an average, by 20.1 % (Fig. 4e, f). This response was similar to that observed with PkCBF and TCBF (−14.0 and −20.0 %, respectively) in the PBO condition. Again, based on previous findings (Martin et al. 2015a), as well as the PkCBF and TCBF response in the PBO condition, the decrease in the RH response was not necessarily surprising, but the differential response to RSE ingestion between limbs was. Given that the microvasculature is compressed (and presumed hypoxic) during occlusion with both the FMD and VOP methods, oxygenation status and/or occlusion cuff placement does not seem a likely explanation. However, regional heterogeneity in the microvascular dilator function response to RSE ingestion is plausible. For example, the regional differences in the relative proportion of type II muscle fibers may potentiate greater nitrate sensitivity due to, in part, lower resting PO2 concentrations improving the magnitude of ischemic activation of nitrate/nitrite conversion to NO (Ferguson et al. 2015; Jones et al. 2016).
Interestingly, and contrary to our hypothesis, there were also no significant changes in FMD conduit artery reactivity measures with RSE or PBO ingestion. We hypothesized that increased availability of nitrate/nitrite/NO with RSE ingestion would potentiate a greater muscular conduit artery (i.e., brachial artery) FMD response compared to baseline. Similar to our findings, Bahra et al. (2012) reported improved vascular compliance with inorganic nitrate ingestion compared to PBO without concomitant changes in FMD measures. Importantly, Bahra and colleagues collected FMD measures (with a distal occlusion cuff position) 3-h post-ingestion of inorganic nitrate while we conducted FMD testing ~45–65-min post-RSE ingestion. While the lack of a RSE-mediated change in brachial artery FMD was surprising to us, plausible explanations exist. For example, induction of RH is paramount to determining vascular reactivity, but differs slightly in how it is traditionally achieved between the VOP and FMD procedures. With FMD, the occlusion cuff is placed downstream from the site of conduit artery imaging (brachial artery herein). Thus, normoxia at the imaging site may have a substantial local effect on the ability to modulate circulating nitrate and nitrite, and ultimately, the FMD response. In addition, one may consider that the absolute volume of tissue in which hypoxia is induced, and acting to potentiate nitrate reduction, is higher with upstream occlusion (e.g., VOP measures) compared to downstream occlusion (e.g., FMD at the brachial artery). However, and importantly, the decrease from baseline in peak and total RH blood flow observed during FMD measurement was similar with both RSE and PBO ingestion suggesting that differential responses in microvascular dilation and/or nitrate conversion were not a primary factor in upstream brachial artery dilation. Future investigations comparing the FMD response of an upper limb (e.g., brachial artery) and lower limb (e.g., popliteal artery) conduit vessel with upstream and downstream occlusion following RSE ingestion are warranted.
Limitations to the present investigation include the timing of venipuncture(s) to elucidate changes in plasma nitrite, the lack of endothelium-independent measures of vascular reactivity, and the application of a single RSE dose for all participants. Indeed, serial plasma measurements would have allowed for a more specific time course of significant and peak increases in plasma nitrate and nitrite. Moreover, alternative methods for determining changes in nitrate/nitrite concentrations with exogenous sources may have been more sensitive (e.g., salivary samples, UPLC quantification methods). Though the differential VOP and FMD responses were surprising to us, this was not likely a product of order of measurements as it was randomized for each subject. However, the effect of occlusion cuff positioning with FMD and inorganic nitrate supplementation warrants further consideration. A 1000 mg RSE dose was utilized in this study and resulted in varying relative doses by bodyweight between participants. However, no significant differences in the responses by sex were observed. Thus, we do not believe that dose played an exceptional role in the observed significant results. Moreover, given the mixed sex population studied in the present investigation differences in vascular reactivity due to phases of the menstrual cycle in female participants cannot be excluded (Adkisson et al. 2010). Finally, the potential effects of other phytochemicals inherent to RSE cannot be excluded.
In conclusion, acute RSE (a form of inorganic nitrate ingestion) significantly increases plasma nitrate 65- to 75-min following ingestion, but not plasma nitrite using the detection methods employed herein. In addition, acute RSE ingestion increased RH PkCBF, but not RH PkFBF or TFBF or brachial artery FMD. Thus, there appears to be regional heterogeneity in the microvascular dilation response to RSE ingestion. Moreover, occlusion cuff positioning and the hypoxic state (or lack thereof) at the site of the imaging for conduit artery reactivity measures (i.e., FMD) may play a role in the results observed herein. Future studies should further characterize the regional specificity and role of local hypoxia in the macro and microvascular responses to RSE ingestion.
Abbreviations
- ΔTr:
-
Systolic duration of the reflected pressure wave
- AgBP:
-
Augmented central pressure
- AgBP@75:
-
Augmented central pressure normalized to a heart rate of 75 bpm
- AIx:
-
Aortic augmentation index
- AIx@75:
-
Aortic augmentation index normalized to a heart rate of 75 bpm
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CBF:
-
Calf blood flow
- PkCBF:
-
Peak CBF
- TCBF:
-
Total calf blood flow
- CDBP:
-
Central diastolic blood pressure
- CMAP:
-
Central mean arterial pressure
- CPP:
-
Central pulse pressure
- CSBP:
-
Central systolic blood pressure
- eNOS:
-
Endothelial nitric oxide synthase
- FBF:
-
Forearm blood flow
- PkFBF:
-
Peak FBF
- TFBF:
-
Total FBF
- FMD:
-
Flow-mediated dilation
- aFMD:
-
Absolute FMD
- %FMD:
-
Relative FMD
- nFMD:
-
FMD normalized to shear rate
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NO x :
-
Total nitrate and nitrite
- PBO:
-
Placebo
- PDBP:
-
Peripheral diastolic blood pressure
- PSBP:
-
Peripheral systolic blood pressure
- PWA:
-
Pulse wave analysis
- RH:
-
Reactive hyperemia
- RSE:
-
Red spinach extract
- T1r:
-
Time to reflection of the reflected pressure wave
- VOP:
-
Venous occlusion plethysmography
References
Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW (2010) Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 235(1):111–118. doi:10.1258/ebm.2009.009186
Bahra M, Kapil V, Pearl V, Ghosh S, Ahluwalia A (2012) Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide 26(4):197–202. doi:10.1016/j.niox.2012.01.004
Bazaral MG, Welch M, Golding LA, Badhwar K (1990) Comparison of brachial and radial arterial pressure monitoring in patients undergoing coronary artery bypass surgery. Anesthesiology 73(1):38–45
Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC (2003) Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol 549(Pt 2):597–605. doi:10.1113/jphysiol.2002.035915
Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H (1994) Stomach NO synthesis. Nature 368(6471):502. doi:10.1038/368502a0
Betik AC, Luckham VB, Hughson RL (2004) Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol 286(1):H442–H448. doi:10.1152/ajpheart.00314.2003
Cao Z, Bell JB, Mohanty JG, Nagababu E, Rifkind JM (2009) Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation. Am J Physiol Heart Circ Physiol 297(4):H1494–H1503. doi:10.1152/ajpheart.01233.2008
Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9(12):1498–1505. doi:10.1038/nm954
Dejam A, Hunter CJ, Schechter AN, Gladwin MT (2004) Emerging role of nitrite in human biology. Blood Cells Mol Dis 32(3):423–429. doi:10.1016/j.bcmd.2004.02.002
Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ (1996) Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol (1985) 81(4):1807–1814
Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch TI, Poole DC (2015) Microvascular oxygen pressures in muscles comprised of different fiber types: impact of dietary nitrate supplementation. Nitric Oxide 48:38–43. doi:10.1016/j.niox.2014.09.157
Gautier C, van Faassen E, Mikula I, Martasek P, Slama-Schwok A (2006) Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochem Biophys Res Commun 341(3):816–821. doi:10.1016/j.bbrc.2006.01.031
Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A (2013) Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension 61(5):1091–1102. doi:10.1161/HYPERTENSIONAHA.111.00933
Gilchrist M, Shore AC, Benjamin N (2011) Inorganic nitrate and nitrite and control of blood pressure. Cardiovasc Res 89(3):492–498. doi:10.1093/cvr/cvq309
Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ (1999) Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci 97(3):291–301
Harris RA, Nishiyama SK, Wray DW, Richardson RS (2010) Ultrasound assessment of flow-mediated dilation. Hypertension 55(5):1075–1085. doi:10.1161/HYPERTENSIONAHA.110.150821
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Kajiyama G, Oshima T (2001) A noninvasive measurement of reactive hyperemia that can be used to assess resistance artery endothelial function in humans. Am J Cardiol 87(1):121–125, A129
Hobbs DA, George TW, Lovegrove JA (2013) The effects of dietary nitrate on blood pressure and endothelial function: a review of human intervention studies. Nutr Res Rev 26(2):210–222. doi:10.1017/S0954422413000188
Hokanson DE, Sumner DS, Strandness DE Jr (1975) An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng 22(1):25–29
Imholz BP, Wieling W, van Montfrans GA, Wesseling KH (1998) Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 38(3):605–616
Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH (2001) A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87(2):212–222
Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO (2008) A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol 4(7):411–417. doi:10.1038/nchembio.92
Jellema WT, Wesseling KH, Groeneveld AB, Stoutenbeek CP, Thijs LG, van Lieshout JJ (1999) Continuous cardiac output in septic shock by simulating a model of the aortic input impedance: a comparison with bolus injection thermodilution. Anesthesiology 90(5):1317–1328
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18(1):111–129
Jones AM, Ferguson SK, Bailey SJ, Vanhatalo A, Poole DC (2016) Fiber type-specific effects of dietary nitrate. Exerc Sport Sci Rev 44(2):53–60. doi:10.1249/JES.0000000000000074
Joyner MJ, Dietz NM, Shepherd JT (2001) From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol (1985) 91(6):2431–2441
Lal SK, Henderson RJ, Cejnar M, Hart MG, Hunyor SN (1995) Physiological influences on continuous finger and simultaneous intra-arterial blood pressure. Hypertension 26(2):307–314
Lundberg JO, Govoni M (2004) Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37(3):395–400. doi:10.1016/j.freeradbiomed.2004.04.027
Lundberg JO, Weitzberg E, Lundberg JM, Alving K (1994) Intragastric nitric oxide production in humans: measurements in expelled air. Gut 35(11):1543–1546
Lundberg JO, Weitzberg E, Gladwin MT (2008) The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7(2):156–167. doi:10.1038/nrd2466
Mahieu D, Kips J, Rietzschel ER, De Buyzere ML, Verbeke F, Gillebert TC, De Backer GG, De Bacquer D, Verdonck P, Van Bortel LM, Segers P, Asklepios I (2010) Noninvasive assessment of central and peripheral arterial pressure (waveforms): implications of calibration methods. J Hypertens 28(2):300–305. doi:10.1097/HJH.0b013e3283340a1a
Martin JS, Beck DT, Gurovich AN, Braith RW (2010) The acute effects of smokeless tobacco on central aortic blood pressure and wave reflection characteristics. Exp Biol Med (Maywood) 235(10):1263–1268. doi:10.1258/ebm.2010.009376
Martin JS, Borges AR, Beck DT (2015a) Peripheral conduit and resistance artery function are improved following a single, 1-h bout of peristaltic pulse external pneumatic compression. Eur J Appl Physiol 115(9):2019–2029. doi:10.1007/s00421-015-3187-8
Martin JS, Borges AR, Christy JBT, Beck DT (2015b) Considerations for SphygmoCor radial artery pulse wave analysis: side selection and peripheral arterial blood pressure calibration. Hypertens Res 38(10):675–683. doi:10.1038/hr.2015.36
Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA (1996) Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol 270(4 Pt 2):H1435–H1440
Murgo JP, Westerhof N, Giolma JP, Altobelli SA (1981) Manipulation of ascending aortic pressure and flow wave reflections with the Valsalva maneuver: relationship to input impedance. Circulation 63(1):122–132
Patterson GC, Whelan RF (1955) The measurement of blood flow during reactive hyperaemia in man. J Physiol 127(1):13–14P
Pauca AL, O’Rourke MF, Kon ND (2001) Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 38(4):932–937
Penaz J, Voigt A, Teichmann W (1976) Contribution to the continuous indirect blood pressure measurement. Zeitschrift fur die gesamte innere Medizin und ihre Grenzgebiete 31(24):1030–1033
Petersson J, Carlstrom M, Schreiber O, Phillipson M, Christoffersson G, Jagare A, Roos S, Jansson EA, Persson AE, Lundberg JO, Holm L (2009) Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med 46(8):1068–1075. doi:10.1016/j.freeradbiomed.2009.01.011
Picone D, Climie R, Keske M, Sharman J (2014) Non-invasive estimation of exercise central blood pressure by radial tonometry may be underestimated due to brachial-to-radial-systolic-blood-pressure-amplification and is related to upper limb blood flow velocity. Artery Res 8(4):124–125. doi:10.1016/j.artres.2014.09.062
Schutte AE, Huisman HW, van Rooyen JM, Malan NT, Schutte R (2004) Validation of the Finometer device for measurement of blood pressure in black women. J Hum Hypertens 18(2):79–84. doi:10.1038/sj.jhh.1001639
Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH (2006) Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 47(6):1203–1208. doi:10.1161/01.HYP.0000223013.60612.72
Shepherd JT (2011) Circulation to skeletal muscle. In: Comprehensive physiology. Wiley, New York. doi:10.1002/cphy.cp020311
Shih YT, Cheng HM, Sung SH, Hu WC, Chen CH (2011) Quantification of the calibration error in the transfer function-derived central aortic blood pressures. Am J Hypertens 24(12):1312–1317. doi:10.1038/ajh.2011.146
Subramanian D, Gupta S (2016) Pharmacokinetic study of amaranth extract in healthy human subjects—a randomized trial. Nutrition. doi:10.1016/j.nut.2015.12.041
Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff H-J, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T (2012) Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation 126(3):325–334. doi:10.1161/circulationaha.111.087155
Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE (2007) Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci 64(1):96–103. doi:10.1007/s00018-006-6374-2
Verbeke F, Segers P, Heireman S, Vanholder R, Verdonck P, Van Bortel LM (2005) Noninvasive assessment of local pulse pressure: importance of brachial-to-radial pressure amplification. Hypertension 46(1):244–248. doi:10.1161/01.HYP.0000166723.07809.7e
Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A (2008) Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51(3):784–790. doi:10.1161/HYPERTENSIONAHA.107.103523
White WB, Berson AS, Robbins C, Jamieson MJ, Prisant LM, Roccella E, Sheps SG (1993) National standard for measurement of resting and ambulatory blood pressures with automated sphygmomanometers. Hypertension 21(4):504–509
Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ (1998) Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 16(12 Pt 2):2079–2084
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ (2000) The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 525(Pt 1):263–270
Zweier JL, Wang P, Samouilov A, Kuppusamy P (1995) Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1(8):804–809
Acknowledgments
This study was financially supported by DolCas Biotech LLC (Landing, NJ, USA) through a contract awarded to J.S.M. The authors wish to thank the participants for their time and compliance with demands associated with the study protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Communicated by David C. Poole.
Rights and permissions
About this article
Cite this article
Haun, C.T., Kephart, W.C., Holland, A.M. et al. Differential vascular reactivity responses acutely following ingestion of a nitrate rich red spinach extract. Eur J Appl Physiol 116, 2267–2279 (2016). https://doi.org/10.1007/s00421-016-3478-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3478-8