Abstract
Purpose
We wanted to explore the specific proprioceptive effect of cervical pain on sensorimotor control. Sensorimotor control comprises proprioceptive feedback, central integration and subsequent muscular response. Pain might be one cause of previously reported disturbances in joint kinematics, head on trunk orientation and postural control. However, the causal relationship between the impact of cervical pain on proprioception and thus on sensorimotor control has to be established.
Methods
Eleven healthy subjects were examined in their ability to reproduce two different head on trunk targets, neutral head position (NHP) and 30° target position, with a 3D motion analyser before, directly after and 15 min after experimentally induced neck pain. Pain was induced by hypertonic saline infusion at C2/3 level in the splenius capitis muscle on one side (referred to as “injected side”).
Results
All subjects experienced temporary pain and the head repositioning error increased significantly during head repositioning to the 30° target to the injected side (p = 0.011). A post hoc analysis showed that pain interfered with proprioception to the injected side during acute pain (p < 0.001), but also when the pain had waned (p = 0.002). Accuracy decreased immediately after pain induction for the 30° target position to the side where pain was induced (3.3 → 5.3°, p = 0.033), but not to the contralateral side (4.9 → 4.1°, p = 0.657). There was no significant impact of pain on accuracy for NHP. A sensory mismatch appeared in some subjects, who experienced dizziness.
Conclusions
Acute cervical pain distorts sensorimotor control with side-specific changes, but also has more complex effects that appear when pain has waned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical pain is assumed to be associated with impaired sensorimotor control (Treleaven 2008). Although there are several reports on changes in muscle spindle sensitivity (Hellstrom et al. 2005; Masri et al. 2005; Thunberg et al. 2001) and muscle activation (Johansson and Sojka 1991; Passatore and Roatta 2006), the causal relationship between the sensory impact of cervical pain and altered sensorimotor control remains to be established.
Orientation and perception of head position in relation to trunk and in space is dependent on integration of several sensory pathways (Cui et al. 2009). Sensorimotor control depends on proprioception, central nervous processing and integration with vestibular and visual cues, weighted against volition and cognition in a continuous feedback with feedforward action and subsequent motor response (Falla et al. 2004a; Gurfinkel et al. 1988; Wolpert et al. 1995).
The multifold of proprioceptive input from joint capsules, ligaments and muscles in the upper cervical segments indicates the special importance of this body-region for sensorimotor control (Boyd-Clark et al. 2002; Holm et al. 2002; Richmond and Bakker 1982; Richmond et al. 1999; Voss 1971; Wyke 1979). Proprioception contributes with sufficient information for continuously adjusted fine-tuned, segmental spinal movements (Holm et al. 2002). Besides the importance on segmental movement control, cervical proprioception contributes to correct head in space and on trunk orientation (Armstrong et al. 2008), as well as to correct body orientation and balance control (Kavounoudias et al. 1999). Therefore, cervical proprioception is important to consider in analyses and treatment of patients with neck pain disorders. Knowledge about how much the pain per se and how much other consequences of a neck pain disorder impact on proprioception is, however, not sufficiently explored. The highly specific interaction between the cervical proprioception, vestibular and visual systems indicates the important collaboration between these systems (Mergner and Rosemeier 1998; Sugita et al. 2004), where adequate proprioception also contributes to dynamic stability of the spine (Panjabi 1992). If these sensory inputs do not concur, this might result in a sensory mismatch, leading to dizziness as a consequence (Reason 1978). Sensory mismatch based on presumed cervical origin is considered to be caused by impaired cervical proprioception and entitled cervicogenic dizziness (Brandt and Bronstein 2001). Several studies suggest this to be the case in at least some patients with neck pain and dizziness (Bracher et al. 2000; Heikkila et al. 2000; Karlberg et al. 1996a, b; Malmstrom et al. 2007; Reid et al. 2008; Treleaven et al. 2003; Wrisley et al. 2000). Impaired postural control as a presumed consequence of sensory mismatch is reported in patients with cervical pain (Falla and Farina 2008; Karlberg et al. 1995; Michaelson et al. 2003; Persson et al. 1996; Stapley et al. 2006; Treleaven 2008; Vuillerme and Pinsault 2009).
The impact of pain has experimentally been studied in several studies. Pain has been induced in the neck-shoulder region (Falla et al. 2007; Madeleine et al. 1999; Vuillerme and Pinsault 2009), in yaw (Capra and Ro 2000; Masri et al. 2005), in upper extremities (Ervilha et al. 2004b; Korotkov et al. 2002; Le Pera et al. 2001), in lumbar region (Hodges et al. 2003) and in lower extremities (Bennell et al. 2005; Farina et al. 2005; Matre et al. 2002). These studies have addressed changes in muscle activity and movement control (Ervilha et al. 2004b; Falla et al. 2007; Farina et al. 2005; Hodges et al. 2003; Madeleine et al. 1999), central nervous modulation (Capra and Ro 2000; Farina et al. 2005; Korotkov et al. 2002; Masri et al. 2005) and postural control (Vuillerme and Pinsault 2009). Two studies explored the effect of induced pain on repositioning ability for lower extremities (Bennell et al. 2005; Matre et al. 2002). They found no detectable changes after pain induction, and Matre and co-workers (2002) suggested this to be due to a robustness in proprioception in the lower extremities. To the best of our knowledge, this is the first study to explore the effect of induced cervical pain on repositioning ability.
It is difficult to quantify both pain and sensorimotor control. While pain may be described and graded subjectively, sensorimotor control is harder to fathom. Head repositioning tests have made indirect evaluation of proprioception possible (Lee et al. 2006; Loudon et al. 1997; Revel et al. 1994; Swait et al. 2007). However, results are diverging, while some studies have reported decreased sensorimotor control in subjects with cervical pain (Feipel et al. 2006; Loudon et al. 1997; Revel et al. 1991; Sterling et al. 2003b; Treleaven 2008; Treleaven et al. 2003), other studies have reported smaller repositioning errors compared to controls, in subclinical neck pain (Kristjansson et al. 2003; Lee et al. 2008). In addition, other studies report no measureable impact on cervical proprioception in conditions with cervical pain (Armstrong et al. 2005; Rix and Bagust 2001; Teng et al. 2007; Woodhouse and Vasseljen 2008).
Such conflicting results suggest both a multifaceted nature of cervical pain (Falla 2004) and possible diverse mechanisms to the variations of sensorimotor control. For example, muscle fatigue and/or muscle tension (Malmstrom et al. 2010; Pedersen et al. 1999) and central nervous modulations (Capra and Ro 2000; Farina et al. 2005; Madeleine et al. 1999) may alter sensorimotor control, the pain itself may interfere with muscle activity (Falla et al. 2007; Sterling et al. 2003b) or adaptation in movement strategy (Cote and Hoeger Bement 2010) and persistent muscle pain may cause morphological changes in muscle composition (O’Leary et al. 2009; Uhlig et al. 1995). Also, the cause of the pain might be considered to have an effect on sensorimotor control, as may the time course, i.e. if pain is acute, sub-acute or persistent. Thus, a multitude of causes, single or in concert may have impact on cervical proprioception and thus sensorimotor control.
The aim was to explore the specific effect of acute cervical pain on cervical proprioception. We also wanted to explore if possible effects were limited to the pain-induced side and if there were any after-effects. Pain can be introduced experimentally by an intramuscular injection of hypertonic saline (Ervilha et al. 2004b; Falla and Farina 2008; Madeleine et al. 1999). Saline acts on nociceptors without affecting electrophysiological properties of the muscle (Farina et al. 2005) and with no effects of the volume of the bolus per se (Falla et al. 2007), thus, resembling a condition with muscular pain without other specific impairments (Madeleine et al. 1998).
The hypothesis was that pain of cervical origin has a direct effect on cervical proprioception and that this impact is side-specific.
Methods
Subjects
We studied eleven healthy young subjects (19–33 years), six men and five women (Table 1). The subjects were recruited through advertisement and were compensated with approximately 85 € for participation. All subjects were informed that they could stop the test at any time and for any reason. Twelve subjects were recruited to the study. One woman did not accomplish the test, due to presyncope, blurred vision and dizziness in connection to the saline infusion. Twenty minutes after the infusion she reported no pain and no other symptoms. The study conforms to the standards set by Declaration of Helsinki, 2004 and was approved by Regional Ethics Review Board (411/2006), Lund University, Lund, Sweden.
Eligible subjects stated themselves as healthy and had no current neck pain and no constant or intermittent neck disability. The ‘neck-healthy’ statement was confirmed by a brief physical examination performed by a physiotherapist (EMM) (palpation of mm trapezius, levator scapulae, sternocleidomastoid, suboccipital muscles; screening by segmental motion of higher cervical levels and the cervico-thoracic junction) and by measuring cervical range of motion (CROM) in horizontal rotation with the Zebris® device (Table 1). The subjects performed four reciprocal maximal CROM and mean values were calculated. The subjects were all right-handed and also stated their physical activity level (Table 1).
Before the test procedure, the subjects were informed in writing and then they received uniform instructions verbally by the test leader, reading aloud from a manual.
After the short examination and CROM test, the subjects were introduced to the position tests during one first test, for familiarization, not used in analyses.
Experimentally induced muscle pain
A bolus of 0.5 ml 5 % preservative-free sterile hypertonic saline infusion (Natrii chloridum, 50 mg/ml, aqua ad iniectabilia) was injected in the paraspinal muscle at C2/3-level on the left side (referred to as “injected side”), most likely the splenius capitis muscle (Kamibayashi and Richmond 1998) during approximately 5 s (Fig. 1). Identification of the splenius muscle was made by palpation, first identifying the lateral rim of the trapezius muscle during arm abduction. Thereafter, during a head protrusion/forward movement, the splenius muscle was identified between the trapezius and sternocleidomastoid muscles, and the location for needle insertion was marked with a pencil on the overlying skin. The site was double-checked before injection, both by palpation by another tester but also by inserting a hypodermic needle (37 mm × 27G; Cardial Health; 5225 Verona Road; Building 2; Madison, WI 53711 USA) connected to an EMG-amplifier (Manufactured for Allergan, Inc.; 2525 Dupont Drive; Irvine; California 92715; USA), with continuous EMG-recording. When the typical EMG-recording from a muscle at rest (low frequency “bursts”) was obtained, the patient was again instructed to make a head protrusion. An increase in the frequency of the “EMG bursts” was regarded as an indication that the tip of the needle was inside the splenius muscle (Fig. 1). This needle was also used for the saline injection; needle depth was measured afterwards with a ruler (Table 1). Immediately after the saline infusion (in connection to position test ‘after I’), the subjects stated the pain intensity to be 53(23) mm [mean (SD)] on a visual analogue scale (VAS: 0 mm = no pain, 100 mm = maximal pain) (Bijur et al. 2001) (n = 11). They also reported the localization of experienced pain on a body chart (Fig. 2). All subjects reported pain localized to the site of injection. Eight of eleven subjects also reported referred pain (up/down from the site of injection/left forehead) (Fig. 2). Other sensations were also probed in an open question. Four subjects then reported either dizziness (1/11), unsteadiness (1/11), nausea (1/11) or presyncope (1/11).
In connection to performing the ‘after II’ test (15 min after the injection) they again reported pain intensity level (n = 9/11, for two subjects recordings were lost). The subjects then stated pain to be 4 (6) mm on a VAS.
After the test procedure, before leaving, no subject suffered from pain when we explicitly asked them and no other consequences were reported. A short-written home programme was introduced with movement exercises, for cervical mobility and muscle stretch.
Test of position sense
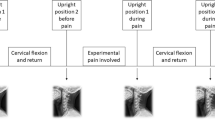
In order to test possible proprioceptive effects of cervical pain on sensorimotor control, the head repositioning ability was tested before (test before) and after (test after I, test after II) the pain induction, ‘pain state’. Repositioning ability was tested by letting the subjects reproduce two goals (‘target’ positions), 30° target in horizontal rotation and neutral head position (NHP) as accurate as possible (Lee et al. 2006; Loudon et al. 1997; Malmstrom et al. 2010; Revel et al. 1991). ‘Test after I’ was started immediately after the pain induction and ‘test after II’ was started 15 min after pain induction.
The head position tests were recorded by a 3D motion analyser Zebris® (Zebris®-CMSHS, with software WinSpine, version 1.78; Zebris Medizintechnik GmbH, Isny, Germany) (Dvir and Prushansky 2000; Lee et al. 2006) which consists of a helmet and a shoulder cap, each fitted with three ultrasound microphones. The helmet was attached on the subject’s head, and the shoulder cap was attached to the right shoulder. The ultrasound microphones on the helmet and shoulder cap received signals from three transmitters on a frame positioned approximately 1 m to the right of the subject. The sampling frequency was 50 Hz. The Zebris® measures distances to the microphones according to the principle of the timing of the intervals between the emission and the reception of ultrasound pulses. The absolute 3D coordinates are then calculated by triangulation.
The subjects were asked to sit on a stool with 10° slope in an upright position. Before the position test, the subjects made the four reciprocal, maximum cervical horizontal rotations to both sides to check for correct device application and to confirm that the CROM values were within normal limits (Table 1) (Malmstrom et al. 2003). Moreover, we wanted to ensure that the 30° target positions were far from end range, i.e. without confounding information from tight structures.
The position test started by determining NHP for each subject, by asking them to focus on a point at 2.5 m in front and leveled with the eyes. The subjects were encouraged to recall this position. Then the subjects were blind-folded and asked to close their eyes (Marx et al. 2003). Thereafter, they focused on keeping head in NHP, to which the Zebris® was calibrated (zero, 0°, in Zebris® registration). This position was regarded as reference NHP (refNHP) in all recordings in each trial.
Thereafter, the 30° target (ref30°) on one side and the NHP (refNHP) were introduced together. The side to start with was randomized and the procedure repeated for the other side. The test leader introduced the targets by moving the subjects head with the hands, guided by real time recording in Zebris®. The subjects were verbally informed when goal positions were attained and each position was held for a couple of seconds. The subjects were explicitly asked to remember the target positions. After the introduction the subjects reproduced the target positions six times at their own pace (30° target on one side and back to NHP in the same performance). The subjects signalled manually, by pressing a manometer connected to the Zebris®, when they considered themselves to be at the target positions. The entire procedure was repeated for each ‘Pain state’.
The subjects made one repositioning test, both sides and both target positions (30° target, NHP) before the test was started, for familiarization. This test was not used in analyses.
All analyses were done on the differences between the reproduced target positions in relation to the introduced positions, i.e. tar30°E= tar30° − ref30° and tarNHPE = tarNHP − refNHP, respectively.
Data processing and statistical analyses
Each subject was represented by their constant error (CE) of each trial set as a measurement of accuracy and directional bias and by their variable error (VE) as a measurement of trial-to-trial variability within six reproduced positions in each trial set (Lee et al. 2006). Constant error was the mean error of six signed differences in each trial set. We considered a value as overshoot (signed positive) when the reproduced position passed the introduced position and as undershoot (signed negative) when the reproduced position underestimated the introduced position and subjects stopped short of the target. Variable error was the standard deviation (SD) of six signed differences of each trial set. Non-parametric statistical tests were used for CE and VE as the Shapiro–Wilk test showed that some of the data sets were not normally distributed.
The following main factors and their interactions were investigated with a full factorial GLM univariate ANOVA (General Linear Model univariate Analysis of Variance) test on CE and VE including main factors:
‘Pain state’: before pain, immediately after pain induction or 15 min after pain induction, degrees of freedom (df) 2; ‘Side’: movement to the injected or non-injected side, side for the 30º target is denoted towards the side it is approached and NHP from the side it is approached, df 1; ‘Target’: target position of 30° or NHP, df 1.
The following main factors and their interactions were investigated with a full factorial GLM univariate ANOVA test on all head reposition assessments made (11 subjects assessed under three ‘Pain state’ conditions, two sides, two target positions (tar30°E and tarNHPE), and six repeated assessments at each trial condition = 792 values):
‘Pain state’: before pain, immediately after pain induction or 15 min after pain induction, df 2; ‘Side’: movement to the injected or non-injected side, side for the 30º target is denoted towards the side it is approached and NHP from the side it is approached, df 1; ‘Target’: target position of 30° or NHP, df 1; ‘Reposition‘: order of the six reproduced positions in each trial set, ranging from 1 to 6; df 5).
The Wilcoxon matched-pairs signed-rank test (two-tailed) was used for statistical evaluation of the CE and VE parameter differences between test conditions and for the statistical comparisons between test conditions in the GLM ANOVA post hoc tests.
In the analysis, p values <0.05 were considered statistically significant.
All statistical tests were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
Results
Head repositioning analysis of accuracy based on CE evaluation
Immediately after pain induction (‘after I’) constant error (CE) was increased for the 30° target position ipsilateral to the side where pain was induced compared with ‘before’ values (p = 0.033), but not for the contralateral side (p = 0.657). After 15 min (‘after II’) there was no significant effect compared with ‘before’ values, neither ipsi- nor contralateral to the side where pain was induced (p = 0.155 injected side, 0.424 non-injected side) (Table 4).
No significant effects were found for NHP, neither for ‘after I’, nor for ‘after II’ when these ‘Pain states’ were compared with ‘before’ (Table 4).
Univariate analysis of accuracy based on CE evaluation
Univariate analysis of CE showed significance for the ‘Target’ position factor, i.e. larger errors for 30° target position (4.4°) than for NHP (0.9°) (p < 0.001). No other main factors or interactions between main factors were significant.
Head repositioning analysis of variability based on VE evaluation
Variable error (VE) was not significantly affected by ‘Pain state’, neither for the 30° target position, nor for NHP (Table 4).
Univariate analysis of variability based on VE evaluation
Univariate analysis of VE showed significance for ‘Target’ position factor, i.e. larger errors for 30° target position (2.5°) than for NHP (1.9°) (p < 0.001). No other main factors or interactions between main factors were significant.
Univariate analyses including all factors defined
Univariate analysis revealed that the main factor ‘Repositioning’, representing the order of the different repositionings (1–6), had neither as a main factor alone (p = 0.955) nor in interaction with other factors any significant influence on the recorded head position. That means that repeated repositioning of the head to the same position under the same condition gave similar results (Table 2).
Other main factors that alone showed significant influence were ‘Side’ and ‘Target’ (Table 2). ‘Side’ (injected and non-injected) had significant (p = 0.015) influence on the accuracy to reproduce the head position. ‘Target’ for the head repositioning (tar30°E and tarNHPE) had significant (p < 0.001) influence on the accuracy to reproduce the head position. Univariate analysis revealed that the factors ‘Pain state’, ‘Side’ and ‘Target’ interacted significantly (Table 2). Therefore, a second set of GLM ANOVA analyses were performed on the individual data from the 30° and NHP target repositioning separately (Table 3).
Univariate analysis of 30° target data
The separate univariate analysis of the 30° target position (Table 3) demonstrated that tar30°E was significantly (p = 0.003) influenced by ‘Pain state’, with the lowest error found ‘before’ pain induction (mean 3.2°), with increased repositioning error immediately after pain induction, ‘after I’ (mean 4.7°) and further increased repositioning error 15 min after pain induction, ‘after II’ (mean 5.4°).
The ‘Side’ towards which repositioning were done, significantly (p = 0.013) influenced the recorded head repositioning error, with smaller error when repositioning the head to the side which was injected (mean 3.8°) than to the contralateral side (mean 5.1°).
However, the significant (p = 0.011) interaction between ‘Pain state’ and ‘Side’ demonstrated that the largest change in head repositioning error was introduced by the ‘Pain state’ change when repositioning the head to the injected side.
Post hoc analysis of ‘Pain state’ considering ‘Side’ factor for 30° target data
Injected and non-injected side combined
Post hoc analysis showed significant increases of tar30°E between ‘before’ and ‘after I’ (p = 0.005) and between ‘before’ and ‘after II’ (p < 0.001). For ‘Pain state’ comparison between ‘after I’ and ‘after II’ no significance was found (p = 0.176) (Fig. 3a).
a Post hoc analysis of the 30° target, n = 396/3 = 132 (green bars injected + non-injected side), n = 396/3/2 = 66 (red bars injected side and blue bars non-injected side). Mean, SEM and p values are reported. b Post hoc analysis of NHP, when returning to NHP from horizontal 30º rotation injected and non-injected side, n = 396/3 = 132 (green bars injected + non-injected side), returning to NHP from horizontal 30º rotation injected and non-injected side, n = 396/3/2 = 66 (red bars injected side and blue bars non-injected side). Mean, SEM and p values are reported
Injected side
Post hoc analysis showed significant increases of tar30°E between ‘before’ and ‘after I’ (p < 0.001) and between ‘before’ and ‘after II’ (p = 0.002). For ‘Pain state’ comparison between ‘after I’ and ‘after II’ no significance was found (p = 0.847) (Fig. 3a).
Non-injected side
Post hoc analysis showed no significance for tar30°E between ‘before’ and ‘after I’ (p = 0.594), between before and ‘after II’ (p = 0.098) but between ‘after I’ and ‘after II’ (p = 0.039) (Fig. 3a).
Univariate analysis of NHP data
Univariate analysis of the NHP (Table 3) showed that tarNHPE was not significantly influenced by ‘Pain state’, neither when the NHP position was approached from 30º horizontal rotation of the injected side, nor from non-injected side (‘Side’ effect). Moreover, the interaction between ‘Pain state’ and ‘Side’ was not significant (Table 3).
Post hoc analysis of ‘Pain state’ considering ‘Side’ factor for NHP data
Injected and non-injected side combined
Post hoc analysis showed no significance for injected and non-injected side combined in pair-wise comparisons (p = 0.864, 0.658 and 0.329) (Fig. 3b).
Injected side
Post hoc analysis showed no significance for injected side in pair-wise comparisons (p = 0.099, 0.146, 0.602) (Fig. 3b).
Non-injected side
Post hoc analysis showed no significance for non-injected side in pair-wise comparisons (p = 0.055, 0.296, 0.386) (Fig. 3b).
Discussion
Experimentally introduced unilateral cervical pain affects the ability to perform a head on trunk reposition test and this probably reflects a pain-associated distortion of cervical proprioception. The clinical implication of this finding is that cervical pain per se has a definite role in proprioception and thus sensorimotor control in the neck and subsequently possibly affects orientation.
The episode of pain changed cervical orientation ability in a complex way. The normal overshoot, seen before pain was inflicted, increased significantly for the 30° target position after saline injection towards the side, where pain was induced. Accuracy, in terms of increased CE (Constant Error), was significantly impaired for this target position (Table 4). Furthermore, the univariate analysis including all main factors suggested that pain interferes with proprioception both in acute pain, but also when the pain had subsided (Tables 2, 3; Fig. 3a).
NHP and 30° target position
The results advocate the two target positions to address different skills in head repositioning tests. The impact of pain was detected in the 30° target position, but not in NHP. Earlier results have reported the lack of correlation between repositioning ability for NHP and positioning during active cervical movement (Swait et al. 2007), suggesting different mechanisms in the different test situations. The 30° target position can be considered a more kinaesthetic test, putting higher demands on the proprioception from the muscles. Enhanced sensations by oscillations during a reposition test leading to decreased CE values have also put light on the importance of kinaesthetic information for the 30° target position (Malmstrom et al. 2009). Hence, providing increased proprioceptive information during the introduction towards a target might increase the sensitivity of the actual movement and positioning, and thus improves proprioception and thus sensorimotor control. Still, others have found NHP, but not the 30° target position, to detect differences between patients and controls (Kristjansson et al. 2003). We state from our results that distorted information from experimentally induced muscle pain does not seem to affect NHP. The higher accuracy and lower variability for the NHP under normal conditions suggest NHP to be less prone to be affected also in the acute ‘Pain state’. Neutral head position seems to be more robust, utilizing other inputs besides the kinaesthesia, in orientation of head on trunk as a reference midpoint (Gurfinkel et al. 1992).
Constant error, variable error and GLM ANOVA
Assessment of CE and VE has previously been established as means to evaluate cervical proprioception (Allison and Fukushima 2003; Lee et al. 2006; Swait et al. 2007). In the CE and VE calculations, where CE represents error with directional bias and accuracy while VE represents variability, each subject is represented by one CE and VE value for each side and target of every trial set.
The CE and VE values before and after pain induction demonstrated changes in proprioception immediately after pain induction, reflected by significant change in CE. The univariate analyses, using at most 792 recorded values, however, may suggest that pain interfered with sensorimotor control in a more complex way. Still, the univariate analyses corroborated the observed impact on sensorimotor control found in CE analysis. There were no significant effects of test order found in the univariate analyses, which also is in line with previous reports on the absence of learning effects, drop in attention and fatigue in similar tests (Rix and Bagust 2001). For a stable result, six repositionings in every trial set have earlier been advocated (Allison and Fukushima 2003; Swait et al. 2007).
Impact of pain
Impaired sensorimotor responses were demonstrated towards the painful side in the acute-state of pain, as well as impaired sensorimotor response in the post-‘Pain state’, although with less prominent side difference than during pain.
The change in CE indicates an induced proprioceptive asymmetry in the acute-state of pain for 30° target position. The significantly impaired accuracy immediately after pain induction could not be explained by bias, due to the lesser median of CE values to left versus right at baseline, i.e. 3.3° compared to 4.9 (Table 4), and post hoc analyses corroborated the results to be an impact of pain rather than caused by an intrinsic side difference (p value 0.213). Sterling and co-workers (2003a) suggested the side of pain to be a possible explanation for side-specific changes in a population of subjects with cervical symptoms after neck trauma. Furthermore, Falla and co-workers (2004b) reported superficial muscles on the same side as the cervical pain to be easier fatigued.
The changes in tar30° immediately after pain induction, but also in the post-‘Pain state’, raise the question if the pain affects locally or centrally. The results can possibly be attributed to local inhibition [less accuracy, i.e. increased CE in comparison before and ‘after I’, with retained variability, i.e. stable VE (Table 4)]. As seen in the latter stage, there is tendency of reduced variability, i.e. decreased VE between ‘after I’ and ‘after II’, injected side (p = 0.050; Table 4) with increasing changes of TARE (successive increases in the univariate analysis for tar30°E, injected and non-injected side combined, Fig. 2a). Earlier studies have reported movement control changes (Ervilha et al. 2004b; Falla et al. 2007; Farina et al. 2005; Hodges et al. 2003; Madeleine et al. 1999), as well as central modulation in experimental pain states (Capra and Ro 2000; Farina et al. 2005; Korotkov et al. 2002; Masri et al. 2005).
With the knowledge that there is a harmonized activation of different muscular layers (Blouin et al. 2007), one might consider other muscles to be affected. The moment arms of the ipsilateral splenius, rectus capitis major and obliques inferior muscles are suitable for rotation in the upper cervical region (Vasavada et al. 1998). The inhibition of the suboccipital agonist muscles in conditions with pain might be explained by sympathetic influence on type I fibres (Roatta and Farina 2011), the predominant muscle type in these muscles (Richmond et al. 1999), additionally abundantly provided with muscle spindles (Voss 1971), and even considered as proprioceptive monitors (McPartland and Brodeur 1999). The minor, deeper suboccipital muscles have a much larger density of muscle spindles than the larger, more superficial cervical muscles, i.e. the splenius muscle (Voss 1971). Still, even larger muscles have experimentally been proved to detect movements of fractions of a degree (Wise et al. 1999) and, therefore, we cannot deduce whether the results derive from the affected splenius alone, from overspill to other muscles or from central changes, or from a combination. However, we can conclude that pain induction causes changes in proprioception and thus impact sensorimotor control.
Neck pain and postural control
Postural control depends on visual, vestibular and proprioceptive information, modified in the central nervous system and executed by motor responses from selected muscles. There is well known interaction between cervical proprioception, vestibular and visual information, necessary for optimal orientation and postural control (Karlberg et al. 1996a, b; Mergner et al. 1993; Persson et al. 1996; Ruhe et al. 2011; Treleaven 2008; Vuillerme and Pinsault 2009). This interaction becomes even more evident when vestibular or visual information fails (Malmstrom et al. 2009; Maurer et al. 2000).
We found neck pain to be capable to impair proprioception in terms of head on trunk orientation. Neck muscle fatigue/tension has also been reported to alter proprioception as well as postural control (Malmstrom et al. 2010; Schieppati et al. 2003; Vuillerme and Pinsault 2009). Taken together, this implies that neck pain may be considered in postural complaints. If cervical proprioception is affected due to pain, this might cause sensory mismatch or disinformation that impairs postural control.
Cervicogenic dizziness
Cervical dizziness is a debated entity (Brandt 1996). The hypothesis on its aetiology considers a proprioceptive or sensorimotor misalignment with vestibular and visual cues (Brandt and Bronstein 2001), the pivotal factor being distorted cervical proprioceptive information (Brandt and Bronstein 2001; Lystad et al. 2011; Malmstrom et al. 2007; Reid and Rivett 2005; Wrisley et al. 2000). Here, pain induction caused a cervical proprioceptive disturbance. Four of the subjects also reported disturbed balance or dizziness. Thus, cervical pain induction led to disturbed proprioception, i.e. affecting orientation, and in 4/11 subjects a perception of dizziness. Therefore, it is feasible to assume that cervical pain may be a cause for both proprioceptive disturbances and perceived imbalance or dizziness, at least in some subjects. One may hypothesize that some people are more sensitive to disturbed proprioception, analogous to visual dependency (Isableu et al. 2003). As the demands on perception of motion are context-dependent (Mergner et al. 1993) and may change after lesions or during ageing (Di Fabio and Emasithi 1997; Isableu et al. 2003; Patel et al. 2010), sensitivity due to cervical disturbances might be different in different individuals. This assumption could explain why some people experience ‘cervicogenic dizziness’ in conditions with neck pain, while most do not.
Clinical consequences: the impact of pain
The main function of pain is to prevent tissue overload or damage. Here it would correspond to reduction of muscular activity in painful muscles (Falla et al. 2007; Farina et al. 2005; Farina et al. 2004; Le Pera et al. 2001; Thunberg et al. 2005) with a shifted activity from deeper painful to more superficial muscles (Falla and Farina 2008). One may hypothesize that in cervical pain such a shift may interfere with normal proprioception. Proprioception and subsequent motor control is important for joint stability (Panjabi 1992), but also for orientation and postural control during motion (Peterka 2002). The impaired ability to sense movements can be one possible explanation why pain maintains itself and becomes persistent. Interference of the motor planning due to impaired sensory inputs can lead to changed movement strategies which can lead to further impairment (Ervilha et al. 2004a). The actual findings, together with previous studies, advocate prevention of further development of a cervical pain condition, as well as support for reduction of cervical pain to be important for optimal muscular performance. If pain has developed, the causes should consequently be analysed and addressed in treatment. The findings do also support the importance for consideration of previously reported pain. Retraining of the sensorimotor function has consequently been suggested as treatment for patient with neck pain (Armstrong et al. 2008; Jull et al. 2007; Roijezon et al. 2008), alone or in combination with other sensory training to improve stability during motion (Treleaven 2008).
The present study demonstrates a prolonged effect of pain on proprioception even after the pain itself had waned. Therefore, cervical pain has to be taken seriously, to improve a disability both in acute and persistent conditions.
Conclusion
The results suggest that cervical pain distorts proprioception, and thus sensorimotor control in terms of accuracy of head on trunk orientation in the acute phase with side-specific changes, but also of a more complex and general nature remaining after the pain has waned.
These results advocate considerations of disturbed sensorimotor control of a complex nature in patients with cervical pain.
References
Allison GT, Fukushima S (2003) Estimating three-dimensional spinal repositioning error: the impact of range, posture, and number of trials. Spine 28:2510–2516
Armstrong, BS, McNair, PJ, Williams, M (2005) Head and neck position sense in whiplash patients and healthy individuals and the effect of the cranio-cervical flexion action. Clin Biomech (Bristol, Avon) 20: 67–684
Armstrong B, McNair P, Taylor D (2008) Head and neck position sense. Sports Med 38:101–117
Bennell K, Wee E, Crossley K, Stillman B, Hodges P (2005) Effects of experimentally-induced anterior knee pain on knee joint position sense in healthy individuals. J Orthop Res 23:46–53
Bijur PE, Silver W, Gallagher EJ (2001) Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 8:1153–1157
Blouin JS, Siegmund GP, Carpenter MG, Inglis JT (2007) Neural control of superficial and deep neck muscles in humans. J Neurophysiol 98:920–928
Boyd-Clark LC, Briggs CA, Galea MP (2002) Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine 27:694–701
Bracher ES, Almeida CI, Almeida RR, Duprat AC, Bracher CB (2000) A combined approach for the treatment of cervical vertigo. J Manipulative Physiol Ther 23:96–100
Brandt T (1996) Cervical vertigo–reality or fiction? Audiol Neurootol 1:187–196
Brandt T, Bronstein AM (2001) Cervical vertigo. J Neurol Neurosurg Psychiatry 71:8–12
Capra NF, Ro JY (2000) Experimental muscle pain produces central modulation of proprioceptive signals arising from jaw muscle spindles. Pain 86:151–162
Cote JN, Hoeger Bement MK (2010) Update on the relation between pain and movement: consequences for clinical practice. Clin J Pain 26:754–762
Cui QN, Razavi B, O’Neill WE, Paige GD (2009) Perception of auditory, visual, and egocentric spatial alignment adapts differently to changes in eye position. J Neurophysiol 103:1020–1035
Di Fabio RP, Emasithi A (1997) Aging and the mechanisms underlying head and postural control during voluntary motion. Phys Ther 77:458–475
Dvir Z, Prushansky T (2000) Reproducibility and instrument validity of a new ultrasonography-based system for measuring cervical spine kinematics. Clin Biomech (Bristol, Avon) 15:658–664
Ervilha UF, Arendt-Nielsen L, Duarte M, Graven-Nielsen T (2004a) Effect of load level and muscle pain intensity on the motor control of elbow-flexion movements. Eur J Appl Physiol 92:168–175
Ervilha UF, Arendt-Nielsen L, Duarte M, Graven-Nielsen T (2004b) The effect of muscle pain on elbow flexion and coactivation tasks. Exp Brain Res 156:174–182
Falla D (2004) Unravelling the complexity of muscle impairment in chronic neck pain. Man Ther 9:125–133
Falla D, Farina D (2008) Neuromuscular adaptation in experimental and clinical neck pain. J Electromyogr Kinesiol 18:255–261
Falla D, Jull G, Hodges PW (2004a) Feedforward activity of the cervical flexor muscles during voluntary arm movements is delayed in chronic neck pain. Exp Brain Res 157:43–48
Falla D, Jull G, Rainoldi A, Merletti R (2004b) Neck flexor muscle fatigue is side specific in patients with unilateral neck pain. Eur J Pain 8:71–77
Falla D, Farina D, Dahl MK, Graven-Nielsen T (2007) Muscle pain induces task-dependent changes in cervical agonist/antagonist activity. J Appl Physiol 102:601–609
Farina D, Arendt-Nielsen L, Merletti R, Graven-Nielsen T (2004) Effect of experimental muscle pain on motor unit firing rate and conduction velocity. J Neurophysiol 91:1250–1259
Farina D, Arendt-Nielsen L, Graven-Nielsen T (2005) Experimental muscle pain decreases voluntary EMG activity but does not affect the muscle potential evoked by transcutaneous electrical stimulation. Clin Neurophysiol 116:1558–1565
Feipel V, Salvia P, Klein H, Rooze M (2006) Head repositioning accuracy in patients with whiplash-associated disorders. Spine 31:E51–E58
Gurfinkel VS, Lipshits MI, Lestienne FG (1988) Anticipatory neck muscle activity associated with rapid arm movements. Neurosci Lett 94:104–108
Gurfinkel V, Lebedev M, Levick Y (1992) What about the so-called neck reflexes in humans? In: Berthoz A, Graf W, Vidal PP (eds.) The head-neck sensory motor system. Oxford University Press, New York, pp 543–547
Heikkila H, Johansson M, Wenngren BI (2000) Effects of acupuncture, cervical manipulation and NSAID therapy on dizziness and impaired head repositioning of suspected cervical origin: a pilot study. Man Ther 5:151–157
Hellstrom F, Roatta S, Thunberg J, Passatore M, Djupsjobacka M (2005) Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Exp Brain Res 165:328–342
Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC (2003) Experimental muscle pain changes feedforward postural responses of the trunk muscles [Clinical Trial Research Support, Non-US Government]. Exp Brain Res 151:262–271
Holm S, Indahl A, Solomonow M (2002) Sensorimotor control of the spine. J Electromyogr Kinesiol 12:219–234
Isableu B, Ohlmann T, Cremieux J, Amblard B (2003) Differential approach to strategies of segmental stabilisation in postural control. Exp Brain Res 150:208–221
Johansson H, Sojka P (1991) Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypotheses 35:196–203
Jull G, Falla D, Treleaven J, Hodges P, Vicenzino B (2007) Retraining cervical joint position sense: the effect of two exercise regimes. J Orthop Res 25:404–412
Kamibayashi LK, Richmond FJ (1998) Morphometry of human neck muscles. Spine 23:1314–1323
Karlberg M, Persson L, Magnusson M (1995) Impaired postural control in patients with cervico-brachial pain. Acta Otolaryngol Suppl 520(Pt 2):440–442
Karlberg M, Johansson R, Magnusson M, Fransson PA (1996a) Dizziness of suspected cervical origin distinguished by posturographic assessment of human postural dynamics. J Vestib Res 6:37–47
Karlberg M, Magnusson M, Malmstrom EM, Melander A, Moritz U (1996b) Postural and symptomatic improvement after physiotherapy in patients with dizziness of suspected cervical origin. Arch Phys Med Rehabil 77:874–882
Kavounoudias A, Gilhodes JC, Roll R, Roll JP (1999) From balance regulation to body orientation: two goals for muscle proprioceptive information processing? Exp Brain Res 124:80–88
Korotkov A, Ljubisavljevic M, Thunberg J, Kataeva G, Roudas M, Pakhomov S, Radovanovic S, Lyskov E, Medvedev S, Johansson H (2002) Changes in human regional cerebral blood flow following hypertonic saline induced experimental muscle pain: a positron emission tomography study. Neurosci Lett 335:119–123
Kristjansson E, Dall’Alba P, Jull G (2003) A study of five cervicocephalic relocation tests in three different subject groups. Clin Rehabil 17:768–774
Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L (2001) Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol 112:1633–1641
Lee HY, Teng CC, Chai HM, Wang SF (2006) Test-retest reliability of cervicocephalic kinesthetic sensibility in three cardinal planes. Man Ther 11:61–68
Lee HY, Wang JD, Yao G, Wang SF (2008) Association between cervicocephalic kinesthetic sensibility and frequency of subclinical neck pain. Man Ther 13:419–425
Loudon JK, Ruhl M, Field E (1997) Ability to reproduce head position after whiplash injury. Spine 22:865–868
Lystad RP, Bell G, Bonnevie-Svendsen M, Carter CV (2011) Manual therapy with and without vestibular rehabilitation for cervicogenic dizziness: a systematic review. Chiropr Man Therap 19:21
Madeleine P, Lundager B, Voigt M, Arendt-Nielsen L (1998) Sensory manifestations in experimental and work-related chronic neck-shoulder pain. Eur J Pain 2:251–260
Madeleine P, Lundager B, Voigt M, Arendt-Nielsen L (1999) Shoulder muscle co-ordination during chronic and acute experimental neck-shoulder pain. An occupational pain study. Eur J Appl Physiol Occup Physiol 79:127–140
Malmstrom EM, Karlberg M, Melander A, Magnusson M (2003) Zebris versus myrin: a comparative study between a three-dimensional ultrasound movement analysis and an inclinometer/compass method: intradevice reliability, concurrent validity, intertester comparison, intratester reliability, and intraindividual variability. Spine 28:E433–E440
Malmstrom EM, Karlberg M, Melander A, Magnusson M, Moritz U (2007) Cervicogenic dizziness—musculoskeletal findings before and after treatment and long-term outcome. Disabil Rehabil 29:1193–1205
Malmstrom EM, Karlberg M, Fransson PA, Lindbladh J, Magnusson M (2009) Cervical proprioception is sufficient for head orientation after bilateral vestibular loss. Eur J Appl Physiol 107:73–81
Malmstrom EM, Karlberg M, Holmstrom E, Fransson PA, Hansson GA, Magnusson M (2010) Influence of prolonged unilateral cervical muscle contraction on head repositioning–decreased overshoot after a 5-min static muscle contraction task. Man Ther 15:229–234
Marx E, Stephan T, Nolte A, Deutschlander A, Seelos KC, Dieterich M, Brandt T (2003) Eye closure in darkness animates sensory systems. Neuroimage 19:924–934
Masri R, Ro JY, Capra N (2005) The effect of experimental muscle pain on the amplitude and velocity sensitivity of jaw closing muscle spindle afferents. Brain Res 1050:138–147
Matre D, Arendt-Neilsen L, Knardahl S (2002) Effects of localization and intensity of experimental muscle pain on ankle joint proprioception. Eur J Pain 6:245–260
Maurer C, Mergner T, Bolha B, Hlavacka F (2000) Vestibular, visual, and somatosensory contributions to human control of upright stance. Neurosci Lett 281:99–102
McPartland J, Brodeur R (1999) Rectus capitis posterior minor: a small but important suboccipital muscle. J bodywork and movement ther 3:30–35
Mergner T, Rosemeier T (1998) Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions–a conceptual model. Brain Res Brain Res Rev 28:118–135
Mergner T, Hlavacka F, Schweigart G (1993) Interaction of vestibular and proprioceptive inputs. J Vestib Res 3:41–57
Michaelson P, Michaelson M, Jaric S, Latash ML, Sjolander P, Djupsjobacka M (2003) Vertical posture and head stability in patients with chronic neck pain. J Rehabil Med 35:229–235
O’Leary S, Falla D, Elliott JM, Jull G (2009) Muscle dysfunction in cervical spine pain: implications for assessment and management. J Orthop Sports Phys Ther 39:324–333
Panjabi MM (1992) The stabilizing system of the spine Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord 5:383–389; discussion 397
Passatore M, Roatta S (2006) Influence of sympathetic nervous system on sensorimotor function: whiplash associated disorders (WAD) as a model. Eur J Appl Physiol 98:423–449
Patel M, Fransson PA, Karlberg M, Malmstrom EM, Magnusson M (2010) Change of body movement coordination during cervical proprioceptive disturbances with increased age. Gerontology 56:284–290
Pedersen J, Lonn J, Hellstrom F, Djupsjobacka M, Johansson H (1999) Localized muscle fatigue decreases the acuity of the movement sense in the human shoulder. Med Sci Sports Exerc 31:1047–1052
Persson L, Karlberg M, Magnusson M (1996) Effects of different treatments on postural performance in patients with cervical root compression. A randomized prospective study assessing the importance of the neck in postural control. J Vestib Res 6:439–453
Peterka RJ (2002) Sensorimotor integration in human postural control. J Neurophysiol 88:1097–1118
Reason JT (1978) Motion sickness adaptation: a neural mismatch model. J R Soc Med 71:819–829
Reid SA, Rivett DA (2005) Manual therapy treatment of cervicogenic dizziness: a systematic review. Man Ther 10:4–13
Reid SA, Rivett DA, Katekar MG, Callister R (2008) Sustained natural apophyseal glides (SNAGs) are an effective treatment for cervicogenic dizziness. Man Ther 13:357–366
Revel M, Andre-Deshays C, Minguet M (1991) Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil 72:288–291
Revel M, Minguet M, Gregoy P, Vaillant J, Manuel JL (1994) Changes in cervicocephalic kinesthesia after a proprioceptive rehabilitation program in patients with neck pain: a randomized controlled study. Arch Phys Med Rehabil 75:895–899
Richmond FJ, Bakker DA (1982) Anatomical organization and sensory receptor content of soft tissues surrounding upper cervical vertebrae in the cat. J Neurophysiol 48:49–61
Richmond FJ, Singh K, Corneil BD (1999) Marked non-uniformity of fiber-type composition in the primate suboccipital muscle obliquus capitis inferior. Exp Brain Res 125:14–18
Rix GD, Bagust J (2001) Cervicocephalic kinesthetic sensibility in patients with chronic, nontraumatic cervical spine pain. Arch Phys Med Rehabil 82:911–919
Roatta S, Farina D (2011) Sympathetic activation by the cold pressor test does not increase the muscle force generation capacity. J Appl Physiol 110:1526–1533
Roijezon U, Bjorklund M, Bergenheim M, Djupsjobacka M (2008) A novel method for neck coordination exercise–a pilot study on persons with chronic non-specific neck pain. J Neuroeng Rehabil 5:36
Ruhe A, Fejer R, Walker B (2011) Altered postural sway in patients suffering from non-specific neck pain and whiplash associated disorder—a systematic review of the literature. Chiropr Man Therap 19:13
Schieppati M, Nardone A, Schmid M (2003) Neck muscle fatigue affects postural control in man. Neuroscience 121:277–285
Stapley PJ, Beretta MV, Dalla Toffola E, Schieppati M (2006) Neck muscle fatigue and postural control in patients with whiplash injury. Clin Neurophysiol 117:610–622
Sterling M, Jull G, Vicenzino B, Kenardy J (2003a) Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. [Research Support, Non-US Government]. Pain 104:509–517
Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R (2003b) Development of motor system dysfunction following whiplash injury [Comparative Study Research Support, Non-US Government]. Pain 103:65–73
Sugita A, Bai R, Imagawa M, Sato H, Sasaki M, Kitajima N, Koizuka I, Uchino Y (2004) Properties of horizontal semicircular canal nerve-activated vestibulospinal neurons in cats. Exp Brain Res 156:478–486
Swait G, Rushton AB, Miall RC, Newell D (2007) Evaluation of cervical proprioceptive function: optimizing protocols and comparison between tests in normal subjects. Spine 32:E692–E701
Teng CC, Chai H, Lai DM, Wang SF (2007) Cervicocephalic kinesthetic sensibility in young and middle-aged adults with or without a history of mild neck pain. Man Ther 12:22–28
Thunberg J, Hellstrom F, Sjolander P, Bergenheim M, Wenngren B, Johansson H (2001) Influences on the fusimotor-muscle spindle system from chemosensitive nerve endings in cervical facet joints in the cat: possible implications for whiplash induced disorders. Pain 91:15–22
Thunberg J, Lyskov E, Korotkov A, Ljubisavljevic M, Pakhomov S, Katayeva G, Radovanovic S, Medvedev S, Johansson H (2005) Brain processing of tonic muscle pain induced by infusion of hypertonic saline. Eur J Pain 9:185–194
Treleaven J (2008) Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther 13:2–11
Treleaven J, Jull G, Sterling M (2003) Dizziness and unsteadiness following whiplash injury: characteristic features and relationship with cervical joint position error. J Rehabil Med 35:36–43
Uhlig Y, Weber BR, Grob D, Muntener M (1995) Fiber composition and fiber transformations in neck muscles of patients with dysfunction of the cervical spine. J Orthop Res 13:240–249
Vasavada AN, Li S, Delp SL (1998) Influence of muscle morphometry and moment arms on the moment-generating capacity of human neck muscles. Spine 23:412–422
Voss H (1971) Tabulation of the absolute and relative muscular spindle numbers in human skeletal musculature. Anat Anz 129:562–572
Vuillerme N, Pinsault N (2009) Experimental neck muscle pain impairs standing balance in humans. Exp Brain Res 192:723–729
Wise AK, Gregory JE, Proske U (1999) The responses of muscle spindles to small, slow movements in passive muscle and during fusimotor activity. Brain Res 821:87–94
Wolpert DM, Ghahramani Z, Jordan MI (1995) An internal model for sensorimotor integration. Science 269:1880–1882
Woodhouse A, Vasseljen O (2008) Altered motor control patterns in whiplash and chronic neck pain. [Research Support, Non-US Government]. BMC Musculoskelet Disord 9:90
Wrisley DM, Sparto PJ, Whitney SL, Furman JM (2000) Cervicogenic dizziness: a review of diagnosis and treatment. J Orthop Sports Phys Ther 30:755–766
Wyke B (1979) Neurology of the cervical spinal joints. Physiotherapy 65:72–76
Acknowledgments
This research was supported by ALF, Department of Oto-Rhino-Laryngology, Lund University Hospital, Lund, Sweden; The Swedish Medical Research Council, Stockholm, Sweden; The Crafoord Foundation, Lund, Sweden and Region Skåne Council’s Research.
Conflict of interest
No benefits in any form have been received by any commercial party related to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fausto Baldissera.
Rights and permissions
About this article
Cite this article
Eva-Maj, M., Hans, W., Per-Anders, F. et al. Experimentally induced deep cervical muscle pain distorts head on trunk orientation. Eur J Appl Physiol 113, 2487–2499 (2013). https://doi.org/10.1007/s00421-013-2683-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2683-y