Abstract
Purpose
Near-infrared spectroscopy (NIRS) is widely used to investigate cerebral oxygenation and/or neural activation during physiological conditions such as exercise. However, NIRS-determined cerebral oxygenated hemoglobin (O2Hb) may not necessarily correspond to intracranial blood flow during dynamic exercise. To determine the selectivity of NIRS to assess cerebral oxygenation and neural activation during exercise, we examined the influence of changes in forehead skin blood flow (SkBFhead) on NIRS signals during dynamic exercise.
Methods
In ten healthy men (age: 20 ± 1 years), middle cerebral artery blood flow velocity (MCA V mean, via transcranial Doppler ultrasonography), SkBFhead (via laser Doppler flowmetry), and cerebral O2Hb (via NIRS) were continuously measured. Each subject performed 60 % maximum heart rate moderate-intensity steady-state cycling exercise. To manipulate SkBFhead, facial cooling using a mist of cold water (~4 °C) was applied for 3 min during steady-state cycling.
Results
MCA V mean significantly increased during exercise and remained unchanged with facial cooling. O2Hb and SkBFhead were also significantly increased during exercise; however, both of these signals were lowered with facial cooling and returned to pre-cooling values with the removal of facial cooling. The changes in O2Hb correlated significantly with the relative percent changes in SkBFhead in each individual (r = 0.71–0.99).
Conclusions
These findings suggest that during dynamic exercise NIRS-derived O2Hb signal can be influenced by thermoregulatory changes in SkBFhead and therefore, may not be completely reflective of cerebral oxygenation or neural activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Near-infrared spectroscopy (NIRS) technique is widely used to investigate cerebral oxygenation or cerebral neural activation (Ekkekakis 2009; Madsen and Secher 1999; Obrig and Villringer 2003). An advantage of NIRS, particularly during dynamic exercise, is that it can be used to measure cerebral oxygenation continuously with good temporal resolution and an acceptable signal-to-noise ratio (Ekkekakis 2009). In the research area of exercise physiology, previous studies (Bhambhani et al. 2007; Subudhi et al. 2009) have used NIRS technique and investigated the relation between cerebral neural activation and exercise performance. Several studies have reported alterations in cerebral oxygenation for meeting the metabolic demands of cerebral neuronal activation during dynamic exercise (Ide et al. 1999; Marshall et al. 2008; Rupp and Perrey 2008; Timinkul et al. 2008). In addition, some studies suggest that reductions in cerebral oxygenation may limit dynamic exercise performance (Bhambhani et al. 2007; Subudhi et al. 2009). However, recent studies (Marshall et al. 2008; Subudhi et al. 2009; Timinkul et al. 2008) have questioned the use of NIRS during exercise. Notably, during heavy exercise cerebral oxygenated hemoglobin (O2Hb) has been shown to increase (Marshall et al. 2008; Subudhi et al. 2009; Timinkul et al. 2008), despite a decrease in intracranial blood flow measured at middle cerebral and carotid artery (Hellstrom et al. 1996; Sato et al. 2011; Sato and Sadamoto 2010). Thus, although cerebral O2Hb has been accepted to be associated with changes in cerebral blood flow (Hoshi et al. 2001), this may not always be the case.
When placed on the forehead, NIR light emanating from the source has to pass through the extracranial tissue, i.e., scalp and skull, to reach the underlying cerebral tissue. Thus, the potential impact of these superficial tissues on NIR light absorption and scattering should be considered. Indeed, several recent studies suggest that the NIRS signals include extracranial contamination (Sorensen et al. 2012; Takahashi et al. 2011). Therefore, a possible explanation for the above mentioned dissociation between NIRS-derived O2Hb and intracranial blood flow during dynamic exercise is the increased forehead skin blood flow (SkBFhead) via exercise-evoked thermoregulatory vasodilatation (Kondo et al. 1998), which could contribute to increases in NIRS-determined O2Hb (Davis et al. 2006). However, to our knowledge, the potential contamination of forehead NIRS measurements by changes in forehead skin blood flow during exercise has not been studied.
Thus, in the present study, we examined the influence of changes in forehead skin blood flow on NIRS signals during dynamic exercise to determine the selectivity of NIRS to assess cerebral oxygenation and neural activation during exercise. To manipulate SkBFhead during steady-state dynamic exercise, we used a facial cooling stimulus, which induces local cutaneous vasoconstriction (Johnson and Kellogg 2010). We tested the hypothesis that thermoregulatory induced changes in skin blood flow in the forehead influence NIRS-derived O2Hb measurements during dynamic exercise.
Materials and methods
Subjects and ethical approval
Ten healthy men with a mean (±SD) age of 20 ± 1 years, height of 170 ± 5 cm, and body mass of 64 ± 9 kg voluntarily participated in this study. Each subject provided written, informed consent after all potential risks and procedures were explained. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the Institutional Review Board of Faculty of Science Engineering, Toyo University (IRB # 2010-R-07). None of the subjects were taking any medications that may have influenced the hemodynamic responses to exercise. All subjects were familiarized with the equipment and procedures before any experimental sessions.
Experimental measurements
Cardiorespiratory measurements
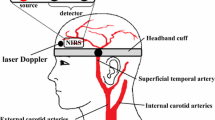
Heart rate (HR) was monitored using a lead II electrocardiogram (ECG). Beat-to-beat arterial blood pressure was measured using finger photoplethysmography (Finometer, Finapres Medical Systems BV, Netherlands). Relative changes in stroke volume (SV) and cardiac output (Q) were estimated using the Modelflow method (Beat Scope 1.1, Finapres Medical Systems BV). This method provides a reliable estimate of changes in SV and Q in healthy humans from rest to submaximal exercise (Sugawara et al. 2003). Expired air was sampled breath-by-breath and end-tidal partial pressure of carbon dioxide (CO2) (PETCO2) was measured with a gas analyzer system (AE-310S, Minato medical science co., Osaka, Japan). Skin blood flow at the right forehead (SkBFhead) was measured by laser Doppler flowmetry (ALF21, Advance, Japan) and the relative changes from the baseline are reported.
Near-infrared spectroscopy (NIRS)
Cerebral oxygenation was measured with a three-wave length (775, 810, 850 nm) high temporal resolution NIRS device (NIRO200, Hamamatsu Photonics, Hamamatsu, Japan), as previously described (Elwell et al. 1994; Ferrari et al. 2004). The probes were attached to the left forehead, approximately 3 cm from the midline and just above the supra-orbital ridge (Kleinschmidt et al. 1996; Obrig et al. 1996). The distance between probe and detector was set to 4 cm. The intensity of incident and transmitted light was recorded continuously at 2 Hz and, along with the specific extinction coefficients and optical pathlength, used for on-line estimation and display of concentration changes (∆μmol/L) in oxygenated (O2Hb), deoxygenated (HHb) and total (THb) hemoglobin according to the Modified-Beer-Lambert law. In addition, forehead tissue oxygenation index (TOI) was measured with the spatial resolved spectroscopy method. The value used for the pathlength factor was 5.92 (van der Zee et al. 1992). Because the laser Doppler flowmetry used a laser with a wavelength of 780 nm, which was close to the wavelength of the NIRS device, the laser Doppler probe (right forehead) was purposely placed with enough distance from the NIRS probe (left forehead) to avoid any potential interference (Takahashi et al. 2011).
Cerebral blood flow measurement
Mean blood flow velocity in the left middle cerebral artery (MCA V mean) was obtained by transcranial Doppler ultrasonography (Multidop T, DWL, Sipplingen, Germany) same as a recent study in our laboratory (Miyazawa et al. 2012). Briefly, a 2-MHz Doppler probe was placed over the left temporal window and fixed with an adjustable headband.
Experimental protocol
On the experimental day, all subjects arrived at the laboratory in the morning 2 h after a light breakfast. The subjects were requested to avoid caffeinated beverages, alcohol, and strenuous physical activity for at least 24 h before the experiment. After the subjects were instrumented, they were seated in a semi recumbent position with a backrest and rested quietly to allow for cardiovascular stability. After 3 min of baseline measurements, subjects performed 4 min of an incremental warm up from an initial work load of 60 W at a pedal rate of 60 revolutions/min. The subjects maintained the pedal rate, and workload was increased 10–30 W every minute until the intensity that corresponded to 60 % of maximum heart rate, calculated from 220 minus age of subject, was reached (approximately 120 bpm). The subject then conducted constant load cycling (129 ± 12 W) for 5 min, after which each subject underwent 3 min of facial cooling with continued cycling exercise. Facial cooling was administered by spraying a mist of cold water (~4 °C) and fanning with a fan placed 50 cm from the subject’s head and positioned facing upwards so as to cool only the head. After 3 min of facial cooling, subjects continued 3 min of exercise at the same workload. Subjects were instructed to breathe as normally as possible throughout the protocol.
Statistics
All signals were sampled continuously at 1 kHz using analog-to-digital converter (PowerLab, ADInstruments, Milford, MA) interfaced with a computer. Mean data for all variables are reported as 1 min averages. Statistical analysis was performed using SigmaStat 3.5 software (Systat Software Inc., Calif, USA). Following confirmation of distribution normality using Shapiro–Wilk W tests, data were analyzed using a one-way repeated measures analysis of variance (ANOVA) with post hoc Fisher’s least significant differences test. Correlation coefficients were obtained to determine the relation between changes in NIRS signals and SkBFhead using a Pearson product moment correlation using 1 min averaged data from baseline to 15 min of dynamic exercise. Data are expressed as mean ± SEM with significance for all two-tailed tests set at P < 0.05.
Results
Mean arterial pressure (MAP), HR, SV, and Q significantly (P < 0.05) increased during exercise (Fig. 1). In response to facial cooling during exercise, MAP and SV significantly (P < 0.05) increased, whereas HR and Q remained unchanged. PETCO2 and MCA V mean significantly (P < 0.05) increased during exercise and remained unchanged with facial cooling. On the other hand, SkBFhead and O2Hb significantly (P < 0.05) increased during exercise, and were significantly (P < 0.05) reduced temporally with facial cooling (Fig. 2). There were no significant difference (P = 0.18) between the percentage decrease in SkBFhead (−68 ± 17 %) and O2Hb (−52 ± 15 %) that occurred with facial cooling (percent changes from 9 to 12 min values). The changes in O2Hb correlated significantly with the relative percent changes in SkBFhead in each individual (r = 0.71–0.99, P < 0.01) (Table 1; Fig. 3). HHb and TOI did not change significantly with either exercise or facial cooling. Likewise, forehead TOI measured with spatially resolved spectroscopy method was also not affected by exercise or facial cooling. Correlation between SkBFhead and HHb and TOI had little correspondence among individuals. In contrast, changes in THb calculated by the summation of O2Hb and HHb were similar with changes in O2Hb and correlated significantly with SkBFhead in each individual (r = 0.70–0.99, P < 0.01).
Mean arterial pressure (MAP) and heart rate (HR) responses along with percent changes in stroke volume (SV) and cardiac output (Q), middle cerebral artery mean blood flow velocity (MCA V mean) and partial pressure of end-tidal carbon dioxide (PETCO2) during exercise and the application of facial cooling during the exercise bout. Values are mean ± SEM. *P < 0.05, different from the baseline value. # P < 0.05, different from the pre-cooling value (9 min)
Percent changes in forehead skin blood flow (SkBFhead) along with cerebral oxyhemoglobin (O2Hb), cerebral deoxyhemoglobin (HHb), cerebral total hemoglobin (THb) and cerebral tissue oxygenation index (TOI) responses during exercise and the application of facial cooling during the exercise bout. Values are mean ± SEM. *P < 0.05, different from the baseline value. # P < 0.05, different from the pre-cooling value (9 min)
Discussion
The salient findings of this study were that during steady-state exercise, the increased MCA V mean remained unchanged with facial cooling, whereas exercise-induced increases in O2Hb and SkBFhead were significantly lowered with facial cooling in equal proportion to each other. The changes in O2Hb correlated significantly and strongly with the relative percent changes in SkBFhead in each individual (r = 0.71–0.99). These findings suggest that during exercise NIRS signals (e.g., O2Hb) might be influenced by thermoregulatory changes in forehead skin blood flow. Therefore, careful consideration must be given to evaluation of cerebral oxygenation via NIRS measurements using the Modified-Beer-Lambert law especially in situations evoking substantial changes in cutaneous blood flow, such as dynamic exercise and heat/cold stress.
Because of methodological advantages including good temporal resolution and an acceptable signal-to-noise ratio (Ekkekakis 2009), the technique of NIRS had been applied to investigate cerebral oxygenation or cerebral neural activation during dynamic exercise (Ide et al. 1999; Marshall et al. 2008; Rupp and Perrey 2008; Timinkul et al. 2008). However, the reliability of NIRS signals during dynamic exercise has been questioned (Marshall et al. 2008; Subudhi et al. 2009; Timinkul et al. 2008). Recent studies suggest that NIRS on the forehead might be affected by changes in the skin blood flow during a verbal fluency task (Takahashi et al. 2011) and administration of norepinephrine (Sorensen et al. 2012). As NIRS-derived O2Hb in forearm muscle has been shown to be influenced by cutaneous vasodilatation during local and whole body heating (Davis et al. 2006), we reasoned that extracranial contamination on the NIRS signals might be occurring during exercise via exercise-evoked thermoregulatory vasodilatation and resultant increases in SkBFhead (Kondo et al. 1998). We applied facial cooling stimulation to manipulate SkBFhead during steady-state dynamic exercise, to determine if the SkBFhead influence NIRS signals during exercise. O2Hb increased with the exercise and was temporally decreased with facial cooling. Furthermore, the percentage decrease in O2Hb (−52 ± 15 %) that occurred with facial cooling were not significantly different from those of SkBFhead (−68 ± 17 %). In contrast, MCA V mean increased during the exercise and remained unchanged with facial cooling. These results demonstrate the potential impact of these superficial tissues on NIR light absorption and scattering in the forehead.
Cerebral blood flow increases during dynamic exercise in an exercise intensity-dependent manner up to moderate intensity (Sato et al. 2011; Subudhi et al. 2008), which is likely to be mediated mainly by elevations in cerebral neuronal activity and the partial pressure of arterial carbon dioxide (PaCO2) (Olin et al. 2011; Sato et al. 2010; Sato and Sadamoto 2010). At heavy exercise intensities, cerebral blood flow and the middle cerebral artery blood flow velocity decrease towards baseline values (Hellstrom et al. 1996; Sato et al. 2011; Sato and Sadamoto 2010) likely due to hyperventilation-induced decrease in partial pressure of arterial carbon dioxide (Nybo et al. 2002; Nybo and Nielsen 2001), high exercise-induced sympathetic nerve activation (Ogoh and Ainslie 2009), and/or a large increase in external carotid artery blood flow (Sato et al. 2011). On the other hand, inconsistent with response of MCA V mean, O2Hb increases during heavy exercise (Marshall et al. 2008; Subudhi et al. 2009; Timinkul et al. 2008). Together with our findings, it could be inferred that substantial changes in SkBFhead partly contributes to the increases in cerebral oxygenation during dynamic exercise potential minimizing its usefulness as an index of cerebral neuronal activation, particularly when exercise is performed in a heated environment.
To the best of our knowledge, this study is the first to attempt extracranial influences on NIRS during dynamic exercise. The previous works that has been done with such quantification were limited to rest including during invasive surgery (Al-Rawi et al. 2001; Davis et al. 2006; Germon et al. 1999; Kirkpatrick et al. 1995; Sorensen et al. 2012; Takahashi et al. 2011). Some studies have attempted to attenuate the influence of changes in extracranial tissue oxygenation on NIRS determination of cerebral O2Hb and HHb. Germon et al. (1999) have compared the effect of increasing the spacing between the transmitter and receiver optodes (range 0.7–5.5 cm) on the sensitivity of NIRS to detect changes in cerebral oxygenation, and found that increasing emitter-detector separation increases sensitivity to cerebral oxygenation while decreasing sensitivity to extracerebral oxygenation. Furthermore, Takahashi et al. (2011) have demonstrated that, with the suppression of the hemodynamic changes in the scalp layer by pressing the skin that covered a pair of optodes, there were no significant influences of skin blood flow changes on NIRS signals. Therefore, future studies using NIRS during dynamic exercise should consider varying the spacing between the transmitter and receiver optodes greater than 4 cm as used in this study. However, the influence of greater distances in optode placement on the sensitivity of NIRS to detect exercise-induced changes needs consideration.
In the present study, TOI was also determined using spatially resolved spectroscopy method, which is considered to quantify oxygen saturation by a subtraction algorithm that helps removes extracerebral sources of light absorption (Al-Rawi et al. 1999; Kirkpatrick 1997; Lam et al. 1997). Indeed, previous reports demonstrated that O2Hb determined by Modified-Beer-Lambert method significantly decreased with vascular clamps to external carotid artery, whereas TOI did not change during carotid endarterectomy (Al-Rawi et al. 2001). In our results, while SkBFhead was increased by exercise and decreased by facial cooling, spatially resolved TOI showed no changes during dynamic exercise and facial cooling (Fig. 2). This result, which was similar to the TOI measures with the Modified-Beer-Lambert law, suggested that spatially resolved spectroscopy method determined TOI was not influenced by SkBFhead. However, the fact remains that TOI did not change during dynamic exercise and therefore, was inconsistent with response of MCA V mean. In the present study, we were unable to obtain O2Hb with spatially resolved spectroscopy and therefore, additional studies using measurements of O2Hb with spatially resolved spectroscopy method during exercise are warranted for clarifying its sensitivity to detect exercise-induced changes in cerebral oxygenation in comparison to O2Hb measured with the Modified-Beer-Lambert law.
There are some methodological considerations in this study that warrant consideration. First, the sham-control condition (e.g., facial cooling during resting condition) was not conducted. Second, although the cerebral circulation is composed of the anterior and posterior circulations, we studied only anterior cerebral circulation. The impact of skin circulation on NIRS signal of posterior cerebral region should be addressed in a future study. Third, we used transcranial Doppler-derived cerebral artery mean velocity as a surrogate measure of cerebral blood flow. Although this assumption is justified as a measure of blood flow only if the vessel diameter remains unchanged (Serrador et al. 2000; Valdueza et al. 1997), less is known about the effect of acute exercise on cerebral artery diameter. However, a few studies suggest that changes in MCA V mean during submaximal dynamic exercise appear to reflect transient changes in cerebral blood flow determined by other exercise validated techniques [e.g., internal carotid artery blood flow (Hellstrom et al. 1996) and 133Xe clearance technique (Jorgensen et al. 1992a, b)]. Fourth, facial microcirculation, including forehead skin blood flow, is asymmetric; skin blood flow may be significantly higher on the right side of the forehead compared to the left (Benedicic et al. 2006). In the present study, because SkBFhead were measured on the right side in all subjects, the asymmetrical effect of SkBFhead was not statistically excluded. This procedure may explain some of the individual variance shown in our results. However, this was necessary to avoid contamination of the NIR signal with the laser Doppler since both devices use similar wavelengths. Furthermore, a significant correlation between changes in right and left side forehead skin blood flow has previously been reported (Benedicic et al. 2006). Therefore, we do not think this methodological constraint limits the interpretation of these data. Lastly, absolute values are difficult to obtain with NIRS (Ekkekakis 2009; Madsen and Secher 1999; Obrig and Villringer 2003) and not possible to obtain with laser Doppler flowmetry (Johnson et al. 1984). Thus, these devices have acknowledged limitation to provide quantitative assessment of cerebral oxygenation and SkBFhead.
Conclusions
In summary, we demonstrate that during exercise NIRS-derived O2Hb using the Modified-Beer-Lambert law can be influenced by thermoregulatory changes in forehead skin blood flow. Although NIRS is widely used to assess cerebral oxygenation in exercising subjects, our new data indicate that changes in SkBFhead may affect the NIRS signal independent of changes in cerebral oxygenation. Therefore, careful consideration must be given to interpreting NIRS measurements of cerebral oxygenation especially in situations in which substantial changes in cutaneous blood flow occur, such as dynamic exercise and heat/cold stress.
Abbreviations
- ECG:
-
Electrocardiogram
- HHb:
-
Deoxygenated hemoglobin
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- MCA V mean :
-
Mean blood flow velocity in the left middle cerebral artery
- NIRS:
-
Near-infrared spectroscopy
- O2Hb:
-
Oxygenated hemoglobin
- PETCO2 :
-
End-tidal partial pressure of carbon dioxide
- Q:
-
Cardiac output
- SkBFhead :
-
Forehead skin blood flow
- SV:
-
Stroke volume
- THb:
-
Total hemoglobin
- TOI:
-
Tissue oxygenation index
References
Al-Rawi PG, Smielewski P, Hobbiger H, Ghosh S, Kirkpatrick PJ (1999) Assessment of spatially resolved spectroscopy during cardiopulmonary bypass. J Biomed Opt 4:208–216
Al-Rawi PG, Smielewski P, Kirkpatrick PJ (2001) Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke 32:2492–2500
Benedicic M, Dolenc VV, Stefanovska A, Bosnjak R (2006) Left-right asymmetry of the facial microvascular control. Clin Auton Res 16:58–60
Bhambhani Y, Malik R, Mookerjee S (2007) Cerebral oxygenation declines at exercise intensities above the respiratory compensation threshold. Respir Physiol Neurobiol 156:196–202
Davis SL, Fadel PJ, Cui J, Thomas GD, Crandall CG (2006) Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J Appl Physiol 100:221–224
Ekkekakis P (2009) Illuminating the black box: investigating prefrontal cortical hemodynamics during exercise with near-infrared spectroscopy. J Sport Exerc Psychol 31:505–553
Elwell CE, Cope M, Edwards AD, Wyatt JS, Delpy DT, Reynolds EO (1994) Quantification of adult cerebral hemodynamics by near-infrared spectroscopy. J Appl Physiol 77:2753–2760
Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29:463–487
Germon TJ, Evans PD, Barnett NJ, Wall P, Manara AR, Nelson RJ (1999) Cerebral near infrared spectroscopy: emitter-detector separation must be increased. Br J Anaesth 82:831–837
Hellstrom G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T (1996) Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol 81:413–418
Hoshi Y, Kobayashi N, Tamura M (2001) Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol 90:1657–1662
Ide K, Horn A, Secher NH (1999) Cerebral metabolic response to submaximal exercise. J Appl Physiol 87:1604–1608
Johnson JM, Kellogg DL Jr (2010) Local thermal control of the human cutaneous circulation. J Appl Physiol 109:1229–1238
Johnson JM, Taylor WF, Shepherd AP, Park MK (1984) Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol 56:798–803
Jorgensen LG, Perko G, Secher NH (1992a) Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol 73:1825–1830
Jorgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH (1992b) Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol 72:1123–1132
Kirkpatrick PJ (1997) Use of near-infrared spectroscopy in the adult. Philos Trans R Soc Lond B Biol Sci 352:701–705
Kirkpatrick PJ, Smielewski P, Whitfield PC, Czosnyka M, Menon D, Pickard JD (1995) An observational study of near-infrared spectroscopy during carotid endarterectomy. J Neurosurg 82:756–763
Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J (1996) Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. J Cereb Blood Flow Metab 16:817–826
Kondo N, Takano S, Aoki K, Shibasaki M, Tominaga H, Inoue Y (1998) Regional differences in the effect of exercise intensity on thermoregulatory sweating and cutaneous vasodilation. Acta Physiol Scand 164:71–78
Lam JM, Smielewski P, al-Rawi P, Griffiths P, Pickard JD, Kirkpatrick PJ (1997) Internal and external carotid contributions to near-infrared spectroscopy during carotid endarterectomy. Stroke 28:906–911
Madsen PL, Secher NH (1999) Near-infrared oximetry of the brain. Prog Neurobiol 58:541–560
Marshall HC, Hamlin MJ, Hellemans J, Murrell C, Beattie N, Hellemans I, Perry T, Burns A, Ainslie PN (2008) Effects of intermittent hypoxia on SaO(2), cerebral and muscle oxygenation during maximal exercise in athletes with exercise-induced hypoxemia. Eur J Appl Physiol 104:383–393
Miyazawa T, Horiuchi M, Ichikawa D, Sato K, Tanaka N, Bailey DM, Ogoh S (2012) Kinetics of exercise-induced neural activation; interpretive dilemma of altered cerebral perfusion. Exp Physiol 97:219–227
Nybo L, Nielsen B (2001) Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol 534:279–286
Nybo L, Moller K, Volianitis S, Nielsen B, Secher NH (2002) Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol 93:58–64
Obrig H, Villringer A (2003) Beyond the visible–imaging the human brain with light. J Cereb Blood Flow Metab 23:1–18
Obrig H, Hirth C, Junge-Hulsing JG, Doge C, Wolf T, Dirnagl U, Villringer A (1996) Cerebral oxygenation changes in response to motor stimulation. J Appl Physiol 81:1174–1183
Ogoh S, Ainslie PN (2009) Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 107:1370–1380
Olin JT, Dimmen AC, Subudhi AW, Roach RC (2011) Cerebral blood flow and oxygenation at maximal exercise: the effect of clamping carbon dioxide. Respir Physiol Neurobiol 175:176–180
Rupp T, Perrey S (2008) Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur J Appl Physiol 102:153–163
Sato K, Sadamoto T (2010) Different blood flow responses to dynamic exercise between internal carotid and vertebral arteries in women. J Appl Physiol 109:864–869
Sato K, Hirasawa A, Tsunoda N, Taniguchi Y, Sadamoto T (2010) Cerebrovascular response during heavy upper body exercise: effect of mode of ventilation on blood flow velocity in the middle cerebral artery. Adv Exp Med Biol 662:347–352
Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T (2011) The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589:2847–2856
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL (2000) MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31:1672–1678
Sorensen H, Secher NH, Siebenmann C, Nielsen HB, Kohl-Bareis M, Lundby C, Rasmussen P (2012) Cutaneous vasoconstriction affects near-infrared spectroscopy determined cerebral oxygen saturation during administration of norepinephrine. Anesthesiology 117:263–270
Subudhi AW, Lorenz MC, Fulco CS, Roach RC (2008) Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circ Physiol 294:H164–H171
Subudhi AW, Miramon BR, Granger ME, Roach RC (2009) Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J Appl Physiol 106:1153–1158
Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M (2003) Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179:361–366
Takahashi T, Takikawa Y, Kawagoe R, Shibuya S, Iwano T, Kitazawa S (2011) Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. Neuroimage 57:991–1002
Timinkul A, Kato M, Omori T, Deocaris CC, Ito A, Kizuka T, Sakairi Y, Nishijima T, Asada T, Soya H (2008) Enhancing effect of cerebral blood volume by mild exercise in healthy young men: a near-infrared spectroscopy study. Neurosci Res 61:242–248
Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhaupl KM (1997) Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. AJNR Am J Neuroradiol 18:1929–1934
van der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, Wyatt JS, McCormick DC, Roth SC, Reynolds EO et al (1992) Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol 316:143–153
Acknowledgments
We appreciate the time and effort spent by our volunteer subjects. This study was supported by the Center for Academic Researches Promotion (Toyo University Research Institution of Industrial Technology, Grant #7).
Conflict of interest
None of the authors have any conflicts of interest associated with this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Narihiko Kondo.
Rights and permissions
About this article
Cite this article
Miyazawa, T., Horiuchi, M., Komine, H. et al. Skin blood flow influences cerebral oxygenation measured by near-infrared spectroscopy during dynamic exercise. Eur J Appl Physiol 113, 2841–2848 (2013). https://doi.org/10.1007/s00421-013-2723-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2723-7