Abstract
The aim of the present study was to test the hypothesis that consuming protein does not attenuate AMPK signalling when exercise is commenced in a glycogen-depleted state. After performing a glycogen-depleting protocol the evening before, the subsequent morning ten active men performed 45 min steady-state cycling at 50 % of peak power output (PPO) followed by an exercise capacity test (1-min intervals at 80 % PPO interspersed with 1-min periods at 40 % PPO). In a repeated measures design, subjects consumed 20 g of a casein hydrolysate solution (PRO) 45 min before exercise, 10 g during and a further 20 g immediately post-exercise, or an equivalent volume of a non-calorie taste matched placebo (PLA). Resting (PRO = 134 ± 29; PLA = 136 ± 28 mmol kg−1) and post-exercise muscle glycogen (PRO = 43 ± 16; PLA = 47 ± 18 mmol kg−1) was not different (P > 0.05) between trials nor was exercise capacity (PRO = 26 ± 9; PLA = 25 ± 10 min, P > 0.05). Phosphorylation of AMPKThr172 increased threefold immediately post-exercise (P < 0.05) and PGC1−mRNA increased sixfold at 3 h post-exercise (P < 0.05), though there were no differences between conditions (P > 0.05). In contrast, there was a trend (P = 0.08) for a divergent response in eEF2Thr56 phosphorylation such that 1.5 fold increases post- and 3 h post-exercise in PLA were blunted with PRO, thus indicative of greater eEF2 activation. We conclude that athletes who deliberately incorporate training phases with reduced muscle glycogen into their training programmes may consume protein before, during and after exercise without negating signalling through the AMPK cascade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
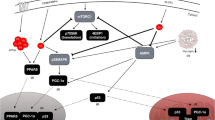
At a molecular level, endurance training-induced increases in skeletal muscle mitochondrial biogenesis are thought to be due to the cumulative effects of transient increases in mRNA transcripts encoding mitochondrial proteins that follow each acute training session (Perry et al. 2010). Contractile-induced disruptions to cell homeostasis (e.g. increased AMP/ATP ratio, Ca2+, reactive oxygen species, reduced substrate availability, etc.) result in the activation of a number of regulatory protein kinases which, in turn, phosphorylate downstream targets such as transcription factors or transcriptional co-activators (Hood 2009). Perhaps the most well-studied signalling kinase that is sensitive to muscle contraction is the adenosine monophosphate activated protein kinase (AMPK), given its role in regulating the proposed master regulator of mitochondrial biogenesis, peroxisome proliferator-activated γ receptor co-activator (PGC-1α) (Irrcher et al. 2008; Jäger et al. 2007). Indeed, PGC-1α expression is increased in human skeletal muscle in an exercise-intensity dependent manner that is associated with upstream signalling through AMPK (Egan et al. 2010). The importance of PGC-1α in regulating mitochondrial content and function is evident from rodent studies demonstrating that overexpression increases oxidative enzyme activity (Lin et al. 2002) and improves exercise capacity (Calvo et al. 2008).
In considering possible signals involved in regulating exercise-induced metabolic gene expression, there is growing evidence that reduction in carbohydrate (CHO) availability is a potent stimulus to enhance oxidative capacity which may be mediated, in part, through AMPK signalling. For example, AMPK displays higher activity at rest and following exercise when glycogen is low (Wojtaszewski et al. 2003; Yeo et al. 2010) and ingestion of glucose during exercise also attenuates exercise-induced AMPK activation (Akerstrom et al. 2005). Furthermore, restricting CHO availability before (Pilegaard et al. 2002), during (Cluberton et al. 2005) and after exercise (Pilegaard et al. 2005) augments the expression of many metabolic genes related to regulation of substrate utilisation. Data from these acute studies therefore suggested that commencing exercise with reduced muscle glycogen, restricting CHO provision during exercise and deliberately minimising post-exercise muscle glycogen re-synthesis may thereby provide an enhanced stimulus to up-regulate oxidative capacity of skeletal muscle. Accordingly, we (Morton et al. 2009) and others (Hansen et al. 2005; Hulston et al. 2010; Yeo et al. 2008) subsequently demonstrated that training in conditions of reduced endogenous and exogenous carbohydrate availability enhances training-induced oxidative adaptations of skeletal muscle. This periodised approach to training and nutrition has since been termed as the train-low compete-high model surmising that selected training sessions be deliberately completed with reduced carbohydrate availability before, during and after exercise but yet competition always be performed with high carbohydrate availability (Burke 2010).
Despite the potential of the train-low approach to enhance skeletal muscle adaptations, there are a number of limitations associated with performing chronic exercise in the face of reduced carbohydrate availability. Indeed, reduced muscle glycogen levels compromises absolute training intensity (Hulston et al. 2010; Yeo et al. 2008) which if performed repeatedly, could lead to a de-training effect. In addition, contractile-induced protein degradation and synthesis are increased and decreased, respectively, to a greater extent when exercise is commenced with reduced glycogen availability compared with elevated glycogen stores (Blomstrand and Saltin 1999; Howarth et al. 2010; Van Hall et al. 1999). In this way, chronic exposure to exercise training with low muscle glycogen availability could also lead to a progressive loss of lean mass thereby also negatively impacting upon physical performance. Indeed, we (Morton et al. 2010) and others (Mettler et al. 2010) have observed that elite athletes can in fact lose skeletal muscle mass during times of heavy training especially when energy intake and CHO availability are considered sub-optimal.
In an attempt to overcome these limitations, one potential strategy may be to consume intact protein or essential amino acids, before, during and/or after exercise. For example, the provision of nutrients in the form of protein (as opposed to CHO) may still allow for the enhanced cell signalling pathways associated with training with reduced carbohydrate availability but yet would also provide an important substrate to promote skeletal muscle remodelling, and potentially minimise any progressive loss of lean mass during training. Indeed, increased protein availability reduces lean mass loss during heavy training periods when energy and CHO availability is restricted (Morton et al. 2010; Mettler et al. 2010). In this regard, accumulating data suggest that protein provision before (Coffey et al. 2011), during (Hulston et al. 2011) and after (Breen et al. 2011; Howarth et al. 2009) endurance or repeated sprint type protocols, enhances post-exercise skeletal muscle protein synthesis as well as activation of some of the signalling molecules regulating translation initiation and elongation. Aside from the additional energy content per se, there is also some evidence (albeit not consistent) that consumption of branched chain amino acids (BCAAs) during exercise improves exercise performance (Blomstrand et al. 1991) and reduces perceptions of effort during sub-maximal exercise that is commenced in a glycogen-depleted state (Blomstrand et al. 1997), possibly through alleviation of central fatigue (Blomstrand 2006). Taken together, the above data suggest that protein ingestion before, during and after exercise performed with reduced CHO availability may therefore prove beneficial as it is likely to still allow for the enhanced cell signalling responses associated with reduced muscle glycogen but yet could also improve net protein balance, and potentially increase training capacity.
The aims of the present study were therefore to test the hypotheses that protein ingestion before, during and after exercise commenced in conditions of reduced muscle glycogen: (1) does not attenuate acute cell signalling responses associated with the regulation of mitochondrial biogenesis, (2) increases translational signalling of skeletal muscle protein synthesis and, (3) increases exercise capacity. In this way, we postulated that our experimental findings would have practical applications for those athletes who deliberately incorporate phases of train-low compete-high into their periodised training and nutritional programmes.
Materials and methods
Subjects
Ten recreationally active males (mean ± SD: age, 22 ± 2 years; body mass, 79 ± 9 kg; height, 1.77 ± 0.05 m; \( \dot{V}{\text{O}}_{{_{{ 2 {\text{peak}}}} }} \), 53 ± 6 ml kg−1 min−1; peak power output, 323 ± 61 W), who cycled on average 1–6 h per week volunteered to take part in the study. The experimental procedures and potential risks associated with the study were explained and subjects gave written informed consent prior to participation. Subjects refrained from additional exercise outside of the study requirements as well as from alcohol and caffeine intake for at least 48 h prior to any of the testing sessions. None of the subjects had history of neurological disease or musculoskeletal abnormality and none were under pharmacological treatment during the course of the study. The study was approved by the Ethics Committee of Liverpool John Moores University.
Overview of experimental design
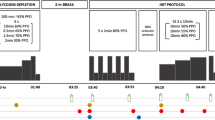
In a randomised, double-blind, cross-over and counterbalanced design and after having initially been assessed for maximal oxygen uptake, participants reported to the laboratory on the evening prior to the main experimental trial in order to perform a glycogen-depleting bout of intermittent exhaustive cycling. At the cessation of exercise, participants were deliberately provided with a low-carbohydrate meal (<50 g) to be consumed within a 30 min period. The following morning, participants arrived at the laboratory in a fasted state and performed 45 min of steady-state cycling and a subsequent bout of intermittent exhaustive cycling as a measure of exercise capacity. Subjects ingested a protein (PRO) or placebo (PLA) beverage 45 min prior to exercise, at regular intervals during exercise and immediately post exercise. Muscle biopsies were sampled from the vastus lateralis immediately before and after exercise and also at 3 h post-exercise. Venous blood samples were also obtained at regular intervals during the experimental protocol. Participants returned to the laboratory 7–10 days after their initial trial so as to complete the second trial.
Assessment of maximal oxygen uptake
Peak oxygen uptake (\( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \)) and peak power output (PPO) were determined using an incremental cycle test to exhaustion on an electrically braked cycle ergometer (Daum Electronic Premium 8i, Furth, Germany). The test commenced with a 5 min warm-up at 50 W at a cadence corresponding to 60 revs min−1 followed by an increase in 30 W every 60 s until volitional exhaustion. Breath-by-breath measurements were obtained throughout exercise using a CPX Ultima series online gas analysis system (Medgraphics, MN, US). \( \dot{V}{\text{O}}_{{ 2 {\text{peak}}}} \) was stated as being achieved by the following end-point criteria: (1) heart rate within 10 beats min−1 of age-predicted maximum, (2) respiratory exchange ratio >1.1, and 3) plateau of oxygen consumption despite increased workload. Peak power output was defined as the highest power output that was able to be maintained for 60 s. The ergometer saddle and handle bar position were recorded for each participant during preliminary testing and replicated during the experimental trials.
Glycogen-depleting protocol
Participants arrived at the laboratory on the evening prior to each main experimental trial (~1800 h) and performed an intermittent bout of cycling to volitional fatigue. After a 5 min warm up at 100 W, participants cycled for 2 min at 90 % PPO, followed immediately by a 2 min recovery period at 50 % PPO. They repeated this work to rest ratio until they could no longer complete 2 min cycling at 90 % PPO, determined as an inability to maintain a cadence of 60 revs min−1. At this point, exercise intensity was lowered to 80 % PPO and when participants could no longer maintain this intensity, it was lowered to 70 % and finally, 60 % PPO with the same work to rest ratio. When the participants were unable to cycle for 2 min at 60 % PPO the exercise protocol was terminated. This intermittent pattern of exercise was employed so as to induce glycogen depletion in both type I and II fibres. Heart rate (HR) was measured continuously throughout exercise (Polar S610i, Kempele, Finland) and ratings of perceived exertion (RPE) were recorded at 2 min intervals during exercise (Borg 1970). The activity pattern and total time-to-exhaustion for the glycogen-depleting ride were recorded for each participant during their first trial and replicated during their second trial. Water intake was consumed ad libitum throughout exercise in the initial trial and was recorded and repeated for the subsequent trial. Participants were deliberately provided with a low carbohydrate meal consisting of 400 g of tomato soup (Heinz, UK: 950 kJ; 26.6 g CHO; 11.8 g fat; 3.6 g protein) within 30 min of completion of the depletion ride so as to induce minimal muscle glycogen re-synthesis prior to the main experimental trial on the subsequent morning. No further nutrient provision was provided after consumption of this meal.
Experimental trial
After an overnight fast, participants entered the laboratory on the subsequent morning (~0630 h) and were fitted with a cannula into an antecubital vein. After obtaining a resting blood sample, the cannula was then flushed with ~2 ml of sterile saline (Kays Medical Supplies, Liverpool, UK) to keep the cannula patent and sterile, a procedure that was completed after each subsequent blood draw. Having obtained a resting blood sample, participants then consumed 1 l of either the PRO (Science in Sport Ltd., Blackburn, UK) beverage-containing casein hydrolysate or a visually identical and taste-matched PLA (see Table 1 for drink compositions). Participants then rested in a supine position for a 45 min period during which further blood samples were obtained at 15 min intervals. After this period, a pre-exercise muscle biopsy was obtained from the vastus lateralis and participants subsequently cycled for 45 min at 50 % PPO. Venous blood samples were obtained at 15 min intervals during exercise and HR (Polar S610i, Kempele, Finland) and \( \dot{V}{\text{O}}_{ 2} \) (Medgraphics, MN, US) were measured continuously during exercise. Rates of carbohydrate and lipid oxidation (g min−1) during exercise were calculated according to the equations presented by Jeukendrup and Wallis (2005). RPE was also measured at 5 min intervals during exercise (Borg 1970). Participants also ingested a further 500 ml of the PRO or PLA beverage during exercise, administered as four equal doses (125 ml) immediately prior to exercise and after completion of 15, 30 and 45 min of exercise. Once the participants had completed the 45 min steady-state cycling protocol, they remained mounted on the ergometer and performed 5 min of active recovery (cycling at 50 W) before commencing the exercise capacity test. This test consisted of intermittent 1-min bout of cycling at intensities corresponding to 40 and 80 % of each subject’s PPO until volitional fatigue. Upon exhaustion, participants dismounted the ergometer and immediately had a post-exercise blood sample and muscle biopsy taken, before consuming a final 1 l bolus of the PRO or PLA solution. In this way, participants ingested a total of 20 g protein prior to exercise, 10 g during exercise and a further 20 g immediately post-exercise. It is acknowledged that protein provision may be prescribed on the basis of g kg−1 body mass, however, on the basis of dose-response studies (albeit on resistance exercise, Moore et al. 2009) and also because 20 g post-exercise is sufficient to stimulate protein synthesis in the hours following steady-state endurance cycling (Breen et al. 2011), we considered an absolute amount of 20 g as a suitable initial dose for which to study. Furthermore, prescribing an absolute dose may also increase practical application for endurance athletes (who usually don’t vary considerably in body mass) given that a 20 g bolus is also an easy to administer dose. Ingestion of 10 g of essential amino acids during exercise (60 min cycling at 70 % \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \)) has also been shown sufficient to stimulate post-exercise protein synthesis (Pasiakos et al. 2011). As such, we considered our chosen dosing strategy as one that has both practical application and supporting rationale and that may be particularly pertinent to exercise in glycogen-depleted states given the increased demands upon amino acid metabolism in this instance. After the post-exercise biopsy, subjects remained seated in the laboratory (performing light activities such as reading or working on a computer) before having a final muscle biopsy sampled at 3 h post exercise. Laboratory conditions remained constant for both experimental trials (19–21 °C, 40–50 % humidity).
Dietary and physical activity control
Participants recorded their diet for 48 h prior to their initial glycogen-depleting exercise protocol and repeated this dietary intake prior to their second trial. Subsequent dietary analysis was performed using a computer software package (Microdiet, Downlee Systems, UK) and the average total daily energy intake was 2,334 ± 528 kcal (52 ± 13 % CHO; 32 ± 12 % fat; 15 ± 5 % protein). Participants were instructed to refrain from ingesting alcohol and caffeine-containing food and drink for 48 h prior to each trial and refrain from physical activity during the 3–5 days preceding each trial.
Blood analysis
Blood samples were drawn from a superficial vein in the anticubital crease of the forearm using a cannula intravenous infusion set (NHS Supply Chain, Alfreton, UK). Samples were collected into vacutainers containing EDTA, lithium heparin or serum separation tubes (SST) and stored on ice or at room temperature (SST for approximately 1 h) until centrifugation at 1,500g for 15 min at 4 °C. Following centrifugation, aliquots of plasma or serum were stored at −80 °C for later analysis. Samples were analysed for plasma glucose, lactate, NEFA and glycerol concentration using commercially available kits (Randox Laboratories, Antrim, UK). Serum insulin concentration was measured using an automated ELISA insulin test kit (Insulin, Cobas, Roche Diagnostics, IN, USA).
Muscle biopsies
Muscle biopsies were obtained from separate incision sites (2–3 cm apart) from the lateral portion of the vastus lateralis muscle pre-, post- and 3 h after the exercise protocol using a Bard Monopty Disposable Core Biopsy Instrument (12 gauge × 10 cm length, Bard Biopsy Systems, Tempe, AZ, USA). Samples were obtained (approximately 40–50 mg) under local anaesthesia (0.5 % marcaine) and immediately frozen in liquid nitrogen and stored at −80 °C for later analysis.
Muscle glycogen concentration
Muscle glycogen concentration was determined according to the method described by Van Loon et al. (2000). Approximately 3–5 mg of freeze dried sample was powdered, dissected free of all visible non-muscle tissue and subsequently hydrolyzed by incubation in 500 μl of 1 M HCl for 3–4 h at 100 °C. After cooling to room temperature, samples were neutralized by the addition of 250 μl 0.12 mol l−1 Tris/2.1 mol l−1 KOH saturated with KCl. Following centrifugation, 150 μl of the supernatant was analysed in duplicate for glucose concentration according to the hexokinase method using a commercially available kit (GLUC-HK, Randox Laboratories, Antrim, UK). Glycogen concentration is expressed as mmol kg−1 dry weight (dw) and intra-assay coefficients of variation was <5 %.
Western blotting
Approximately 20 mg of frozen muscle was ground to powder and homogenised in 120 μl of ice cold lysis buffer [25 mM Tris/HCl (pH 7.4), 50 mM NaF, 100 mM NaCl, 5 mM EGTA, 1 mM EDTA, 10 mM Na-pyrophosphatase, 1 mM Na3VO4, 0.27 M sucrose, 1 % Triton X-100, 0.1 % 2-mercaptoethanol] and supplemented with a protease inhibitor tablet (Complete mini, Roche Applied Science, West Sussex, UK). Homogenates were centrifuged at 14,000g for 10 min at 4 °C and the supernatant was collected. The protein content of the supernatant was determined using a bicinchoninic acid assay (Sigma, UK). Each sample was diluted with an equal volume of 2× Laemmli buffer (National Diagnostics, USA) and boiled for 5 min at 100 °C. 50 μg of protein from each sample was then separated in Tris–glycine running buffer (10× Tris/Glycine, Geneflow Ltd, Staffordshire, UK) using self-cast 4 % stacking and 10 % separating gels (National Diagnostics, USA). Gels were transferred semi-dry onto nitrocellulose membrane (Geneflow Ltd, Staffordshire, UK) for 2 h at 200 V and 45 mA per gel in transfer buffers (anode 1; 0.3 M Tris, 20 % methanol, pH 10.4; anode 2; 0.25 M Tris, 20 % methanol, pH 10.4; cathode; 0.4 M 6-amino hexanoic acid, 20 % methanol, pH 7.6). After transfer, membranes were blocked for 1 h at room temperature in Tris-buffered saline (TBST: 0.19 M Tris pH 7.6, 1.3 M NaCl, 0.1 %Tween-20) with 5 % non-fat milk. The membranes were then washed for 3 × 5 min in TBST before being incubated overnight at 4 °C with antibodies for anti-phospho AMPKThr172, eEF2Thr56 as well as total protein content of AMPK, GAPDH, eEF2 (Cell Signalling, UK), and PGC-1α (Calbiochem, Merck Chemicals, UK), all at concentrations of 1:1000 in 1× TBST. The next morning, membranes were washed for a further 3 × 5 min in TBST and subsequently incubated with anti-species horseradish peroxidise-conjugated secondary antibody (Bio-Rad, UK or Dako, UK) for 1 h at room temperature. After a further 3 × 5 min washes in TBST, membranes were exposed in a chemiluminescence liquid (SuperSignal, Thermo Fisher Scientific, Rockford, IL, USA) for 5-min. Membranes were visualised using a Bio-Rad Chemi-doc system, and band densities were determined using Quantity One image-analysis software. Comparative samples from each subject for both exercise protocols were run on the same gel and all gels were run in duplicate to verify responses. It should be noted that all raw densitometry data were used for statistical analysis purposes so as to compare within-subject responses to both the PRO and PLA trials.
Real-time RT-PCR
Total RNA was isolated from muscle biopsies (20 mg) using Trizol reagent (Life Technologies/Invitrogen, USA), according to the manufacturer’s protocol. For further purification of the RNA, Qiagen All Prep DNA/RNA Micro Kit (Qiagen, UK) was used using manufacturer’s recommended guidelines. The final volume of the purified RNA samples was 14 μl. RNA quality and quantity were determined using Implen Nanophotometer (Implen, Munchen, Germany) and the RNA was stored at −80 °C. The cDNA was synthesised using random hexamers (Applied Biosystems, Foster City, USA) and Superscript III enzyme (Life Technologies/Invitrogen, USA), using manufacturer’s protocol. Gene-specific expression data were obtained using custom-designed primers, supplied by Eurofins (MWG Eurofins, Germany). For PGC-1α, forward primer 5′-TGAGAGGGCCAAGCAAAG-3′ and reverse primer 5′-ATAAATCACACGGCGCTCTT-3′ were used, and for GAPDH, forward primer 5′-AGCCACATCGCTCAGACAC-3′ and reverse primer 5′-GCCCAATACGACCAAATCC-3′ were applied. Each cDNA sample was analysed in triplicate with negative controls using AB 7500 Real-Time Quantitative PCR instrument (Applied Biosystems) and Agilent Brilliant II qPCR Master Mix with Low ROX (Agilent Technologies, USA). One microliter of cDNA together with 200 nM of final concentration of primers was used for each reaction with total volume of 20 μl. The following cycling parameters were used: 50 °C for 2 min, initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/elongation at 60 °C for 1 min. Data were collected and analysed using proprietary software for the instrument (SDS version 1.43, Applied Biosystems, Foster City, USA). Changes in mRNA content were calculated according to the 2−∆∆Ct method where GAPDH was used as the housekeeping gene.
Statistical analysis
Statistical analysis was conducted using the Statistical Package for Social Sciences software programme (version 15). Data were analysed using a two way repeated measures general linear model where the within factors were time and exercise condition (i.e. PLA versus PRO). Where there was a significant interaction effect or main effect of time, Bonferroni post hoc tests were used to locate specific differences. Student’s t test for paired samples was also used to compare average physiological responses during the glycogen-depletion protocol as well as exercise capacity and physiological variables at the point of exhaustion. All data in text, figures and tables are presented as means (SD) with P values ≤0.05 indicating statistical significance.
Results
Physiological responses to the glycogen-depleting exercise protocol
Exercise time to exhaustion for the glycogen-depletion protocol for both the placebo and protein condition was 64.1 ± 6.4 min. Mean HR and RPE for the placebo and protein conditions during exercise were 160 ± 15 versus 163 ± 14 b min−1 and 14.7 ± 1.2 versus 15 ± 1.4, respectively. There was no significant difference in either average heart rate (P = 0.106) or RPE (P = 0.175) between trials.
Physiological responses to the steady-state exercise and exercise capacity test
Muscle glycogen
The glycogen depletion and nutritional protocol was successful in ensuring that participants commenced the steady-state exercise protocol with reduced glycogen availability (placebo and protein conditions = 136 ± 28 and 134 ± 29 mmol kg dw−1, respectively). Exercise decreased muscle glycogen similarly in both trials (P < 0.001) to levels <50 mmol kg dw−1 and there` was also no apparent muscle glycogen re-synthesis at 3 h post-exercise (P = 0.328) in either condition (see Fig. 1).
Substrate oxidation
CHO oxidation significantly decreased during steady-state exercise (P < 0.001) though there was no difference (P = 0.12) between trials (see Table 2). Similarly, lipid oxidation increased (P < 0.001) during exercise though no difference (P = 0.23) was apparent between protein and placebo ingestion (see Table 2). Both \( \dot{V}{\text{O}}_{2} \) and RPE increased (both P < 0.001) during steady-state cycling though the magnitude of increase was not different (P = 0.89 and 0.92, respectively) between conditions (see Table 2).
Plasma metabolites
Plasma glucose, lactate, NEFA, glycerol and serum insulin concentrations are presented in Table 3. Plasma glucose concentration decreased throughout the experimental protocol (P < 0.001) in both conditions with no effect of protein ingestion (P = 0.98). Exercise-induced increases in plasma lactate (P < 0.001) during the protocol were also not different between conditions (P = 0.18). Plasma NEFA concentration increased during exercise under both placebo and protein ingestion (P = 0.001) with a tendency for significant differences between trials (P = 0.075). Similarly, plasma glycerol concentration increased during exercise (P < 0.001) and also displayed significant effects for condition (P = 0.03) and interaction (P = 0.003). In accordance with the provision of amino acids in the protein trial, serum insulin concentration displayed a significant main effect for time (P = 0.001), condition (P = 0.01) and interaction (P = 0.002).
Exercise capacity test and physiological responses
Following the completion of the 45 min steady-state cycling protocol, there was no difference in time to exhaustion (P = 0.61) between trials during the subsequent exercise capacity test (24.7 ± 10.2 and 25.6 ± 8.7 min for placebo and protein, respectively, see Fig. 2). Heart rate (160 ± 16 and 163 ± 15 beats min−1, P = 0.30), RPE (16 ± 1 and 16 ± 1, P = 0.76) and plasma glucose (4.6 ± 0.97 and 4.6 ± 0.72 mmol l−1, P = 0.88), lactate (4.5 ± 1.95 and 5.4 ± 2.55 mmol l−1, P = 0.60), glycerol (421.4 ± 115.4 and 390.8 ± 109.8 mmol l−1, P = 0.30) and NEFA (1.35 ± 0.59 and 1.36 ± 0.56 mmol l−1, P = 1.0) were also not different at the point of exhaustion between the placebo and protein trials, respectively.
Skeletal muscle cell signalling responses
Phosphorylation of AMPKThr172 increased by approximately threefold post-exercise (P = 0.03) with no difference (P = 0.545) between conditions and returned to basal levels at 3 h post-exercise (see Fig. 3). There was a statistical trend (P = 0.08) for greater activation of eEF2Thr following the ingestion of protein, compared to the placebo solution, as illustrated by the greater dephosphorylation of eEF2Thr56 in the protein condition (see Fig. 4).
PGC-1α mRNA and protein content
Muscle PGC-1α mRNA content increased sixfold at 3 h following exercise (P = 0.001) with no difference between conditions (P = 0.77; Fig. 5). Total protein content of PGC-1α did not change at any time-point post-exercise (P = 0.81) in either trial (see Fig. 6).
Discussion
Although recent data demonstrate that training with reduced CHO availability enhances oxidative adaptations of skeletal muscle (Hansen et al. 2005; Hulston et al. 2010; Morton et al. 2009; Yeo et al. 2008), incorporating this approach to training may have detrimental effects if performed long-term such as reduced absolute training intensity (Hulston et al. 2010; Yeo et al. 2008) and potentially loss of lean mass (Morton et al. 2010). With this in mind, we postulated that provision of nutrients in the form of protein (as opposed to CHO) may still allow for the activation of acute cell signalling pathways associated with regulating the enhanced training response in CHO restricted states but yet also provide important substrate to promote skeletal muscle remodelling. To this end, we employed an experimental design in which subjects performed acute exercise in a glycogen-depleted state but with consumption of protein or placebo before, during and after exercise. Given that subjects replicated the precise pattern of intensities and duration of the glycogen-depletion protocol between trials (thus resulting in similar heart rates and RPE) and that pre-exercise glycogen availability prior to the main experimental trial was almost identical, our data indeed suggest that subjects commenced the experimental trial in a similar metabolic state and with similar CHO availability. Importantly, we provide novel data by demonstrating that protein ingestion before and during glycogen-depleted exercise does not impair the magnitude of AMPKThr172 phosphorylation or PGC-1α gene expression. Additionally, we observed that protein feeding before, during and after exercise induced a trend (which we consider indicative of physiological significance) for greater dephosphorylation and hence, activation of eEF2. These data were therefore considered to have important practical implications in that athletes who deliberately incorporate the train-low compete-high model into their periodised training programmes may consume protein in close proximity to the exercise stimulus without apparently negating AMPK mediated signalling.
A contractile-induced increase in AMPK activation is a well-documented component of a signalling cascade thought to regulate the early transcriptional responses to exercise (Hood 2009). Given that glycogen-binding domains have been observed on AMPK subunits (McBride et al. 2009), commencing exercise with reduced muscle glycogen levels increases the activity of AMPK α2 (Wojtaszewski et al. 2003) and AMPKThr172 phosphorylation in human skeletal muscle (Yeo et al. 2008). Augmented signalling through AMPK in a glycogen-depleted state has therefore been suggested as a contributory mechanism underpinning the enhanced oxidative adaptations associated with training with reduced carbohydrate availability (Hansen et al. 2005; Hulston et al. 2010; Morton et al. 2009; Yeo et al. 2008). Although the present study design did not incorporate a high glycogen trial, the threefold increases in AMPKThr172 phosphorylation immediately post-exercise is consistent with the magnitude of increase observed by other authors when exercise has been commenced in a glycogen-depleted state compared with high glycogen availability (Yeo et al. 2010). Importantly, consuming 20 g of protein 45 min before exercise and a further 10 g during exhaustive exercise does not cause any impairment in AMPK signalling, thus demonstrating that protein ingestion still allows for the increased magnitude of AMPK phosphorylation associated with reduced glycogen availability.
In accordance with the role of AMPK as an upstream signalling kinase regulating PGC-1α expression (Jäger et al. 2007), we observed a similar sixfold increase in PGC-1α mRNA between trials at 3 h post-exercise with no concomitant changes in PGC-1α protein. This time-course of PGC-1α expression is consistent with data from our laboratory (Bartlett et al. 2012) and others (Little et al. 2010, 2011) and indeed, it is now well documented that increased PGC-1α protein is typically only observed in the days following an acute exercise bout (Little et al. 2010, 2011) or with repeated days and weeks of exercise (Perry et al. 2010). In this regard, future studies should include additional sampling points in an extended recovery period (i.e. >24 h post-exercise) and/or with repeated bouts of glycogen-depleted exercise (i.e. exercise training) so as to ascertain the role of increased protein availability in stimulating up-regulation of oxidative proteins within a more physiologically relevant time-course. We also acknowledge that measurement of nuclear and mitochondrial translocation of PGC-1α as well as acetylation status would have also been advantageous to have been measured here (Philp et al. 2011) though the limited tissue obtained by our chosen biopsy technique did not permit this analysis.
There is now a growing body of literature examining the regulation of skeletal muscle protein synthesis in the early recovery period (i.e. <4 h) following acute endurance type exercise as opposed to the more frequently studied resistance protocols. It is generally thought muscle protein synthesis is suppressed during exercise, possibly due to Ca2+ induced activation of eEF2 kinase (Rose et al. 2009) such that eEF2 becomes phosphorylated (Rose et al. 2005) and hence deactivated during exercise thereby suppressing translational elongation. In contrast, endurance exercise elevates muscle protein synthesis in the recovery period even when the subjects remain in a fasted state (Mascher et al. 2011), though it is well documented that exercise and post-exercise feeding act synergistically to elevate synthesis rates and ensure that net protein balance becomes positive. Indeed, Hulston et al. (2011) observed that co-ingestion of protein with carbohydrate during 3 h cycling exercise and throughout recovery increased net leg protein balance during exercise and recovery as well as enhancing post-exercise mixed muscle protein synthesis when compared with carbohydrate per se. Interestingly, Breen et al. (2011) also observed that the enhanced post-exercise protein synthesis (in a 4 h recovery period) induced by co-ingestion of protein with carbohydrate immediately post-exercise is specifically due to increased rates of myofibrillar and not mitochondrial protein synthesis. The latter data are of particular importance for the present design given that our participants commenced exercise in a glycogen-depleted state, a condition which exacerbates contractile-induced increases and decreases in muscle protein degradation and synthesis, respectively (Howarth et al. 2010). The repeated absence of protein provision in the early recovery period following glycogen-depleting exercise may therefore lead to a progressive loss of myofibrillar protein, muscle mass and hence, an impaired capacity to generate force.
Although the present study is limited in that we did not directly measure muscle protein turnover via stable isotope methods, we chose to measure the phosphorylation status of eEF2 given its role as a molecular regulator of elongation. Making inferences on protein turnover based on snapshots of signalling molecules is of course not without limitations. However, it is important to note that Breen et al. (2011) observed that the enhanced myofibrillar protein synthesis induced by post-exercise protein feeding was associated with reduced phosphorylation (i.e. hence increased activation) of eEF2Thr56 at 4 h post-exercise, therefore supporting the role of eEF2 as a key regulator of muscle protein synthesis. Our data are in agreement with these authors as we also observed that protein feeding apparently reduces phosphorylation of eEF2Thr56 at 3 h post-exercise (we acknowledge that our chosen sample size may have been underpowered to achieve full statistical significance). Furthermore, we also provide novel data by demonstrating that protein feeding before and during glycogen-depleting exercise appears to reduce eEF2Thr56 phosphorylation immediately post-exercise, thereby suggestive of improved metabolic conditions to attenuate the contraction-induced phosphorylation of eEF2Thr56 (Rose et al. 2005) and hence reduce the suppression in muscle protein synthesis rates which occur during exercise (Rose et al. 2009). Future studies utilising stable isotope methodology are now required to fully test this hypothesis. Unfortunately, the limited tissue obtained by our chosen biopsy technique also did not permit us to measure other important signalling nodes regulating protein synthesis (e.g. mTOR, p70S6 kinase etc.) or indeed, markers of muscle protein degradation such as the atrogenes.
Given that training power outputs are reduced in a glycogen-depleted state compared with normal glycogen availability (Hulston et al. 2010; Yeo et al. 2008), we also tested the hypothesis that protein ingestion before and during glycogen-depleting exercise would be able to restore exercise capacity whilst simultaneously allowing for the enhanced signalling responses associated with low glycogen stores. Aside from additional energy per se, this hypothesis was based on a small body of literature demonstrating that branched chain amino acid supplementation (BCAAs) reduces central fatigue and enhances performance, as evidenced by decreased ratings of perceived exertion (RPE) (Blomstrand et al. 1997; Greer et al. 2007) and improved time to exhaustion and time-trial performance (Blomstrand et al. 1991; Crowe et al. 2006). Ergogenic mechanisms of action in these studies have been postulated to be due to increased plasma concentrations of BCAA relative to free tryptophan (f-Trp) such that f-Trp entry to the brain is reduced thereby suppressing the synthesis of the neurotransmitter 5-hydroxytryptamine (5-HT) and ultimately reducing central fatigue (Blomstrand 2006; Newsholme and Blomstrand 2006). Although we did not measure plasma amino acid concentrations, Watson et al. (2004) observed BCAA ingestion neither reduce RPE nor improve exercise capacity at 50 % \( \dot{V}{\text{O}}_{{ 2 {\text{max}}}} \) when commenced in a glycogen-depleted state, despite a fourfold reduction in the plasma concentration of f-TRP to BCAA. When taken together, both the present data and that of the aforementioned researchers suggest that essential amino acid provision offers no advantage to exercise capacity or subjective feelings of perceived exertion when exercise is commenced in a glycogen-depleted state.
A limitation of our chosen experimental design was that post-exercise biopsies were sampled at the point of exhaustion and not at matched time-points across both the PLA and PRO trials. Indeed, although we observed no significant differences in mean exercise capacity between trials (25 ± 10 and 26 ± 9 min for PLA and PRO, respectively, P = 0.61), it is important to consider individual differences in exercise capacity (range: −9 to 7 min difference between trials) and as such, differences in total exercise duration may have contributed to subtle differences in cell signalling responses. Nevertheless, because one of our experimental aims was to assess the effects of protein ingestion on exercise capacity, we felt that disrupting the subject from their natural flow of exercise (as well as including a brief rest period in order to perform a time-matched biopsy) followed by subsequent exercise until exhaustion may not have allowed us to appropriately address this experimental aim. Future studies may therefore benefit from adopting a time-matched biopsy approach.
In summary, we provide novel data by demonstrating that protein ingestion before, during and after exercise does not attenuate AMPK signalling in human skeletal muscle when exercise is commenced in a glycogen-depleted state. As such, we therefore consider the present data to be of practical implications for those athletes who deliberately incorporate the train-low compete-high approach into their training programmes as protein does not attenuate activation of a cell signalling cascade thought to contribute to the enhanced training response observed when restricting CHO before, during and after training. Further acute studies utilising a greater portfolio of measures of protein synthesis and degradation are now warranted as well as longitudinal studies investigating the effects of protein ingestion on training adaptations (and estimates of lean muscle mass) when in glycogen-depleted states.
References
Akerstrom TC, Birk JB, Klein DK, Erikstrup C, Plomgaard P, Pedersen BK, Wojtaszewski J (2005) Oral glucose ingestion attenuates exercise-induced activation of 5-AMP activated protein kinase in human skeletal muscle. Biochem Biophys Res Commun 342:949–955
Bartlett JD, Hwa-Joo C, Jeong TS, Louhelainen J, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B, Morton JP (2012) Matched work high-intensity interval running and continuous running induce similar increases in PGC1 mRNA, AMPK, p38 and p53 phosphorylation in human skeletal muscle. J Appl Physiol 112:1135–1143
Blomstrand E (2006) A role for branched-chain amino acids in reducing central fatigue. J Nutr 136:544–547
Blomstrand E, Saltin B (1999) Effect of muscle glycogen on glucose, lactate and amino acid metabolism during exercise and recovery in human subjects. J Physiol 514:293–302
Blomstrand E, Hassmen P, Ekblom B, Newsholme EA (1991) Administration of branched-chain amino acids during sustained exercise: effects on performance and on plasma concentration of some amino acids. Eur J Appl Physiol 63:83–88
Blomstrand E, Hassmén P, Ek S, Ekblom B, Newsholme EA (1997) Influence of ingesting a solution of branched-chain amino acids on perceived exertion during exercise. Acta Physiol Scand 159:41–49
Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2:92–98
Breen L, Philp A, Witard OC, Sarah R, Selby A, Smith K, Baar K, Tipton KD (2011) The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J Physiol 589:4011–4025
Burke LM (2010) Fueling strategies to optimize performance: training high or training low? Scand J Med Sci Sports 20:48–58
Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM (2008) Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol 104:1304–1312
Cluberton LJ, McGee SL, Murphy RM, Hargreaves M (2005) Effect of carbohydrate ingestion on exercise-induced alterations in metabolic gene expression. J Appl Physiol 99:1359–1363
Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, Phillips SM, Hawley JA (2011) Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol 111:1473–1483
Crowe MJ, Weatherson JN, Bowden BF (2006) Effects of dietary leucine supplementation on exercise performance. Eur J Appl Physiol 97:664–672
Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ (2010) Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor γ coactivator-1α mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol 10:1779–1790
Greer BK, Woodard JL, White JP, Arguello EM, Haymes EM (2007) Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. Int J Sport Nutr Exerc Metab 17:595–607
Hansen AK, Fischer CP, Plomgaard P, Andersen JL, Saltin B, Pedersen BK (2005) Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol 98:93–99
Hood DA (2009) Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab 34:465–472
Howarth KR, Moreau NA, Phillips SM, Gibala MJ (2009) Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol 106:1394–1402
Howarth KR, Phillips SM, MacDonald MJ, Richards D, Moreau NA, Gibala MJ (2010) Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J Appl Physiol 109:431–438
Hulston CJ, Venables MC, Mann CH, Martin C, Philp A, Baar K, Jeukendrup AE (2010) Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med Sci Sports Exerc 40:2046–2055
Hulston CJ, Wolsk E, Grøndahl TS, Yfanti C, Van Hall G (2011) Protein intake does not increase vastus lateralis muscle protein synthesis during cycling. Med Sci Sports Exerc 49:1635–1642
Irrcher I, Ljubicic V, Kirwan AF, Hood DA (2008) AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS One 3:3614
Jäger S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104:12017–12022
Jeukendrup AE, Wallis GA (2005) Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26:28–37
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801
Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ (2010) Acute endurance exercise increases the nuclear abundance of PGC-1alpha in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 298:912–917
Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ (2011) An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1alpha and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300:1303–1310
Mascher H, Ekblom B, Rooyackers O, Blomstrand E (2011) Enhanced rates of muscle protein synthesis and elevated mTOR signalling following endurance exercise in human subjects. Acta Physiol 202:175–184
McBride A, Ghilagaber S, Nikolaev A, Hardie DG (2009) The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab 9:23–34
Mettler S, Mitchell N, Tipton KD (2010) Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exer 42:326–337
Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnapolsky MA, Phillips SM (2009) Ingested protein dose response of muscle albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89:161–168
Morton JP, Croft L, Bartlett JD, Maclaren DPM, Reilly T, Evans L, McArdle A, Drust B (2009) Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J Appl Physiol 106:1513–1521
Morton JP, Robertson C, Sutton L, MacLaren DP (2010) Making the weight: a case study from professional boxing. Int J Sport Nutr Exerc Metab 20:80–85
Newsholme EA, Blomstrand E (2006) Branched-chain amino acids and central fatigue. J Nutr 136:274–276
Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ, Pilosky MA, Rood JC, Fielding RA, Young AJ (2011) Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr 94:809–818
Perry CGR, Lally J, Holloway GP, Heigenhauser GJF, Bonen A, Spriet LL (2010) Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588:4795–4810
Philp A, Chen A, Lan D, Meyer GA, Murphy AN, Knapp AE, Olfert M, McCurdy CE, Marcotte GR, Hogan MC, Baar K, Schenk (2011) Sirtuin 1 (SIRT1) deacteylase activity is not required for mitochondrial biogenesis or peroxisome proliferator activated receptor coactivator-1α (PGC1-α) deacetylation following endurance exercise. J Biol Chem 286:30561–30570
Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Neufer PD (2002) Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol 541:261–271
Pilegaard H, Osada T, Andersen LT, Helge JW, Saltin B, Neufer PD (2005) Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54:1048–1055
Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA et al (2005) Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol 569:223–228
Rose AJ, Alsted TJ, Jensen TE, Kobberø JB, Maarbjerg SJ, Jensen J, Richter EA (2009) A Ca(2+)-calmodulin-eEF2K-eEF2 signalling cascade, but not AMPK, contributes to the suppression of skeletal muscle protein synthesis during contractions. J Physiol 587:1547–1563
Van Hall G, Saltin B, Wagenmakers AJ (1999) Muscle protein degradation and amino acid metabolism during prolonged knee-extensor exercise in humans. Clin Sci 97:557–567
Van Loon LJC, Saris WH, Kruijshoop M, Wagenmakers AJ (2000) Maximizing postexercise muscle glycogen synthesis: carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am J Clin Nutr 72:106–111
Watson P, Shirreffs SM, Maughan RJ (2004) The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur J Appl Physiol 93:306–314
Wojtaszewski JFP, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA (2003) Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab 284:813–822
Yeo WK, Paton CD, Garnham AP, Burke LM, Carey AL, Hawley JA (2008) Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J Appl Physiol 105:1462–1470
Yeo WK, McGee SL, Carey AL, Paton CD, Garnham AP, Hargreaves M, Hawley JA (2010) Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp Physiol 95:351–358
Acknowledgments
This study was supported by a research grant from Science in Sport, UK. We also extend our appreciation to all the subjects who took part in the study for their efforts during demanding exercise protocols.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Martin Flueck.
Rights and permissions
About this article
Cite this article
Taylor, C., Bartlett, J.D., van de Graaf, C.S. et al. Protein ingestion does not impair exercise-induced AMPK signalling when in a glycogen-depleted state: implications for train-low compete-high. Eur J Appl Physiol 113, 1457–1468 (2013). https://doi.org/10.1007/s00421-012-2574-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2574-7