Abstract
Recent studies have suggested that dietary inorganic nitrate (NO3 −) supplementation may improve muscle efficiency and endurance exercise tolerance but possible effects during team sport-specific intense intermittent exercise have not been examined. We hypothesized that NO3 − supplementation would enhance high-intensity intermittent exercise performance. Fourteen male recreational team-sport players were assigned in a double-blind, randomized, crossover design to consume 490 mL of concentrated, nitrate-rich beetroot juice (BR) and nitrate-depleted placebo juice (PL) over ~30 h preceding the completion of a Yo–Yo intermittent recovery level 1 test (Yo–Yo IR1). Resting plasma nitrite concentration ([NO2 −]) was ~400 % greater in BR compared to PL. Plasma [NO2 −] declined by 20 % in PL (P < 0.05) and by 54 % in BR (P < 0.05) from pre-exercise to end-exercise. Performance in the Yo–Yo IR1 was 4.2 % greater (P < 0.05) with BR (1,704 ± 304 m) compared to PL (1,636 ± 288 m). Blood [lactate] was not different between BR and PL, but the mean blood [glucose] was lower (3.8 ± 0.8 vs. 4.2 ± 1.1 mM, P < 0.05) and the rise in plasma [K+] tended to be reduced in BR compared to PL (P = 0.08). These findings suggest that NO3 − supplementation may promote NO production via the nitrate-nitrite-NO pathway and enhance Yo–Yo IR1 test performance, perhaps by facilitating greater muscle glucose uptake or by better maintaining muscle excitability. Dietary NO3 − supplementation improves performance during intense intermittent exercise and may be a useful ergogenic aid for team sports players.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emerging research suggests that dietary inorganic nitrate (NO3 −) supplementation may improve muscle efficiency and fatigue resistance (Bailey et al. 2009, 2010; Hernández et al. 2012; Larsen et al. 2007, 2011). It has been shown that nitrate ingestion results in 15–25 % increase in time-to-exhaustion during severe-intensity, constant work-rate exercise (Bailey et al. 2009, 2010; Lansley et al. 2011b). In addition, some studies have reported a 1–2 % improvement in time-trial performance during high-intensity endurance exercise (Cermak et al. 2012a; Lansley et al. 2011a; Murphy et al. 2012), although these findings have not been confirmed in well-trained athletes (Bescós et al. 2012; Christensen et al. 2013; Wilkerson et al. 2012). Consequently, nitrate supplementation, in the form of nitrate-rich beetroot products, has rapidly gained popularity as an ergogenic aid for athletic performance.
Research to date has primarily focused on the use of nitrate to improve performance in continuous, endurance-type exercise ranging from ~6 min to ~2 h. This neglects team sport players or other athletes whose aim is to maximize performance in intermittent high-intensity exercise. The rapid, repeated transitions from a low to a high metabolic rate in team sport exercise place divergent demands on the energy transfer system compared to continuous endurance exercise (Bangsbo et al. 2007; Krustrup et al. 2003, 2006; Mohr et al. 2007). It is presently not known whether the performance-enhancing effects of dietary nitrate that have been observed during continuous performance tests may also be present during team sport-specific intense intermittent exercise.
Phosphocreatine and glycogen degradation is greater in type II than type I muscle fibres during maximal exercise (Greenhaff et al. 1994). Type II muscle fibres are heavily recruited during a transition from low to high metabolic rate (Krustrup et al. 2004, 2009). As this pattern is repeated during multiple sprint sports, the rate at which fatigue develops in type II fibres plays a key role in determining intermittent exercise performance (Colliander et al. 1988; Krustrup et al. 2003, 2006). High-intensity intermittent exercise also results in a high O2 demand relative to O2 delivery which may result in the development of muscle hypoxia, as well as low muscle pH and disturbances of ionic balance, factors which likely also contribute to the fatigue process (Krustrup et al. 2003; Mohr et al. 2004).
The physiological effects of nitrate supplementation are likely mediated by the reduction of inorganic NO3 − to nitrite (NO2 −) and nitric oxide (NO) (Lundberg and Weitzberg 2010; Lundberg et al. 2011). NO has numerous physiological signalling functions including the regulation of blood flow, muscle contractility, glucose and calcium homeostasis, and mitochondrial O2 consumption (Stamler and Meissner 2001), factors which may impact on muscle fatigue and performance (Bailey et al. 2011). The reduction of NO2 − to NO is facilitated when O2 availability is limited (Lundberg and Weitzberg 2010; Lundberg et al. 2011) and nitrate supplementation appears to be particularly effective in enhancing performance in hypoxia and ischaemia (Kenjale et al. 2011; Vanhatalo et al. 2011; Masschelein et al. 2012). Recent studies suggest that nitrate supplementation may specifically improve calcium handling and force production in type II muscle fibres (Hernández et al. 2012) and result in preferential distribution of blood flow to type II muscles (Ferguson et al. 2013). Given the importance of type II fibre recruitment and metabolism to performance in high-intensity intermittent exercise, and the likelihood that tissue hypoxia develops to a greater extent during such exercise compared to continuous low-intensity exercise, these recent studies therefore provide a clear rationale for nitrate supplementation to enhance performance during multiple sprint sports.
It is important that the influence of nitrate supplementation on intermittent exercise performance is examined using a valid and reliable test. The Yo–Yo Intermittent Recovery, level 1 (IR1) test has been specifically developed to mimic the high-intensity running bouts in football match-play (Bangsbo et al. 2008) and to assess an athlete’s fatigue resistance during intermittent exercise taxing both aerobic and anaerobic energy systems (Krustrup et al. 2003). This test has a high reproducibility and is sensitive to changes in fitness status and the competitive level of team sport players (Krustrup et al. 2003; Mohr et al. 2003; Ingebrigtsen et al. 2012) and to training interventions (Iaia et al. 2008; Mohr et al. 2007).
The purpose of this study was to assess the physiological and performance effects of dietary nitrate supplementation on submaximal and exhaustive intermittent exercise using a well-established and ecologically valid field performance test. We hypothesized that dietary nitrate supplementation would increase the distance covered in the Yo–Yo IR1 test.
Methods
Subjects
Fourteen male, recreational team-sport players (mean ± SD: age 22 ± 2 years, body mass 83 ± 10 kg, height 1.80 ± 0.10 m, \( \dot V{\text{O}}{}_{2\max } \): 52 ± 7 mL kg−1 min−1) familiar with intense intermittent exercise volunteered to participate in this study. Subjects gave their written informed consent to participate after the experimental procedures, associated risks, and potential benefits of participation had been explained in detail. The study was approved by the Institutional Research Ethics Committee and conformed to the code of ethics of the Declaration of Helsinki.
Experimental design
The subjects reported to the laboratory on three separate occasions over a 12-day period. On visit 1, subjects were familiarized to the Yo–Yo intermittent recovery level 1 test (Yo–Yo IR1; see Krustrup et al. 2003). Subjects were then assigned in a double-blind, randomized, crossover design to receive either NO3 −-rich beetroot juice (BR) or NO3 −-depleted BR (PL). The PL was created by passing standard beetroot juice through an ion-exchange resin specific for NO3 − (Lansley et al. 2011b). On each experimental visit (2 and 3), the first 5 min of the Yo–Yo IR1 test was first completed to familiarize the subjects with the experimental procedures (including blood drawing from an intravenous cannula) to be employed during the main test. This also served to assess the reproducibility of the physiological responses to repeated intermittent exercise. Then, after a 45 min passive rest period, the full Yo–Yo IR1 test was completed. Blood samples were obtained before, during and after the tests as previously described (Krustrup et al. 2003) and heart rate (HR) was recorded continuously throughout the experiment (Polar RS400, Polar Electro Oy, Kempele, Finland).
The experimental trials were carried out at the same time of the day (±1 h). Subjects were asked to record their food intake in the 24 h preceding the first experimental trial and to replicate this same diet in the 24 h preceding the subsequent trial. Subjects were instructed to arrive at the laboratory in a rested and fully hydrated state, ≥3 h post-prandial, and to avoid strenuous exercise in the 24 h preceding each experimental trial. Subjects were also asked to refrain from caffeine and alcohol in the preceding 24 h and to abstain from using antibacterial mouthwash and chewing gum throughout the study because this is known to prevent the reduction of nitrate to nitrite in the oral cavity (Govoni et al. 2008).
Experimental protocol
Upon arrival at the laboratory, a cannula (Insyte-W™, Becton–Dickinson, Madrid, Spain) was inserted into the subject’s antecubital vein to enable frequent blood sampling before, during and after the test. The Yo–Yo IR1 test was performed indoors on a wooden surface on running lanes with a width of 2 m and a length of 20 m. The Yo–Yo IR1 test has been described and evaluated previously (Bangsbo et al. 2008; Krustrup et al. 2003). In brief, it consists of repeated 20 m runs at a progressively increased speed controlled by audio bleeps from a CD player. Each running bout is interspersed by a 10-s active recovery period where the subject jogs around a marker placed 5 m behind the starting line (for details see Bangsbo and Mohr 2012). When a subject twice fails to reach the finishing line in time, the distance covered is recorded and this represents the test result.
Blood was sampled at rest (baseline) prior to both the submaximal and exhaustive Yo–Yo IR1 tests, as well as after 160, 440 and 600 m during the submaximal test, and at 160, 440, 600, 760, 920, 1,080, 1,240, 1,400 and 1,560 m during the exhaustive test. In addition, blood was collected at 1, 3, 5 and 45 min post the submaximal test, and at exhaustion and at 1, 3, 5, 10 and 15 min post the exhaustive test. Blood lactate and glucose concentrations were analyzed in all samples. Plasma [NO2 −] and [NO3 −] were analyzed in samples collected at baseline before both tests; at 600 m during and at 5 and 45 min post the submaximal test; and at 600 m, exhaustion, and at 15 min post the exhaustive test. Plasma [K+] and [Na+] were measured in a subsample of the subjects (n = 7). Plasma [K+] and [Na+] were analyzed in samples collected at baseline; at 600 m during and at 5 and 45 min post the submaximal test; and at 600 m, 1,080 m, exhaustion, and at 5 min post the exhaustive test.
Supplementation
After completion of the familiarization test, the subjects were assigned in a double-blind, randomized, crossover design to consume concentrated NO3 −-rich beetroot juice (BR; organic beetroot juice containing ~4.1 mmol of NO3 − per 70 mL; Beet it, James White Drinks Ltd., Ipswich, UK) and NO3 −-depleted beetroot juice (PL; organic beetroot juice containing ~0.04 mmol NO3 − per 70 mL; Beet it, James White Drinks Ltd., Ipswich, UK). On the day before each experimental trial, subjects were instructed to consume 2 × 70 mL of the beverage in the morning (~10 a.m.) and 2 × 70 mL in the evening (~7 p.m.). On each experimental day, subjects consumed a further 2 × 70 mL 2.5 h prior to and 1 × 70 mL 1.5 h prior to the start of the exercise protocol. A minimum of 72-h washout period separated the supplementation periods.
Blood analyses
Blood samples were drawn into 5-mL heparin syringes (Terumo Corporation, Leuven, Belgium). 200 μL of blood was immediately haemolyzed in 200 μL of ice-cold Triton X-100 buffer solution (Triton X-100, Amresco, Salon, OH) and analyzed to determine blood [lactate] and [glucose] within ~5 min of collection (YSI 2300, Yellow Springs Instruments, Yellow Springs, OH). The remaining whole blood was then centrifuged at 4,000 rpm for 3 min (Hettich EBA 20, Germany) before plasma was extracted and stored on ice for ~30 min prior to being frozen at −80 °C for later analysis of plasma [K+] and [Na+] (9180 Electrolyte Analyzer, F. Hoffmann-La Roche, Basel, Switzerland). Blood samples for the determination of plasma [NO2 −] and [NO3 −] were collected into lithium-heparin tubes (Vacutainer, Becton–Dickinson, New Jersey, USA) and centrifuged at 4,000 rpm and 4 °C for 5 min within 3 min of collection. Plasma was extracted and immediately frozen at −80 °C for later analysis of [NO2 −] and [NO3 −].
All glassware, utensils, and surfaces were rinsed with deionized water to remove residual NO prior to [NO2 −] and [NO3 −] analysis. The [NO2 −] of the undiluted (non-deproteinized) plasma was determined by its reduction to NO in the presence of glacial acetic acid and 4 % (w/v) aqueous NaI. The spectral emission of electronically excited nitrogen dioxide product, from the NO reaction with ozone, was detected by a thermoelectrically cooled, red-sensitive photomultiplier tube housed in a Sievers gas-phase chemiluminescence nitric oxide analyzer (Sievers NOA 280i. Analytix Ltd, Durham, UK). The [NO2 −] was determined by plotting signal (mV) area against a calibration plot of 100 nM to 1 μM sodium nitrite. Prior to determination of [NO3 −], samples were deproteinized using zinc sulphate/sodium hydroxide precipitation. 400 μL of 10 % (w/v) aqueous ZnSO4 and 400 μL of 0.5 M NaOH were added to 200 μL of sample and vortexed for 30 s before being left to stand at room temperature for 15 min. Thereafter, samples were centrifuged at 4,000 rpm for 5 min, and the supernatant was removed for subsequent analysis. The [NO3 −] of the deproteinized plasma sample was determined by its reduction to NO in the presence of 0.8 % (w/v) VCl3 in 1 M HCl. The production of NO was detected using the chemiluminescence nitric oxide analyzer, as described above. The [NO3 −] was determined by plotting signal (mV) area against a calibration plot of 500 nM to 15 nM sodium nitrate.
Statistical analyses
Differences between PL and BR in distance covered and HR during the Yo–Yo IR1 test were analyzed using a paired-samples t test. Differences between PL and BR in blood and plasma variables were analyzed using a two-way repeated measures ANOVA (supplement x time). Significant effects were further analyzed using simple contrasts with the α-level adjusted via Fisher’s LSD. Relationships between (changes in) plasma [NO2 −] and (changes in) performance were analyzed using Pearson product moment correlation coefficients. Statistical significance was accepted at P < 0.05. Results are presented as mean ± SD unless stated otherwise.
Results
There were no significant differences between the initial 600 m submaximal Yo–Yo IR1 test and the initial 600 m portion of the subsequent exhaustive Yo–Yo IR1 test for plasma [NO3 −], [NO2 −], [Na+] or [K+] or blood [glucose] or [lactate].
Plasma [NO2 −] and [NO3 −]
There were significant main effects by supplement and time, and an interaction effect on the plasma [NO2 −] (all P < 0.001) and [NO3 −] (all P < 0.05). At resting baseline the [NO2 −] was 118 ± 44 nM in PL and 584 ± 343 nM in BR (P < 0.05). Overall, [NO2 −] was greater in BR than PL at each measurement time point (Fig. 1), and was ~377 % greater, on average, across the entire protocol. At resting baseline the [NO3 −] was 25 ± 9 μM in PL and 768 ± 180 μM in BR (P < 0.05). [NO3 −] was greater in BR than PL at each measurement time point (Fig. 2) and was ~2,833 % greater, on average, across the entire protocol. During the submaximal test, the [NO2 −] and [NO3 −] were not significantly altered relative to baseline in either the PL or BR conditions. During the exhaustive test, however, the [NO2 −] declined by 20 ± 26 nM (~20 %) in PL (P < 0.05) and by 288 ± 221 nM (~54 %) in BR (P < 0.05) relative to the pre-exercise baseline (Fig. 1). Concurrently, [NO3 −] rose by 4.4 ± 3.8 μM (~17 %) in PL (P < 0.05) and by 71.8 ± 82.3 μM (~10 %) in BR (P < 0.05; Fig. 2). The reduction in [NO2 −] and the rise in [NO3 −] were significantly greater in BR than PL (both P < 0.05).
Plasma [NO2 −] did not change during the submaximal test in either condition (panels a and b), but decreased significantly during the exhaustive test in both BR (filled circle) and PL (open circle) (panels c and d) with the decline being greater in BR. Error bars indicate the SE. *P < 0.05 compared to PL; # P < 0.05 compared to baseline
Plasma [NO3 −] did not change during the submaximal test (panels a and b), but increased during the exhaustive Yo–Yo IR1 test in both BR (filled circle) and PL (open circle) (panels c and d) with the increase being greater in BR. Error bars indicate the SE. *P < 0.05 compared to PL; # P < 0.05 compared to baseline
Yo–Yo IR1 performance
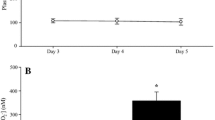
The distance covered in the Yo–Yo IR1 test was 4.2 % greater (P < 0.05) in BR (1,704 ± 304 m) compared to PL (1,636 ± 288 m) (Fig. 3). There were no differences between conditions in HR at the end of either the submaximal test (PL: 170 ± 7 vs. BR: 168 ± 4 b min−1; P > 0.05) or the exhaustive test (PL: 198 ± 5 vs. BR 194 ± 11 b min−1; P > 0.05). Following BR ingestion, the baseline plasma [NO2 −] tended to be correlated with Yo–Yo IR1 test performance (r = 0.48; P = 0.08) and the change in plasma [NO2 −] from baseline to end-exercise also tended to be correlated with Yo–Yo IR1 test performance (r = 0.46; P = 0.09).
Of the 14 subjects tested, ten improved their Yo–Yo IR1 performance (by between 40 and 280 m), one was unchanged, and three became slightly worse (by 40–80 m). In the three subjects whose Yo–Yo IR1 test performance became worse, baseline plasma [NO2 −] was substantially elevated by BR compared to PL (by 131, 205 and 668 %). However, in two of these subjects, the decline in plasma [NO2 −] from baseline to exhaustion was similar in BR and PL (difference of −30 and −12 nM) compared to a substantially greater fall of plasma [NO2 −] in BR compared to PL in those subjects whose performance improved (−31 ± 49 nM in PL vs. −388 ± 358 nM in BR, n = 10).
Blood [lactate] and [glucose]
Blood [lactate] increased significantly during both the submaximal and exhaustive Yo–Yo IR1 tests (P < 0.05) but there were no differences between supplements (Fig. 4a). There was a trend towards a significant interaction effect on blood [glucose] during the submaximal test (P = 0.06), suggesting that blood [glucose] decreased to a greater extent during the BR trial compared to PL (Fig. 4b). The follow-up tests indicated that blood [glucose] was lower in BR than PL after 600 m and at 1 min into recovery (P < 0.05 for both). The mean blood [glucose] during the submaximal test was greater in PL (4.1 ± 1.1 mM) compared to BR (3.7 ± 0.7 mM) (P < 0.01, paired samples t test including all measurements). Blood [glucose] declined with time during the exhaustive Yo–Yo IR1 test in both BR and PL condition (P < 0.05). Although there was no significant interaction or main effect by supplement during the exhaustive test, the mean blood [glucose] was significantly greater in PL (4.2 ± 1.1 mM) compared to BR (3.8 ± 0.8 mM) (P < 0.001, paired samples t test including all measurements) (Fig. 4b).
Blood [lactate] during the submaximal and exhaustive Yo–Yo IR1 test was not different between BR (filled circle) and PL (open circle) (panel a). There was a greater decline in blood [glucose] in BR than PL during the submaximal test and the mean blood [glucose] was lower in BR than PL during both the submaximal and the exhaustive Yo–Yo IR1 tests (panel B). Error bars indicate the SE. *P < 0.05 compared to PL; ¥ P < 0.05 compared to PL (mean of all samples)
Plasma [Na+] and [K+]
The plasma [Na+] was not significantly different between PL and BR during the submaximal (Fig. 5c) or exhaustive Yo–Yo IR1 tests (Fig. 5d). The plasma [K+] was not significantly different between BR and PL during the submaximal Yo–Yo IR1 test (P > 0.05; Fig. 5a). However, there was a trend for a significant interaction effect on plasma [K+] during the exhaustive Yo–Yo IR1 test (P = 0.08; Fig. 5b) suggesting that the rise in plasma [K+] was blunted with BR compared to PL. Post hoc analysis indicated that plasma [K+] was lower in BR than PL after 600 m (P < 0.05).
Plasma [K+] during the submaximal test was not different between BR (filled circle) and PL (open circle) (panel a; note that the data points for BR and PL at rest are superimposed), but there was a trend towards an attenuated rise in plasma [K+] in BR (filled circle) relative to PL (open circle) during the exhaustive Yo–Yo IR1 test (panel b; note that the data points for BR and PL at 5-min recovery are superimposed). Plasma [Na+] during the submaximal (panel c) and exhaustive (panel d) Yo–Yo IR1 test was not different between BR (filled circle) and PL (open circle). Error bars indicate the SE. *P < 0.05 compared to PL (n = 7)
Discussion
The principal original finding of this study was that dietary nitrate supplementation, in the form of beetroot juice, significantly improved intense intermittent exercise performance as measured by an increased distance covered in the Yo–Yo IR1 test. This finding is consistent with our experimental hypothesis and suggests that increasing dietary nitrate intake might be an effective nutritional strategy to improve performance in intermittent sports such as association football. Plasma [NO2 −] declined over the course of the Yo–Yo IR1 test in both the PL and BR trials; however, plasma [NO2 −] was greater with BR prior to the onset of the test and the magnitude of the decline in plasma [NO2 −] was greater in this condition. Concomitantly, plasma [NO3 −] increased during the Yo–Yo IR1 test in both PL and BR, but plasma [NO3 −] was greater with BR prior to the onset of the test and the magnitude of the increase in plasma [NO3 −] was greater with BR. Blood [lactate] and plasma [Na+] were not significantly impacted by dietary NO3 − supplementation across the Yo–Yo IR1, but there were trends for blood [glucose] and plasma [K+] to be lower with BR compared to PL.
Influence of dietary NO3 − supplementation on intermittent exercise performance
In recreationally active and trained but sub-elite subjects, short-term dietary nitrate supplementation has been shown to increase exercise tolerance at a fixed sub-maximal work rate (Bailey et al. 2009, 2010; Lansley et al. 2011b) and to increase power output during self-paced endurance exercise leading to improved cycle time-trial performance (Cermak et al. 2012a; Lansley et al. 2011a). It has recently been reported that dietary nitrate supplementation might also improve performance during repeated high-intensity intervals on a rowing ergometer (Bond et al. 2012). Specifically, these authors asked trained rowers to complete 6 × 500 m rowing ergometer repetitions (~90 s completion time per repetition) with 90-s recovery and observed a higher mean power output with nitrate supplementation. These results might have implications for improving performance in high-intensity interval training sessions. However, no study to date has investigated the influence of nitrate supplementation on team sport-specific, intense intermittent exercise performance.
To assess the potential ergogenic effects of dietary nitrate supplementation on intermittent exercise performance where the interval and recovery durations are characteristic of those encountered during team sports, we measured the distance covered during the Yo–Yo IR1 test after supplementation with BR and PL. Performance (distance covered) in the Yo–Yo IR1 test has been shown to closely correlate with high-intensity running during soccer games (Krustrup et al. 2003), which is a key determinant of soccer performance (Bradley et al. 2011; Mohr et al. 2003). Moreover, since performance in the Yo–Yo IR1 test has been shown to discriminate between players of different ability levels in different team sports (Atkins, 2006; Bangsbo et al. 2008; Veale et al. 2010; Vernillo et al. 2012), improved Yo–Yo IR1 test performance would be expected to contribute towards improved performance in invasion games. In this study, we observed a 4.2 % increase in the total distance covered in the Yo–Yo IR1 test with BR compared to PL. This suggests that dietary nitrate supplementation might enhance performance in intermittent team sports, at least in recreationally active subjects. Further research is required to establish whether nitrate supplementation can enhance intermittent exercise performance in highly trained team sport athletes. Nitrate supplementation has been reported be less effective in enhancing endurance exercise performance in highly trained endurance athletes in some (Bescós et al. 2012; Cermak et al. 2012b; Christensen et al. 2013; Peacock et al. 2012; Wilkerson et al. 2012) but not all (Bond et al. 2012; Cermak et al. 2012a) studies.
Although dietary nitrate supplementation produced a statistically significant improvement in Yo–Yo IR1 test performance that is likely to be practically meaningful during team sports which rely on intermittent high-intensity exercise (Bangsbo et al. 2008), the size of the improvement was less than has been reported following other interventions. Specifically, caffeine ingestion (6 mg/kg body weight) resulted in a 16 % mean improvement in performance in the Yo–Yo IR2 test (Mohr et al. 2011) whereas sprint and speed endurance training resulted in 10 and 29 % improvements in Yo–Yo IR2 performance, respectively (Mohr et al. 2007). There is evidence that the improvements in Yo–Yo test performance following these interventions are related, in part, to better control of muscle K+ homeostasis (Iaia et al. 2008; Mohr et al. 2007, 2011). In contrast, short-term dietary supplementation with l-arginine, a substrate for NO production via nitric oxide synthase, altered neither plasma [NO3 −] and [NO2 −] nor high-intensity intermittent exercise performance in judo players (Liu et al. 2009).
Influence of dietary NO3 − supplementation on NO metabolism
In this study dietary nitrate supplementation increased the resting baseline plasma [NO3 −] and [NO2 −] by ~3,000 and ~400 %, respectively. While several previous reports have shown that dietary nitrate supplementation can increase resting plasma [NO3 −] and/or [NO2 −] (Bailey et al. 2009, 2010; Bescós et al. 2012; Larsen et al. 2007, 2010; Lansley et al. 2011a; Vanhatalo et al. 2010a, 2011), the magnitude of the increase has typically been smaller than the values attained in the present study. Indeed, in previous studies, plasma [NO3 −] has been reported to increase by ~400–600 % (Bescós et al. 2012; Larsen et al. 2007, 2010) and plasma [NO2 −] by ~50–150 % (Bailey et al. 2009, 2010; Bescós et al. 2012; Larsen et al. 2007, 2010; Lansley et al. 2011a; Vanhatalo et al. 2010a) after dietary nitrate supplementation. The greater increases in plasma [NO3 −] and [NO2 −] in the present study is most likely a consequence of the higher nitrate dose (~29 mmol in ~36 h) administered compared to our previous studies using beetroot juice (~5–6 mmol/day; Bailey et al. 2009, 2010; Lansley et al. 2011b; Vanhatalo et al. 2010a).
It should be noted that while the daily nitrate dose used in this study was approximately four times greater than the recommended dietary allowance in the UK, it is similar to that used in the study of Kapil et al. (2010) who investigated the dose–response relationship between nitrate ingestion and the reduction of resting blood pressure. The nitrate supplementation regimen we used also resulted in a similar nitrate intake to the influential ‘Dietary Approaches to Stop Hypertension’ (DASH) diet (Hord et al. 2009) and traditional Mediterranean and Japanese diets (Sobko et al. 2010) which are promoted for their cardiovascular health benefits. We used a relatively high nitrate dose and selected the nitrate ingestion regimen in this study to ensure that plasma [NO2 −] remained elevated throughout the relatively lengthy testing protocol, which included an initial submaximal Yo–Yo IR1 test and a subsequent exhaustive Yo–Yo IR1 test. We have previously reported improvements in endurance exercise tolerance using a nitrate dose of ~5–6 mmol/day for 4–6 days (Bailey et al. 2009, 2010; Lansley et al. 2011b). It is unclear whether the smaller nitrate dose used in our previous studies might be ergogenic during intermittent exercise or whether the nitrate dose administered herein was optimal for enhancing intermittent exercise performance. Moreover, since we have shown that performance in certain exercise tests is improved with more chronic (15 days) nitrate supplementation (Vanhatalo et al. 2010a), further research is required to determine whether intermittent exercise performance can be increased to a greater extent, safely, with longer term nitrate supplementation.
It is now known that NO3 −, which was once considered to be an inert product of NO oxidation, can be reduced in vivo to bioactive NO2 − and further to NO and other reactive nitrogen species (Lundberg and Weitzberg 2010; Lundberg et al. 2011). The reduction of NO2 − to NO is potentiated by acidosis and hypoxia (Lundberg and Weitzberg 2010; Lundberg et al. 2011). NO2 − reduction to NO would be anticipated during the Yo–Yo IR1 test because the test results in blood (Rampinini et al. 2010) and muscle (Krustrup et al. 2003) acidosis, and it is known that high-intensity exercise reduces muscle PO2 (Richardson et al. 1999). It has been reported that exercise may both increase (Allen et al. 2010; Gladwin et al. 2000) and decrease (Cosby et al. 2003; Dreissigacker et al. 2010; Larsen et al. 2010) plasma [NO2 −]. In the present study we found that plasma [NO2 −] declined and that plasma [NO3 −] increased during exhaustive intermittent exercise. Importantly, the decline in plasma [NO2 −] and the increase in plasma [NO3 −] were significantly greater with BR compared to PL. Given that plasma [NO2 −] can serve as a circulating reservoir for hypoxic NO production (Lundberg and Weitzberg 2010), a decline in venous plasma [NO2 −] may be interpreted to represent a reduction of NO2 − to NO within the muscle or in the surrounding microvasculature. On this basis, it is possible that increasing plasma [NO2 −] via dietary nitrate supplementation might augment the synthesis of NO during high-intensity intermittent exercise. This interpretation is not straightforward, however. Because NO2 − is both an oxidation product of NO generated via the ‘conventional’ nitric oxide synthase pathway and a substrate for NO production in hypoxia, the net NO3 −–NO2 −–NO ‘flux’ during submaximal and high-intensity exercise is unclear.
In the present study we found that, following BR ingestion, the baseline plasma [NO2 −] and the change in plasma [NO2 −] from baseline to end-exercise tended to be correlated with Yo–Yo IR1 test performance. Other studies have also suggested that baseline plasma [NO2 −] and/or the change in plasma [NO2 −] during exercise may be related to exercise performance. It has been shown that plasma [NO3 −] and [NO2 −] are significantly higher in trained athletes compared to untrained controls (Poveda et al. 1997). Dreissigacker et al. (2010) reported that plasma [NO2 −] decreased from the start to the end of exercise and that the magnitude of this reduction was correlated with exercise capacity at 80 % of maximal work rate. Totzeck et al. (2012) also showed that baseline plasma [NO2 −] was correlated with lactate threshold and predicted exercise capacity during an incremental cycle test in highly trained athletes. Collectively, these results suggest that both a high baseline plasma [NO2 −] and the capacity to ‘utilize’ [NO2 −] during exercise may be related to high-intensity exercise performance.
The possibility that there may be ‘responders’ and ‘non-responders’ to nitrate supplementation has been suggested previously (Christensen et al. 2013; Wilkerson et al. 2012). Wilkerson et al. (2012) reported that, while group mean 50-mile cycle time-trial performance was not significantly improved by acute nitrate supplementation, subjects who evidenced a >30 % increase in plasma [NO2 −] (‘responders’) improved their performance whereas those whose plasma [NO2 −] changed by <30 % did not improve their performance. In the present study, we found that nitrate supplementation resulted in a substantial elevation of plasma [NO2 −] in all subjects (range 131–746 % increase with BR compared to PL), presumably because the amount of nitrate ingested was relatively high. However, we also found that while the majority of subjects exhibited a substantially greater decline of plasma [NO2 −] with BR compared to PL during the exhaustive Yo–Yo IR1 test, this was not the case in two of the three subjects whose test performance did not improve with BR. The explanation for this difference is not clear but might relate to inter-individual differences in muscle oxygenation and acidosis during exercise, factors which might, in turn, be linked to differences in effort, anaerobic capacity or muscle fibre-type distribution.
Mechanisms for enhanced Yo–Yo IR1 test performance after NO3 − supplementation
Fatigue in the Yo–Yo IR1 test is accompanied by a significant reduction in muscle [phosphocreatine] ([PCr]), [glycogen] and pH, and a significant increase in muscle [lactate] (Krustrup et al. 2003). Therefore, delaying the attainment of this limiting intramuscular milieu might be expected to improve performance in the Yo–Yo IR1 test. Existing evidence suggests that dietary nitrate supplementation can attenuate the decline in muscle PCr and the accumulation of muscle adenosine diphosphate and inorganic phosphate (Bailey et al. 2010), metabolites linked to the process of muscle fatigue (Allen et al. 2008). Blunting the rate of change of these substrates and metabolites would be expected to delay the attainment of a ‘critical’ intramuscular environment and to extend the tolerable duration of high-intensity constant-work-rate exercise (Burnley and Jones 2007; Vanhatalo et al. 2010b). In the present study, there were no significant differences in blood lactate accumulation with BR compared to PL. However, blood [glucose] was lower over the Yo–Yo IR1 test with BR compared to PL. To our knowledge, this is the first study to show that dietary nitrate supplementation lowers blood [glucose] during exercise. Among its various signalling properties, NO is known to play an important role in skeletal muscle glucose uptake (Merry et al. 2010). Therefore, it is possible that a greater increase in NO synthesis following nitrate supplementation enhanced skeletal muscle glucose uptake during the Yo–Yo IR1 test. If so, it is possible that this might have contributed to the enhanced intermittent exercise performance with BR by sparing muscle glycogen utilization (Tsintzas and Williams 1998) in individual (especially type II) fibres or fibre compartments. Further research is required to determine whether dietary nitrate supplementation can increase skeletal muscle glucose uptake during exercise using more specific experimental techniques.
Fatigue during intense exercise has been linked to depolarized muscle membrane potential induced by disturbances in muscle ion homeostasis where interstitial K+ accumulation is the main component (McKenna et al. 2008; Mohr et al. 2011; Nielsen et al. 2003). In the present study, there was a trend (P = 0.08) for the rise in plasma [K+] during the exhaustive Yo–Yo IR1 test to be attenuated with BR compared to PL, with post hoc analysis showing that plasma [K+] was significantly lower with BR compared to PL after 600 m of the test. Therefore, assuming that fatigue during intermittent high-intensity exercise is related, in part, to reduced muscle excitability due to a net loss of muscle K+ (McKenna et al. 2008; Nielsen and de Paoli, 2007), these results suggest that performance in the Yo–Yo IR1 test may have been enhanced by BR due to a reduced muscle K+ efflux and accumulation in the extracellular fluids. The muscle K+ efflux appears to be accelerated by acidosis (Mohr et al. 2004; Nordsborg et al. 2003). While there was no difference in blood [lactate] between BR and PL during the exhaustive Yo–Yo IR1 test, it is recognized that measurements of blood [lactate] are not sufficiently sensitive to reflect possible differences in muscle anaerobic energy turnover and H+ accumulation between BR and PL.
It has been reported that glycogen content is reduced in a high proportion of type II muscle fibres during the Yo–Yo IR1 test (Krustrup et al. 2003) and that most type II fibres are depleted or almost depleted of glycogen after a football game (Krustrup et al. 2006). This suggests that type II fibres are heavily recruited and that a significant metabolic perturbation in these fibres may coincide with exhaustion during the Yo–Yo IR1 test. Recent research indicates that type II muscle fibres might be specifically impacted by dietary nitrate supplementation (Ferguson et al. 2013; Hernández et al. 2012). Ferguson et al. (2013) reported that dietary nitrate supplementation increased hind limb skeletal muscle blood flow in rats, with this additional blood flow being preferentially distributed to type II muscle fibres. An increase in muscle O2 delivery might be expected to increase the proportional energy contribution from oxidative metabolism (Bangsbo et al. 2001), particularly in type II muscle fibres where O2 requirements may outstrip O2 supply (Behnke et al. 2003; McDonough et al. 2005) and to blunt the rate of PCr decline and accumulation of fatigue-related metabolites (Hogan et al. 1999; Vanhatalo et al. 2010b). Increased muscle O2 delivery may also hasten the restoration of homeostasis in type II muscle fibres in the recovery periods between exercise bouts during the Yo–Yo IR1 test. For example, greater muscle O2 availability would be expected to speed PCr resynthesis in the recovery phase of intermittent exercise (Paganini et al. 1997; Vanhatalo et al. 2011). It has also been reported that dietary nitrate supplementation increases sarcoplasmic reticulum calcium release and force production, specifically in type II fibres (Hernández et al. 2012). Assuming these findings are applicable to humans, an enhanced force production in type II muscle fibres might lower energy turnover by reducing the number of muscle fibres recruited to generate a given submaximal force production during the Yo–Yo IR1 test. Therefore, changes in skeletal muscle contractile properties and motor unit recruitment, as well as changes in bulk blood flow and its distribution, might account, at least in part, for the improved intermittent exercise performance with BR compared to PL in this study.
In conclusion, this study has shown for the first time that short-term dietary nitrate supplementation can improve intermittent exercise performance in recreationally active adults. Plasma [NO2 −] was elevated prior to exercise with BR compared to PL and declined to a greater extent with BR compared to PL during the exhaustive Yo–Yo IR1 test, suggesting that NO2 − may have served as a substrate for NO production during high-intensity exercise. Nitrate supplementation resulted in strong trends for reductions in blood [glucose] and plasma [K+] during the Yo–Yo IR1 test, suggesting that changes in muscle glucose uptake and muscle excitability may have contributed to the increased fatigue resistance. The results of this study suggest that nitrate may be an effective ergogenic aid for intermittent high-intensity exercise performance in recreational team sport players.
References
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH (2010) Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3 months of exercise training. Free Radic Biol Med 49:1138–1144
Atkins SJ (2006) Performance of the Yo–Yo intermittent recovery test by elite professional and semiprofessional rugby league players. J Strength Cond Res 20:222–225
Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM (2009) Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107:1144–1155
Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM (2010) Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol 109:135–148
Bailey SJ, Vanhatalo A, Winyard PG, Jones AM (2011) The nitrate-nitrite-nitric oxide pathway: its role in human exercise physiology. Eur J Sport Sci 12:309–320
Bangsbo J, Mohr M (2012) Fitness testing in football. Bangsbosport ISBN 978-87-994880-0-1, Copenhagen, Denmark
Bangsbo J, Krustrup P, González-Alonso J, Saltin B (2001) ATP production and efficiency of human skeletal muscle during intense exercise: effect of previous exercise. Am J Physiol Endocrinol Metab 280:E956–E964
Bangsbo J, Iaia FM, Krustrup P (2007) Metabolic response and fatigue in soccer. Int J Sports Physiol Perform 2:111–127
Bangsbo J, Iaia FM, Krustrup P (2008) The Yo–Yo intermittent recovery test: a useful tool for evaluation of physical performance in intermittent sports. Sports Med 38:37–51
Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC (2003) Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol 549:597–605
Bescós R, Ferrer-Roca V, Galilea PA, Roig A, Drobnic F, Sureda A, Martorell M, Cordova A, Tur JA, Pons A (2012) Sodium nitrate supplementation does not enhance performance of endurance athletes. Med Sci Sports Exerc 44:2400–2409
Bond H, Morton L, Braakhuis AJ (2012) Dietary nitrate supplementation improves rowing performance in well-trained rowers. Int J Sport Nutr Exerc Metab 22:251–256
Bradley PS, Mohr M, Bendiksen M, Randers MB, Flindt M, Barnes C, Hood P, Gomez A, Andersen JL, Di Mascio M, Bangsbo J, Krustrup P (2011) Sub-maximal and maximal Yo–Yo intermittent endurance test level 2: heart rate response, reproducibility and application to elite soccer. Eur J Appl Physiol 111:969–978
Burnley M, Jones AM (2007) Oxygen uptake kinetics as determinant of sports performance. Eur J Sport Sci 7:63–79
Cermak NM, Gibala MJ, van Loon LJ (2012a) Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab 22:64–71
Cermak NM, Res P, Stinkens R, Lundberg JO, Gibala MJ, van Loon L JC (2012b) No improvement in endurance performance following a single dose of beetroot juice. Int J Sport Nutr Exerc Metab 22:470–478
Christensen PM, Nyberg M, Bangsbo J (2013) Influence of nitrate supplementation on VO(2) kinetics and endurance of elite cyclists. Scand J Med Sci Sports 23:e21–e31
Colliander EB, Dudley GA, Tesch PA (1988) Skeletal muscle fiber type composition and performance during repeated bouts of maximal, concentric contractions. Eur J Appl Physiol Occup Physiol 58:81–86
Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9:1498–1505
Dreissigacker U, Wendt M, Wittke T, Tsikas D, Maassen N (2010) Positive correlation between plasma nitrite and performance during high-intensive exercise but not oxidative stress in healthy men. Nitric Oxide 23:128–135
Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC (2013) Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591:547–557
Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO 3rd (2000) Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci USA 97:11482–11487
Govoni M, Jansson EA, Weitzberg E, Lundberg JO (2008) The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19:333–337
Greenhaff PL, Nevill ME, Soderlund K, Bodin K, Boobis LH, Williams C, Hultman E (1994) The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol 478:149–155
Hernández A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H (2012) Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol 590:3575–3583
Hogan MC, Richardson RS, Haseler LJ (1999) Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol 86:1367–1373
Hord NG, Tang Y, Bryan NS (2009) Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90:1–10
Iaia FM, Thomassen M, Kolding H, Gunnarsson T, Wendell J, Rostgaard T, Nordsborg N, Krustrup P, Nybo L, Hellsten Y, Bangsbo J (2008) Reduced volume but increased training intensity elevates muscle Na+–K+ pump alpha1-subunit and NHE1 expression as well as short-term work capacity in humans. Am J Physiol Regul Integr Comp Physiol 294:R966–R974
Ingebrigtsen J, Bendiksen M, Randers MB, Castagna C, Krustrup P, Holtermann A (2012) Yo–Yo IR2 testing of elite and sub-elite soccer players: performance, heart rate response and correlations to other interval tests. J Sports Sci 30:1337–1345
Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A (2010) Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56:274–281
Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD (2011) Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol 110:1582–1591
Krustrup P, Mohr M, Amstrup T, Rysgaard T, Johansen J, Steensberg A, Pedersen PK, Bangsbo J (2003) The Yo–Yo intermittent recovery test: physiological response, reliability, and validity. Med Sci Sports Exerc 35:697–705
Krustrup P, Söderlund K, Mohr M, Bangsbo J (2004) The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch 447:855–866
Krustrup P, Mohr M, Steensberg A, Bencke J, Kjaer M, Bangsbo J (2006) Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc 38:1165–1174
Krustrup P, Söderlund K, Relu MU, Ferguson RA, Bangsbo J (2009) Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: effect of thigh occlusion. Scand J Med Sci Sports 19:576–584
Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM (2011a) Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc 43:1125–1131
Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM (2011b) Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 110:591–600
Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B (2007) Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 91:59–66
Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B (2010) Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med 48:342–347
Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E (2011) Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13:149–159
Liu TH, Wu CL, Chiang CW, Lo YW, Tseng HF, Chang CK (2009) No effect of short-term arginine supplementation on nitric oxide production, metabolism and performance in intermittent exercise in athletes. J Nutr Biochem 20:462–468
Lundberg JO, Weitzberg E (2010) NO-synthase independent NO generation in mammals. Biochem Biophys Res Commu 396:39–45
Lundberg JO, Carlström M, Larsen FJ, Weitzberg E (2011) Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res 89:525–532
Masschelein E, Van Thienen R, Wang X, Van Schepdael A, Thomis M, Hespel P (2012) Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J Appl Physiol 113:736–745
McDonough P, Behnke BJ, Padilla DJ, Musch TI, Poole DC (2005) Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563:903–913
McKenna MJ, Bangsbo J, Renaud JM (2008) Muscle K+, Na+, and Cl disturbances and Na+–K+ pump inactivation: implications for fatigue. J Appl Physiol 104:288–295
Merry TL, Lynch GS, McConell GK (2010) Downstream mechanisms of nitric oxide-mediated skeletal muscle glucose uptake during contraction. Am J Physiol Regul Integr Comp Physiol 299:R1656–R1665
Mohr M, Krustrup P, Bangsbo J (2003) Match performance of high standard soccer players with special reference to development of fatigue. J Sports Sci 21:519–528
Mohr M, Krustrup P, Nielsen JJ, Nybo L, Rasmussen MK, Juel C, Bangsbo J (2007) Effect of two different intense training regimens on skeletal muscle ion transport proteins and fatigue development. Am J Physiol Regul Integr Comp Physiol 292:R1594–R1602
Mohr M, Nordsborg N, Nielsen JJ, Pedersen LD, Fischer C, Krustrup P, Bangsbo J (2004) Potassium kinetics in human muscle interstitium during repeated intense exercise in relation to fatigue. Pflugers Arch 448:452–456
Mohr M, Nielsen JJ, Bangsbo J (2011) Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J Appl Physiol 111:1372–1379
Murphy M, Eliot K, Heuertz RM, Weiss E (2012) Whole beetroot consumption acutely improves running performance. J Acad Nutr Diet 112:548–552
Nielsen OB, de Paoli FV (2007) Regulation of Na+–K+ homeostasis and excitability in contracting muscles: implications for fatigue. Appl Physiol Nutr Metab 32:974–984
Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J, Juel C (2003) Localization and function of ATP-sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 284:R558–R563
Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J (2003) Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol 285:R143–R148
Paganini AT, Foley JM, Meyer RA (1997) Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol 272:C501–C510
Peacock O, Tjønna AE, James P, Wisløff U, Welde B, Böhlke N, Smith A, Stokes K, Cook C, Sandbakk O (2012) Dietary nitrate does not enhance running performance in elite cross-country skiers. Med Sci Sports Exerc 44:2213–2219
Poveda JJ, Riestra A, Salas E, Cagigas ML, López-somoza C, Amado JA, Berrazueta JR (1997) Contribution of nitric oxide to exercise-induced changes in healthy volunteers: effects of acute exercise and long-term physical training. Eur J Clin Invest 27:967–971
Rampinini E, Sassi A, Azzalin A, Castagna C, Menaspà P, Carlomagno D, Impellizzeri FM (2010) Physiological determinants of Yo–Yo intermittent recovery tests in male soccer players. Eur J Appl Physiol 108:401–409
Richardson RS, Leigh JS, Wagner PD, Noyszewski EA (1999) Cellular PO2 as a determinant of maximal mitochondrial O2 consumption in trained human skeletal muscle. J Appl Physiol 87:325–331
Sobko T, Marcus C, Govoni M, Kamiya S (2010) Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide 22:136–140
Stamler JS, Meissner G (2001) Physiology of nitric oxide in skeletal muscle. Physiol Rev 81:209–237
Totzeck M, Hendgen-Cotta UB, Rammos C, Frommke LM, Knackstedt C, Predel HG, Kelm M, Rassaf T (2012) Higher endogenous nitrite levels are associated with superior exercise capacity in highly trained athletes. Nitric Oxide 27:75–81
Tsintzas K, Williams C (1998) Human muscle glycogen metabolism during exercise. Effect of carbohydrate supplementation. Sports Med 25:7–23
Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM (2010a) Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299:R1121–R1131
Vanhatalo A, Fulford J, DiMenna FJ, Jones AM (2010b) Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95:528–540
Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM (2011) Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 589:5517–5528
Veale JP, Pearce AJ, Carlson JS (2010) The Yo–Yo intermittent recovery test (level 1) to discriminate elite junior Australian football players. J Sci Med Sport 13:329–331
Vernillo G, Silvestri A, Torre AL (2012) The Yo–Yo intermittent recovery test in junior basketball players according to performance level and age group. J Strength Cond Res 26:2490–2494
Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM (2012) Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur J Appl Physiol 112:4127–4134
Acknowledgments
The authors thank Jamie R Blackwell for advice and assistance with the plasma nitrate and nitrite analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David C. Poole.
Rights and permissions
About this article
Cite this article
Wylie, L.J., Mohr, M., Krustrup, P. et al. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur J Appl Physiol 113, 1673–1684 (2013). https://doi.org/10.1007/s00421-013-2589-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2589-8