Abstract
Purpose
Sex hormones have profound effects on the nervous system in vitro and in vivo. The present study examines the effect of the menstrual cycle on maximal isometric force (MVC) and tremor during an endurance task.
Methods
Nine eumenorrheic females participated in five study visits across their menstrual cycle. In each menstrual phase, an MVC and an endurance task to failure were performed. Tremor across the endurance task was quantified as the coefficient of variation in force and was assessed in absolute time and relative percent time to task failure.
Results
MVC decreases 23 % from ovulation to the mid luteal phase of the menstrual cycle. In absolute time, the mid luteal phase has the highest initial tremor, though the early follicular phase has substantially higher tremor than other phases after 150 s of task performance. In relative time, the mid luteal phase has the highest level of tremor throughout the endurance task.
Conclusions
Both MVC and tremor during an endurance task are modified by the menstrual cycle. Performance of tasks and sports which require high force and steadiness to exhaustion may be decreased in the mid luteal phase compared to other menstrual phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult fertility and reproductive function in females is controlled by the hypothalamic–pituitary–gonadal axis, resulting in large and predictable oscillations of estradiol and progesterone (Fig. 1) across the menstrual cycle. Sex steroid hormonal oscillations at the plasma level affect the central nervous system since steroids can easily traverse the blood–brain barrier due to their high lipid solubility (Stoffel-Wagner 2001). Estradiol binds to estrogen receptor α (ERα) sites on γ-aminobutyric acid (GABA) releasing neurons resulting in decreased GABA transmission (Schultz et al. 2009) and enhanced cerebellar neuron discharge during rodent locomotion (Smith et al. 1989). Therefore, it is unsurprising that studies utilizing transcranial magnetic stimulation have found increased corticospinal tract excitability during the late follicular phase compared to the early follicular and mid luteal phases of the menstrual cycle (Smith et al. 1999, 2002). There is an initial depression in cortical excitability in the early follicular phase which may be due to high GABA concentrations at the cortex (Epperson et al. 2002; Harada et al. 2011). There is also lower corticospinal tract excitability at the mid luteal phase (Smith et al. 2002) which may be an effect of progesterone metabolite potentiation of GABAA receptors (Callachan et al. 1987).
While the aforementioned in vitro and transcranial magnetic stimulation studies indicate that estradiol and progesterone metabolites could have profound effects on motor activity, the effect of the menstrual cycle on voluntary force generation in vivo is equivocal. Sarwar et al. (1996) found that both quadriceps and handgrip strength were elevated around the ovulatory phase of the menstrual cycle; effects which were attributed to increased estradiol levels. Phillips et al. (1996) also reported increases in maximal voluntary contraction (MVC) in the follicular phases with a precipitous drop after ovulation. The decrease in MVC in the luteal phase may be due to the neuroinhibitory effects of progesterone on the motor cortex and our group has already demonstrated clear effects of the menstrual cycle phase on both the motor (Tenan et al. 2013) and autonomic nervous systems (Tenan et al. 2014). Conversely, many studies have failed to find any systematic change in muscular strength across the menstrual cycle (Dibrezzo et al. 1988; Lebrun et al. 1995; Birch and Reilly 1999; Janse de Jonge et al. 2001; Abt et al. 2007; Kubo et al. 2009; Montgomery and Shultz 2010). Additionally, Greeves et al. (1997) also failed to find any effect of supraphysiologic levels of estradiol on force production in women undergoing in vitro fertilization. The differential results may be due, in part, to diurnal variation in maximal force generation which is depressed in the morning of the luteal phase but elevated in the afternoon (Birch and Reilly 2002) or an over-reliance on discrete hormonal data that is sampled 2–3 times over the course of a study. The present study examines the holistic effects of the menstrual cycle on MVC by determining menstrual phase from daily monitoring of basal body temperature, a technique which has been previously shown to produce menstrual effects on MVC (Phillips et al. 1996).
Gross athletic performance and motor tasks, such as rowing, running and cycling, have been examined across the menstrual cycle. In a rowing study, Vaiksaar et al. (2011) showed no difference between the follicular and luteal phases for either maximal power or VO2max. On the contrary, Lebrun et al. (1995) suggest VO2max is elevated in the follicular phase of runners, and Jurkowski et al. (1981) showed that women have a greater time to exhaustion during cycling in the luteal phase. While gross athletic performance is important for a relatively small, specific segment of the population, the ability to maintain a given level of force output without substantial levels of tremor has applicability to athletes, soldiers and sedentary individuals hoping to perform activities of daily life. While a few studies have examined the sex differences in force steadiness (Yoon et al. 2014) and time to fatigue in controlled tasks (Fulco et al. 1999; Hunter et al. 2004), these aspects have not been assessed across the menstrual cycle. Given the variations in motor activity that are known to occur across the menstrual cycle (Hampson and Kimura 1988; Hampson 1990; Tenan et al. 2013), the ability to maintain force without undesired levels of tremor may be compromised.
Therefore, the purpose of the present study was to examine the effect of the menstrual cycle on MVC, time to fatigue and force tremor during a fatiguing contraction of the knee extensors. We hypothesized that a decrease in MVC would be observed after ovulation (Phillips et al. 1996), a result of previously reported decrease in corticospinal tract excitability (Smith et al. 2002), and that differential effects of time to fatigue and force tremor would also be observed around this time point.
Methods
Participants
Nine eumenorrheic females (24.7 ± 4.5 years) participated in five study visits. The self-reported activity levels of the participants were homogeneous and recreationally active. The study visits were performed in the morning, and time was standardized within each participant. All participants were free from neurologic, endocrine and metabolic disorders and had no history of previous leg surgery, immobilizations, arthritis or other chronic injury to the dominant leg. All participants had not used hormonal contraceptives for at least 6 months prior to testing and reported a history of clinically normal menstrual cycles. All participants gave their informed consent in accordance with the Helsinki Declaration and all experimental procedures were approved by the University of Texas at Austin Institutional Review Board.
Determination of menstrual cycle phase
Study visits were performed at five points in the menstrual cycle corresponding to distinct menstrual phases: early follicular, late follicular, ovulatory, mid luteal and late luteal. The first data collection for each subject was randomized, resulting in a pseudo-counterbalanced design. This design approach attempts to account for any effect of time or training effect on the overall study analyses. The ovulatory phase had one participant which started data collection in this phase and all other phases had two participants initiating data collection. Determining menstrual cycle phase via basal body temperature (BBT) in the present cohort has been previously described (Tenan et al. 2013, 2014).
Briefly, BBT was obtained via an oral thermometer (BD Basal, Franklin Lakes, NJ) for each participant 1 month prior to data collection. A biphasic BBT response is normal and ovulation is operationally defined as the nadir prior to the luteal phase temperature rise (de Mouzon et al. 1984). If the BBT was poorly defined, a second BBT was obtained from the following cycle before admission to data collection. If a clear biphasic response was not obtained after two consecutive BBTs, the participant did not enter data collection. The menstrual cycle was divided into two distinct major phases (follicular—pre-ovulation and luteal—post-ovulation) which were of variable length per subject [based upon a 3-day ovulation period; (Prior et al. 1990)]. The follicular and luteal phase were then each sub-divided into equally spaced phases of early follicular, late follicular, mid luteal and late luteal (Fig. 1 provides an example). The data collection points (n = 5) were equally spaced within the middle of each determined five phases we constructed within the menstrual cycle. The BBT was assessed and then confirmed independently by two trained investigators. One participant did not have their data analyzed during their last study visit in the mid luteal phase because they exhibited a short luteal defect; however, the data from that participant’s other trials were assessed because the late luteal trial was collected in the preceding cycle. A second participant was found to be anovulatory, defined by a lack of biphasic response in the BBT, in their last study visit during the ovulatory phase; therefore, that participant only had four study visits because their data collection started in the mid luteal phase.
Experimental protocol
Participants were instructed to not perform strenuous physical activity 48 h prior to testing and avoid alcohol and caffeine 8 h prior to testing, there were no additional dietary or exercise restrictions over the course of the study. No participants reported any subjective feelings of withdrawal from alcohol or caffeine over the course of the study. Participants were seated in an adjustable chair with the dominant hip and knee flexed at 90°. The waist and dominant thigh were immobilized with pads and straps. Participants then performed a light warm-up consisting of 12 dynamic submaximal knee extensions without resistance. After warm-up completion, the dominant ankle was strapped to a restraint with a strain gauge (Entran Sensors & Electronics, Fairfield, NJ) that was analog to digital converted (Micro 1401, Cambridge Electronic Design, Cambridge, England) at 1000 Hz. The participant was instructed to perform a MVC of the knee extensor muscles for 3 s. Auditory encouragement was given to the individual during the MVC, but they had no auditory or visual feedback on performance during the MVC. MVCs were performed until 3 MVCs of commensurate values were obtained. All MVCs were separated by at least 60 s of rest. The MVCs were highly reproducible with an intraclass correlation of 0.991 (95 % confidence interval: 0.986–0.995); therefore, the average of the three MVCs for that trial is reported and was used for calculations in the isometric knee extension endurance protocol.
During the endurance task, the participant was situated with a computer screen facing them with only their force generation provided as visual feedback. The participant was blinded to their absolute force level as well as the time performing the endurance task. They were instructed to trace a line on the screen that corresponded to 25 % of MVC. Force steadiness was practiced until participants were comfortable maintaining a constant force output. The participant then performed a slow ramp up to 25 % MVC and held that target force until instructed to terminate the trial by the investigator. The trial was terminated by the investigator when online force oscillations exceeded ±5 % MVC or the participant was unable to maintain target force for 3 s.
Force primary data reduction
Offline analysis of force data from the endurance task was performed in Matlab R2013a (Mathworks, Natick, MA, USA). Analysis of the endurance task was initiated 2 s after attaining target force. A 0.001 s non-overlapping sliding window algorithm determined when force was <80 % of target force and this time point was termed task failure. The offline algorithm renders a more conservative estimate of task failure than employed during online data collection. The use of the sliding window algorithm also limits investigator variability between trials and ensures data reproducibility. A second non-overlapping sliding window algorithm (window length: 0.5 s) determined force coefficient of variation across the endurance task.
Statistical analysis
All statistical analyses were performed in SAS 9.3 (SAS Institute, Cary, NC, USA) and graphics were created in R (R Core Team 2014) using the ggplot2 (Wickham 2009), dplyr (Wickham and Francois 2015) and car packages (Fox and Weisberg 2011). MVC and time to task failure were assessed via repeated-measures ANOVAs with post hoc comparisons adjusted via the Tukey–Kramer method to limit type 1 error inflation.
Force coefficient of variation data was found to be highly skewed, resulting in regressions violating normality of errors and constant variance. Force coefficient of variation was normal after a natural logarithm transform, resulting in valid regression analyses. Therefore, all regression analyses on coefficient of variation data were performed on transformed data; however, figures reflect model estimates and confidence bands that have been exponentiated back to original units to facilitate interpretation.
The force coefficient of variation was assessed in both absolute time (s) and in relative time (percent of time to task failure). Each of these were assessed with a multilevel regression where each menstrual phase was nested within the participant with an unstructured variance covariance structure and the repeated measures taken during the endurance task were assumed to have a first-order autoregressive variance covariance structure. The use of a multilevel model is generally a more conservative analytic method compared to an ANOVA as it accounts for variance at multiple levels that may adversely affect hypothesis testing the fixed effects of interest. Each regression was fit with fixed effects for menstrual phase, time (relative or absolute, depending on model), time self-interaction (i.e., quadratic effect for time) and the interaction of menstrual phase and time. Trial MVC was allowed to co-vary, allowing for extraction of time and menstrual phase effects on force variation apart from changes in MVC across the menstrual cycle.
Results
Maximal voluntary contraction and time to task failure
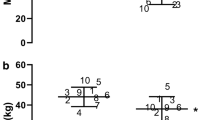
MVC changes across the menstrual cycle were significant (p = 0.004). The MVC in the mid luteal phase is significantly lower than late follicular (p = 0.011), ovulatory (p = 0.015), and late luteal phases (p = 0.004). There was a clear trend towards significance when comparing the mid luteal and early follicular phases (p = 0.061). The MVC model estimates from the repeated-measures ANOVA are in Fig. 2. There is, however, no effect of menstrual phase on time to task failure (p = 0.300; Fig. 3).
Force variability during an endurance task
Menstrual phase, time (both linear and quadratic terms) and the interaction of time and menstrual phase all significantly affect force coefficient of variation (p < 0.009) during an endurance task in absolute time. Visual inspection of model estimates and 95 % confidence intervals (Fig. 4) indicate that the mid luteal phase initially has higher force variation but that the early follicular phase has a substantially greater increase in force variability when the task exceeds 150 s.
Menstrual phase, the quadratic term for relative time and the interaction of menstrual phase and time significantly (p < 0.001) affect force coefficient of variation in relative time. Visual inspect of model estimates and 95 % confidence intervals (Fig. 5) demonstrate that the mid luteal phase has elevated force tremor compared to the other phases. After 25 % of time to fatigue, the late follicular and late luteal phases have the lowest level of force variation.
Discussion
The aim of this study was to examine the effect of menstrual cycle phase upon maximal voluntary force production, time to fatigue and force tremor during an endurance task. Our findings show clinically substantial decreases in maximal force generation of the knee extensors at the mid luteal phase. Tremor increases during an endurance task; however, the interpretation of how the menstrual cycle affects tremor depends on the method in which it is assessed.
Other studies have shown MVC changes across the menstrual cycle (Phillips et al. 1996; Sarwar et al. 1996), focusing on an apparent increase in MVC around ovulation. Sarwar et al. (1996) determined menstrual phase by retrospectively estimating back to the first day of bleeding and assuming that 14 days prior to menstruation was ovulation. While this strategy has an inherent level of variability in phase prediction, their data show a clear increase in MVC up to mid cycle, comparable to our ovulatory phase. They demonstrate a sharp decrease after mid cycle. This data agrees with our results which also demonstrate a sharp decrease at the mid luteal phase, resulting in forces statistically indistinguishable from early follicular. However, Sarwar et al. (1996) did report that MVC remains depressed at late luteal, whereas our data show a return to baseline. This different finding is possibly a result of timing in data collection within the late luteal phase. Our late luteal phase was collected within the last 3–4 days before menses onset, around the time of corpus luteum degeneration and progesterone fall (see Fig. 1). If progesterone is the MVC depressing factor and Sarwar’s data was collected before corpus luteum degeneration, it would explain why they report continued MVC force depression at the late luteal phase. Using methods similar to ours, Phillips et al. (1996) determined menstrual phase prospectively via basal body temperature mapping. In line with our data, they reported, a 7.6 % decrease in MVC around ovulation. While Philips et al. (1996) demonstrate MVC decreases after ovulation, they note that the exact timing of this MVC decrease post-ovulation is unclear. Our study shows a clear time course whereby MVC is lower when progesterone is at its theoretical peak, and MVC recovers as progesterone decreases in the late luteal phase (Bulun and Adashi 2011). In the present study, the observed decrease in MVC did not result in a significant change in time to fatigue, though a visual assessment of the data does suggest a small link between decreases in MVC and increase in time to fatigue when the endurance task’s force is relative to the MVC. This apparent link is consistent with the previously reported exponential relationship between MVC and time to fatigue (Hunter et al. 2004).
There is also a sizable body of literature which suggests that there is no change in force generation ability across the menstrual cycle (Dibrezzo et al. 1988; Lebrun et al. 1995; Birch and Reilly 1999; Janse de Jonge et al. 2001; Abt et al. 2007; Kubo et al. 2009; Montgomery and Shultz 2010). Such a great wealth of data opposing the findings of the present study cannot be ignored. Indeed, the diversity of results in response to a remarkably simple research question suggests that non-reproductive effects of sex hormones are poorly understood. The disagreement in the literature may be based on diurnal oscillations of hormones within the menstrual cycle (Birch and Reilly 2002), abnormal menstrual cycles assumed to present normally (Hoffman et al. 2008), the complicated interactions between hormones and their tissue receptors (Burris et al. 2013) or some other yet unrecognized variables. Studies using the BBT method appear to have a greater propensity for showing changes across the menstrual cycle (Lebrun et al. 1995; Phillips et al. 1996), which suggests a daily measurement that considers the menstrual cycle as a holistic process may be the best route of characterizing menstrual cycle effects.
The appropriate application of the tremor data in the present study depend on the given task. For tasks which are performed to task failure, such as a weightlifting, the relative time to fatigue is the most appropriate metric. In this instance, tremor is always highest in the mid luteal phase and lowest in the late luteal and late follicular phases. When considered in concert with the MVC data, performance in the mid luteal phase may be decreased for tasks which require both high levels of strength and steadiness. However, the majority of sport and occupational performance does not require constant force generation for greater than 100 s without rest. In these instances, absolute time is the appropriate way to view the present data. The absolute time data indicate that an elevated level of tremor occurs in the mid luteal phase within the first 100 s of force generation; whereas, all other phases have similar levels of tremor. The small difference in tremor between the phases should be negligible in all but the finest motor tasks such as handwriting, sewing, baiting a fishing hook, archery or sharpshooting. To the knowledge of the authors, none of these tasks have been assessed across the menstrual cycle. The tremor data in the present study should not be compared with males since previous studies have indicated females matched for MVC with males have a similar time to fatigue (Hunter et al. 2004) and that brain areas controlling tremor are not different between the sexes (Yoon et al. 2014).
The timing of both MVC and tremor changes, occurring in the mid luteal phase, suggest a mediating action of progesterone. A simple hypothesis whereby decreased corticospinal tract excitability (Herzog et al. 2001; Smith et al. 2002) due to the neuroinhibitory effects of progesterone’s first-order metabolite (Callachan et al. 1987), pregnanolone, could be proposed. This hypothesis would explain the decrease in MVC due to an inability to maximally discharge all lower motor neurons innervating the quadriceps. The decreased corticospinal tract excitability would also explain the increased tremor due to increased motor unit synchronization from greater levels of spatial summation versus temporal summation at the lower motor neuron. While this hypothesis “fits” with the current study, it over-extrapolates from the present findings and ignores existing literature which do not consistently show motor changes in the luteal phase (Dibrezzo et al. 1988; Lebrun et al. 1995; Birch and Reilly 1999; Janse de Jonge et al. 2001; Abt et al. 2007; Kubo et al. 2009; Montgomery and Shultz 2010). Thus, there is a clear need for a cohesive program of research which examines voluntary and involuntary muscle activity and muscle mechanics during natural hormonal menstrual cycles (e.g., eumenorrheic menstrual cycle), naturally perturbed menstrual cycles (e.g., amenorrhea, oligomenorrhea, etc.) and chemically perturbed menstrual cycles (e.g., oral contraceptive, GnRH agonist with supplementation of sex hormones, etc.) in otherwise healthy women. Additional, subject-level variability may be accounted for by examining other aspects such as age, training status or tissue receptors for sex hormones and other genotypic information that is not evident from studies examining purely behavioral outcomes.
Conclusions
The summation of the present study suggests that, with the exception of the mid luteal phase, isometric muscular performance is stable across the menstrual cycle. Performance in the mid luteal phase may be subject to decreases in maximal force and increased force tremor. However, this is the first study to examine tremor across the menstrual cycle and there is considerable dissent within the scientific community regarding changes in maximal force. Future research should focus on why seemingly well-controlled studies examining non-reproductive effects of the menstrual cycle can have substantially differing results. Novel biomarkers such as sex hormone binding globulin or sex hormone metabolites should be investigated; it may be appropriate to consider menstrual cycle variations to be a product of more than simply changes in estradiol and progesterone.
Abbreviations
- ANOVA:
-
Analysis of variance
- BBT:
-
Basal body temperature
- ERα:
-
Estrogen receptor alpha
- GABA:
-
Γ-aminobutyric acid
- MVC:
-
Maximal voluntary contraction
References
Abt JP, Sell TC, Laudner KG, McCrory JL, Loucks TL, Berga SL, Lephart SM (2007) Neuromuscular and biomechanical characteristics do not vary across the menstrual cycle. Knee Surg Sports Traumatol Arthrosc 15(7):901–907
Birch K, Reilly T (1999) Manual handling performance: the effects of menstrual cycle phase. Ergonomics 42(10):1317–1332
Birch K, Reilly T (2002) The diurnal rhythm in isometric muscular performance differs with eumenorrheic menstrual cycle phase. Chronobiol Int 19(4):731–742
Bulun S, Adashi E (2011) The physiology and pathology of the female reproductive axis. In: Melmed S, Polonsky K, PR L, Kronenberg H (eds) Williams textbook of endocrinology, vol 12. Elsevier Saunders, Philadelphia
Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, Lundasen T, Hughes T, Kojetin DJ (2013) Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev 65(2):710–778
Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA (1987) Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci 231(1264):359–369
de Mouzon J, Testart J, Lefevre B, Pouly JL, Frydman R (1984) Time relationships between basal body temperature and ovulation or plasma progestins. Fertil Steril 41(2):254–259
Dibrezzo R, Fort IL, Brown B (1988) Dynamic strength and work variations during three stages of the menstrual cycle. J Orthop Sports Phys Ther 10(4):113–116
Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH (2002) Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 59(9):851–858
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks
Fulco C, Rock P, Muza S, Lammi E, Cymerman A, Butterfield G, Moore L, Braun B, Lewis S (1999) Slower fatigue and faster recovery of the adductor pollicis muscle in women matched for strength with men. Acta Physiol Scand 167(3):233–240
Greeves JP, Cable NT, Luckas MJ, Reilly T, Biljan MM (1997) Effects of acute changes in oestrogen on muscle function of the first dorsal interosseus muscle in humans. J Physiol 500(Pt 1):265–270
Hampson E (1990) Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 15(2):97–111
Hampson E, Kimura D (1988) Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci 102(3):456–459
Harada M, Kubo H, Nose A, Nishitani H, Matsuda T (2011) Measurement of variation in the human cerebral GABA level by in vivo MEGA-editing proton MR spectroscopy using a clinical 3 T instrument and its dependence on brain region and the female menstrual cycle. Hum Brain Mapp 32(5):828–833
Herzog AG, Friedman MN, Freund S, Pascual-Leone A (2001) Transcranial magnetic stimulation evidence of a potential role for progesterone in the modulation of premenstrual corticocortical inhibition in a woman with catamenial seizure exacerbation. Epilepsy Behav 2(4):367–369
Hoffman M, Harter RA, Hayes BT, Wojtys EM, Murtaugh P (2008) The interrelationships among sex hormone concentrations, motoneuron excitability, and anterior tibial displacement in women and men. J Athl Train 43(4):364–372
Hunter SK, Critchlow A, Shin I-S, Enoka RM (2004) Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol 96(1):195–202
Janse de Jonge XA, Boot CR, Thom JM, Ruell PA, Thompson MW (2001) The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol 530(Pt 1):161–166
Jurkowski J, Jones NL, Toews CJ, Sutton JR (1981) Effects of menstrual cycle on blood lactate, O2 delivery, and performance during exercise. J Appl Physiol 51(6):1493–1499
Kubo K, Miyamoto M, Tanaka S, Maki A, Tsunoda N, Kanehisa H (2009) Muscle and tendon properties during menstrual cycle. Int J Sport Med 30(02):139–143
Lebrun C, McKenzie D, Prior J, Taunton J (1995) Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc 27(3):437–444
Montgomery MM, Shultz SJ (2010) Isometric knee-extension and knee-flexion torque production during early follicular and postovulatory phases in recreationally active women. J Athl Train 45(6):586–593
Phillips SK, Sanderson AG, Birch K, Bruce SA, Woledge RC (1996) Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J Physiol 496(Pt 2):551–557
Prior J, Vigna Y, Schulzer M, Hall J, Bonen A (1990) Determination of luteal phase length by quantitative basal temperature methods: validation against the midcycle LH peak. Clin Invest Med 13(3):123–131
Sarwar R, Niclos BB, Rutherford OM (1996) Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol 493(Pt 1):267–272
Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB (2009) Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci 29(6):1897–1903
Smith SS, Woodward DJ, Chapin JK (1989) Sex steroids modulate motor-correlated increases in cerebellar discharge. Brain Res 476(2):307–316
Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM (1999) Menstrual cycle effects on cortical excitability. Neurology 53(9):2069–2072
Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM (2002) Effects of ovarian hormones on human cortical excitability. Ann Neurol 51(5):599–603
Stoffel-Wagner B (2001) Neurosteroid metabolism in the human brain. Eur J Endocrinol 145(6):669–679
R Core Team (2014) R: a language and environment for statistical computing
Tenan MS, Peng Y-L, Hackney AC, Griffin L (2013) Menstrual cycle mediates vastus medialis and vastus medialis oblique muscle activity. Med Sci Sports Exerc 45(11):2151–2157
Tenan MS, Brothers RM, Tweedell AJ, Hackney AC, Griffin L (2014) Changes in resting heart rate variability across the menstrual cycle. Psychophysiol 51(10):996–1004
Vaiksaar S, Jürimäe J, Mäestu J, Purge P, Kalytka S, Shakhlina L, Jürimäe T (2011) No effect of menstrual cycle phase and oral contraceptive use on endurance performance in rowers. J Strength Cond Res 25(6):1571–1578
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Wickham H, Francois R (2015) dplyr: a grammar of data manipulation R package version 0.4.1
Yoon T, Noven MLV, Nielson KA, Hunter SK (2014) Brain areas associated with force steadiness and intensity during isometric ankle dorsiflexion in men and women. Exp Brain Res 232:3133–3145
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report. All research involving humans was supervised by the local ethics board and included informed consent.
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Tenan, M.S., Hackney, A.C. & Griffin, L. Maximal force and tremor changes across the menstrual cycle. Eur J Appl Physiol 116, 153–160 (2016). https://doi.org/10.1007/s00421-015-3258-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3258-x