Abstract
Equivocal findings have been reported in the few studies that examined the impact of ambient temperature (T a) and hypohydration on cognition and dynamic balance. The purpose of this study was to determine the impact of acute exposure to a range of ambient temperatures (T a 10–40 °C) in euhydration (EUH) and hypohydration (HYP) states on cognition, mood and dynamic balance. Thirty-two men (age 22 ± 4 years, height 1.80 ± 0.05 m, body mass 85.4 ± 10.8 kg) were grouped into four matched cohorts (n = 8), and tested in one of the four T a (10, 20, 30, 40 °C) when EUH and HYP (−4 % body mass via exercise–heat exposure). Cognition was assessed using psychomotor vigilance, 4-choice reaction time, matching to sample, and grammatical reasoning. Mood was evaluated by profile of mood states and dynamic postural balance was tested using a Biodex Balance System. Thermal sensation (TS), core (T core) and skin temperature (T sk) were obtained throughout testing. Volunteers lost −4.1 ± 0.4 % body mass during HYP. T sk and TS increased with increasing T a, with no effect of hydration. Cognitive performance was not altered by HYP or thermal stress. Total mood disturbance (TMD), fatigue, confusion, anger, and depression increased during HYP at all T a. Dynamic balance was unaffected by HYP, but 10 °C exposure impaired balance compared to all other T a. Despite an increase in TMD during HYP, cognitive function was maintained in all testing environments, demonstrating cognitive resiliency in response to body fluid deficits. Dynamic postural stability at 10 °C appeared to be hampered by low-grade shivering, but was otherwise maintained during HYP and thermal stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Workplace accidents and injuries have been reported to increase in summer months (Vernon 1928; Kenefick and Sawka 2007) when fluid turnover is highest and individuals are more likely to become hypohydrated (acute body water deficit). However, the physiological or psychological factors contributing to this increase have not been fully explored. It is possible that thermal stress and/or hydration status might impair cognitive function, decision making skills, or postural balance, thereby affecting the ability to perform tasks and possibly contributing to accidents and injuries in the workplace. Potential cognitive and postural balance impairments may be related to factors such as increased perceived exertion, unpleasant thirst and thermal sensations, alterations in mood and overall discomfort commonly experienced with hypohydration (HYP) and/or exposure to extreme environments (Gagge et al. 1969; Ganio et al. 2011; Gopinathan et al. 1988; Lieberman et al. 2009). The effect of environmental temperature or hydration state on cognition, balance, and perceptual variables have been studied singly but have not been explored in concert, or across a range of environmental temperatures.
Most studies investigating the impact of HYP on cognitive function have reported significant alterations in cognition with body mass losses as low as 1–2 % (Cian et al. 2001; Gopinathan et al. 1988; Lieberman 2007; Ganio et al. 2011). However, due to differing methodologies used to achieve HYP and the type of cognitive tests administered, findings are inconsistent and may be confounded by factors such as exercise-related fatigue, elevated body core temperature due to environmental exposure, and learning effects due to repeated testing (Benton 2011). Recent reviews in the area of HYP and cognition (Grandjean and Grandjean 2007; Lieberman 2007) suggest that future research should examine the possible confounding and interacting effects of HYP procedures and testing environment. In addition, it has also been recommended that research focuses on cognitive tests with proven sensitivity to environmental and nutritional factors (Lieberman 2007) and that adequate pre-experimental training be employed to minimize potential learning effects (Grandjean and Grandjean 2007). To date no known study has systematically evaluated HYP and cognition in a range of environmental temperatures using established cognitive tests and well-trained volunteers, while allowing adequate recovery from the procedures and environment used to induce HYP.
The influence of HYP on standing balance has been studied previously (Derave et al. 1998; Gauchard et al. 2002; Patel et al. 2007) and findings are equivocal. Two investigations (Derave et al. 1998; Gauchard et al. 2002) reported a decline in the ability to maintain postural balance when volunteers were hypohydrated (−2 to −3 % of body mass). Conversely one investigation (Patel et al. 2007) observed no effect despite fluid losses ranging from 2 to 4 % body mass. Since balance testing occurred less than 30 min after exercise-induced fluid loss in these studies, the results may be confounded by local muscle fatigue (Derave et al. 1998; Gauchard et al. 2002; Patel et al. 2007). Additionally, it is possible that factors associated with HYP, such as orthostatic intolerance and cerebral blood flow (Carter et al. 2006; Wilson et al. 2006), could alter postural balance (Adolph and Associates 1947). Whether these hydration-related alterations manifest as changes in static or dynamic balance is not clear. Studies on the impact of environmental temperature on postural stability are also exceedingly limited. Mäkinen et al. (2005) demonstrated a decline in postural stability following acute and repeated cold (10 °C) exposure. However, the impact of environmental heat exposure on dynamic postural stability, alone or combined with HYP, has not been examined.
Therefore, the purpose of this study was to quantify the effects of moderate HYP (−4 % body mass) on cognitive performance, mood, and dynamic balance in volunteers well trained on cognitive and balance tasks, over a range of environmental temperatures from 10 to 40 °C. It was hypothesized that additive effects of environmental temperature and HYP would negatively impact cognitive performance, mood state and dynamic postural balance, with the largest impact in 10 °C and 40 °C environments. As cognitive function and postural balance are critical to successful and safe completion of various tasks, these findings have potential application to workers, military personnel, or athletes who work, train or compete in various environmental conditions when hypohydrated.
Methods
Subjects
Four groups of eight healthy, non-heat acclimated men (total n = 32; age 22 ± 4.2 years, height 1.80 ± 0.05 m, body mass 85.4 ± 10.8 kg) volunteered to participate in this investigation. Appropriate institutional review boards approved this study. Before participation, each volunteer attended briefings informing them of the purpose of the experiment and possible risks and signed a written informed consent document. Investigators adhered to the policies for the protection of human subjects as prescribed in US Army Medical research and Materiel Command Regulation 70-25. The research was conducted in adherence with the provisions of 45 Code of Federal Regulations Part 46.
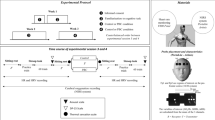
Testing took place over a 3-week period, with the first week used for training/familiarization in cognition and balance tasks, followed by euhydration (EUH) and HYP trials separated by 1 week in a counter-balanced design. EUH and HYP trial days began with exercise–heat exposure (3 h at 50 °C) with (EUH trial) and without (HYP trail) fluid replacement, followed by a 90-min break, then cognition and balance testing took place in the selected environment (10, 20, 30, or 40 °C).
Preliminary procedures and familiarization
One week of familiarization, preliminary testing and hydration assessment preceded the experimental trials. For five consecutive days during familiarization, volunteers consumed 2 l of sports drink in the evening in addition to normal food and fluid intake on the day preceding testing. On each subsequent morning, volunteers provided a first morning urine sample to measure urine specific gravity (USG). In addition, nude body mass was measured for each volunteer using a precision electronic scale (±0.05 kg, Mettler Toledo, Model WSI-600, Toledo, OH, USA) before breakfast and after voiding. The mean of the 5-day measures of body mass was used to establish a reliable baseline to determine EUH, confirmed by a USG < 1.02 and a variation in daily body mass of 1 % (Sawka et al. 2007).
During preliminary testing, volunteers performed five cognitive practice sessions (PRACT) in order to establish a baseline and to reduce training and learning effects. The day-to-day variability of scores provided the typical measurement noise against which trial scores could be compared when significant effects were found. Scores were compiled for each variable over the last 3 days of a 5-day practice period, and a coefficient of variation (%CV) was calculated for each volunteer. (Within-subjects %CV = (standard deviation PRACT/mean PRACT) × 100.) A mean %CV for all 32 volunteers was calculated and considered an a priori ‘zone of indifference’ for determining meaningful changes during trial days (Cheuvront et al. 2005), which are described in detail below. Each familiarization session took place in a ~22 °C, 20–30 % RH environment.
Cognitive testing occurred during each day of familiarization and on each trial day. The computer-based cognitive battery consisted of a psychomotor vigilance test (PVT; Dinges and Powell 1985), 4-choice reaction time test (4-Choice; Lieberman et al. 2002), matching to sample test (Match; Lieberman et al. 2002), grammatical reasoning (GR; Baddeley 1968), and the profile of mood states (POMS) questionnaire (McNair et al. 1971). The PVT and 4-choice tested reaction time (RT), while Match and GR assessed short-term spatial memory and grammar-based logical reasoning, respectively. These tests have been previously used and found to be sensitive to changes observed with HYP (Ganio et al. 2011; Armstrong et al. 2012), environmental exposure, and other physiological stresses such as sleep deprivation and military training (Lieberman et al. 2009). The POMS is a standardized inventory of self-reported mood states, with adjectives that factor into six mood sub-scales (tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/inertia, and confusion/bewilderment), which can be combined to determine TMD. Scores for TMD are calculated such that tension/anxiety, depression/dejection, anger/hostility, fatigue/inertia, and confusion/bewilderment are given a positive score while vigor/activity is assigned a negative score, then summed to form a global aggregate measure of mood. Higher TMD scores are associated with high levels of tension, depression, fatigue, and confusion and lower TMD scores are associated with an improved mood state.
On each of the 5 days of familiarization and on the days of experimental testing, volunteers additionally performed ‘Dynamic Balance’ tests on the Biodex Balance System (BBS; Biodex Medical Systems, Shirley, NY, #945-300) (Hinman 2000) after completing cognitive testing. This test is an indicator of dynamic body control and allows a quantitative measurement of the subject’s ability to control their center of gravity and balance using visual feedback via an LCD screen. During familiarization and experimental testing, each volunteer stood bilaterally on a circular platform. Foot placement on the platform was self-chosen by the volunteer during the first familiarization session and was maintained throughout familiarization and testing by lining up the volunteer’s feet with a pre-drawn grid on the platform. Each day of familiarization and testing consisted of three consecutive, 20 s trials with the stability of the platform set at level 2 (1 = least stable, 8 = most stable). After a short count down, the platform was released to the set level of instability. Familiarization and experimental trials were completed with eyes open so volunteers could receive feedback via an LCD screen depicting the movement of their center of gravity.
The LCD screen depicted the area of the platform represented by four concentric zones labeled A, B, C and D, with the A zone being the innermost zone. The goal of the ‘Dynamic Balance’ test was to hold the unstable platform in a level position for the duration of the 20 s test through anterior/posterior and medial/lateral movement of the feet and ankles. The LCD screen presented a cursor interfaced with the movement of the platform. Balance scores were given based on three different variables; time spent in region A (Time in A), mean deflection (MD), and overall stability index (OSI). Time in A was defined as the amount of test time the cursor was held within the A zone, where the platform is most stable. MD was defined as the average position of the volunteer in all motions throughout the test where a greater value indicates more motion and less dynamic stability. OSI was defined as the variance of the platform displacement from level, measured in degrees, where a greater value indicates more displacement and less balance stability. These three scores are the standard output of the dynamic balance test.
Experimental design and testing
Following familiarization, the 32 volunteers were grouped into 4 cohorts matched for aerobic fitness (n = 8 each; Table 1). Each cohort then performed experimental testing in one of the four environments: 10, 20, 30, and 40 °C while in EUH as well as HYPO (−4 % body mass) states. EUH and HYP trials took place in the same selected environment for each volunteer and were randomly assigned and separated by 1 week in a counter-balanced design. All trials occurred at the same time of day, and physical exercise was restricted for 24 h prior to testing. On the morning of each trial, nude body mass and USG were measured for comparison to the preceding week’s 5-day mean. In addition, a small blood sample was taken to measure plasma osmolality. Volunteers with a combination of at least two measures of EUH (USG < 1.02, plasma osmolality <290 mmol/kg, and nude body mass within 1 % of the 5-day mean) were considered euhydrated (Sawka et al. 2007) and met the criteria to initiate testing. Following hydration assessment, volunteers consumed a standardized breakfast of two nutritional bars (total of 540 kcal, 16 g fat, 94 g carbohydrate, 8 g protein) and 250 ml of water before entering the environmental chamber set at 50 °C, ~20 % relative humidity, 1.6 m/s air speed. Volunteers were semi-nude (shirtless with shorts, socks, and sneakers) for the duration of exercise–heat exposure and afternoon cognition and balance testing. Core temperature (T core) was measured during testing with a telemetric temperature sensor (VitalSense Jonah Ingestible Capsule; Minimitter inc., Bend, OR) inserted as a suppository 8–10 cm beyond the anal sphincter prior to exercise–heat exposure.
Exercise–heat exposure
Volunteers began a 3-h work/rest cycle which consisted of 30 min of walking on a treadmill at 1.34 m/s and 3.5 % grade, followed by 30 min of seated rest repeated three times. The purpose of light walking exercise was to initiate sweating and produce ~4 % HYP when fluids were not replaced, while the periods of seated rest allowed core temperature to remain below the laboratory safety cutoff of 39.5 °C for the duration of exposure. Body mass was measured every 30 min, and sweat volume and body mass losses were considered equivalent so that during trial EUH, volunteers drank 1 ml of 0.05 % NaCl solution to replace every 1 g of mass that was lost between each weigh-in. No fluids were replaced during trial HYP so that progressive fluid loss occurred over the 3-h heat exposure. A 90-min break followed heat exposure where volunteers showered, relaxed, and ate a small snack (granola bar; 140 kcal). The purpose of this break was to allow T core to return to pre-exercise heat exposure levels thus allowing assessment of T a and HYP effects independent of extreme changes in T core (Kenefick et al. 2009). Following the break, nude body mass was again measured and this value was compared to the pre-heat exposure value. In trial EUH, if body mass did not equal pre-heat exposure values, additional fluid was provided to maintain mass within 0.05 kg pre- to post-heat exposure. These fluids were consumed 20–30 min prior to entering the environmental chamber to begin testing. Similarly, in trial HYP, if volunteers exceeded −4.5 % body mass loss, fluid was provided to bring their net fluid loss to −4 % of pre-exercise body mass before cognitive testing began. At the conclusion of the break, a second blood sample was drawn to measure plasma osmolality.
Cognition and balance experimental testing
After the 90-min rest period, volunteers entered their selected environmental condition while EUH or HYP and were instrumented with skin thermistors while resting in the environmental chamber. Subjects then began a computer-based cognitive battery lasting approximately 30 min. Immediately upon completion of cognitive testing, volunteers performed the balance testing as described above. Upon completion of cognitive and balance testing, core and skin temperatures were measured and recorded. Skin temperature was assessed by thermistors (YSI, Yellow Springs, OH) at four different sites (left calf, thigh, forearm, and chest) and a weighted average (Ramanathan 1964) was used to calculate mean skin temperature. Volunteers were asked to rate their thermal sensation (TS) using an 8-point Likert scale with verbal anchors from 0 (unbearably cold) to 8 (unbearably hot). Thirst was assessed using a similar 8-point Likert scale with verbal anchors from 1 (not thirst at all) to 9 (very, very thirsty) (Gagge et al. 1969; Engell et al. 1987).
Statistical analysis
All cognitive, balance, physiological and perceptual variables were analyzed using a 2 × 4 mixed model ANOVA (repeated measures for hydration state within an environment; comparison among environments with different volunteers) and Newman–Keuls post hoc test when a significant F value was present. Significance was accepted at the p < 0.05 level. Sample size for a 2 × 4 mixed model ANOVA was calculated based on the desire to detect an effect size >1.0 for main and interaction effects. Eight subjects per group provided the necessary statistical power (>0.80) to see an effect of equal or greater magnitude to the typical measurement variation (%CV, coefficient of variation), assuming a modest autocorrelation parameter (0.50) between two repeated measures (hydration) when comparing four groups (environment) (Park and Schutz 1999).
In addition to conventional statistical analyses, all significant effects were compared to the magnitude of the variability of the test during training. Since all tests were practiced five times during training without any hydration or environment manipulations, the first 2 days were used for familiarization and the coefficient of variation (%CV) was calculated for each individual for the last 3 days of training. A mean CV was compiled for each test for all 32 volunteers for any variable where significant differences were found. Changes seen with environment and hydration manipulations were considered meaningful only if the magnitude of change exceeded the typical variability. All data are presented as mean ± standard deviation.
Results
Hydration
All volunteers were euhydrated prior to heat exposure, as their body mass was within 1 % of the 5-day average, plasma osmolality averaged ≤290 mmol/kg (EUH 289 ± 4, HYP 290 ± 4 mmol/kg), and USG was below 1.020 (Sawka et al. 2007). In the HYP trials, exposure to heat resulted in a −4.1 ± 0.5 %, −4.2 ± 0.2 %, −4.0 ± 0.3 % and −4.1 ± 0.5 % reduction in body mass in the 10, 20, 30 and 40 °C trials, while post-heat exposure plasma osmolality increased to 298 ± 6 mmol/kg during HYP. To maintain EUH during heat exposure for the subsequent EUH trials, volunteers drank 3.7 ± 0.6 l of 0.05 % NaCl and water solution and were fully hydrated prior to entering the environmental chamber to begin testing, with mean plasma osmolality 285 ± 5 mmol/kg.
Immediately prior to cognitive testing, T core values had recovered from exercise–heat exposure, as a result of the 90-min break. Although there was an elevation in core temperature (0.6 ± 0.3 °C) when HYP (main effect of hydration), absolute T core remained below 38 °C (Table 1). Overall skin temperatures and TS ratings within each environment were similar between the EUH and HYP trials, and were stable (±1.0 °C) within 15 min of entering the environmental chamber, which coincided (±5 min) with the start of cognitive testing. Skin temperature increased ~4.0 °C with each 10 °C increase in environment tested and TS was markedly different (p < 0.05) in each environment, with no interaction of hydration and environment (Table 1). Thirst ratings were significantly higher (p < 0.05) during HYP in all environments with no interaction effects between hydration and environment.
Cognitive testing
Regardless of the environment or hydration state, no effect was observed on reaction time during the PVT or the number of correct responses during the 4-choice, Match, and GR tests (Table 2). Additionally, no interaction effects of environment and hydration were observed.
POMS
There was a significant main effect of hydration state on TMD (p < 0.01; Fig. 1), with post hoc tests revealing significantly increased mood disturbance during HYP. TMD scores were 23 % higher during HYP compared with EUH, while the typical practice variation observed for TMD during training was 19.5 %. Main effects for hydration state were also observed for ratings of anger/hostility (p < 0.05), confusion/bewilderment (p < 0.05), depression/dejection (p < 0.05), and fatigue (p < 0.01). No significant main effects for hydration state were observed for vigor/activity and tension/anxiety. Additionally, no main effect of environment or interaction of environment/hydration state was observed (Table 3), nor were any trial order effects (p = 0.73).
Total mood disturbance (TMD) ratings during EUH and HYP trials (all environments combined). Higher TMD scores are associated with a worsened mood state and lower TMD scores are associated with an improved mood state. †Significant difference (p < 0.05) between EUH and HYP. Data are presented as mean ± standard deviation
Balance testing
A main effect of environment was found in all three balance variables (MD, Time in A, and OSI). Post hoc tests indicated significantly impaired balance at 10 °C compared to all other environments for MD, Time in A, and OSI (Table 4). For OSI (Fig. 2), scores at 10 °C were 85 % higher while scores were 96 % higher for MD (with a higher score indicating decreased stability for OSI and MD), and 23 % lower for Time in A (with a lower score indicating decreased stability) at 10 °C, respectively, compared to all other environments. As seen in Fig. 2, balance performance was similar at 20 °C, 30 °C, and 40 °C, while mean scores showed significant impairment and increased variability at 10 °C. No effect of hydration state was observed for MD and OSI (Table 4). However, a significant main effect of hydration was found for Time in A, with a slight improvement in performance during HYP. When viewed relative to the day-to-day variation of this test, the hydration-related change was not considered meaningful as the %CV for Time in A was 9.8 % and the mean difference between EUH and HYP for Time in A was only 6 %.
Discussion
This study is the first to examine the potential additive effects of HYP and acute exposure to a range of environmental temperatures (10–40 °C) on mood state, cognition, and dynamic balance. The experimental design mitigated the confounding effects of exercise–heat exposure used to induce HYP by providing a 90-min recovery period prior to cognitive and balance testing, and learning effects were minimized by training each volunteer with a week of practice sessions using well-established cognitive tests and dynamic balance assessment. The primary findings of this investigation were: (1) cognitive performance was not impaired by −4 % HYP or by acute thermal stress; (2) total mood scores and sub-scores of anger/hostility, confusion/bewilderment, depression/dejection, and fatigue were adversely affected by −4 % HYP but not affected by environment; (3) HYP did not alter dynamic balance, but balance was impaired in the 10 °C environment.
Several other investigations have also reported minimal or no effect of exposure to hot or cold environments on cognitive performance (Adam et al. 2008; Ramsey et al. 1975) and when hypohydrated (Adam et al. 2008; Patel et al. 2007; Szinnai et al. 2005). The small impacts of HYP on cognition reported by previous studies were often observed when testing occurred immediately after exercise–heat exposure (Patel et al. 2007), indicating a possible role of latent fatigue or hyperthermia (elevated body core temperature). Studies that found minimal changes in cognition following dehydration employed either an extensive recovery period following exercise/heat stress (Adam et al. 2008) or a passive model of fluid loss (Szinnai et al. 2005). Similarly, it has been posited that the duration of environmental exposure and resulting change in body core temperature play an important role in observed changes in cognition with thermal stress (Hancock and Vasmatzidis 2003). In the present study, the ample recovery period between exercise–heat exposure and cognitive testing allowed core temperature to return to near pre-heat exposure levels, and the acute thermal stress changed skin temperature and thermal comfort but did not alter basal body temperature. While there was a small increase in T core in all HYP trials, body temperatures remained below levels associated with hyperthermia-induced changes in cognitive function (Hocking et al. 2001).
Gagge et al. (1969) demonstrated the potential for thermal stimuli to produce sensory distraction related to skin and ambient temperatures, and mood disturbance with HYP has been well-documented in previous work (Ganio et al. 2011; Gopinathan et al. 1988; Patel et al. 2007; Armstrong et al. 2012). In the present study, volunteers reported elevated sensory distracters of thirst and uncomfortable thermal sensations in addition to large environment-induced changes in skin temperature and modest HYP-induced changes in core temperature. However, despite the negative influence of thirst and thermal discomfort, cognitive task performance was not affected. The ability to overcome the negative effects of stressors and maintain an effective level of performance is termed ‘cognitive resilience’ within the cognitive psychology literature. Sinclair and Mark (1995) reported that negative mood states tended to enlist greater effort and decisions made in these conditions were more often correct. This finding suggests that the negative mood reported by the volunteers in the present study may have facilitated greater effort and detailed attention such that cognition was unaffected by the challenges of thermal stress and HYP. However, it is also possible that marginal, but statistically significant changes in cognition seen in previous work (Ganio et al. 2011; Armstrong et al. 2012) were not observed due to the relatively small number of volunteers in each environmental cohort.
Dynamic balance was not altered by HYP (−4 %) achieved via exercise in the heat. Other investigations (Derave et al. 1998; Gauchard et al. 2002; Patel et al. 2007) have demonstrated equivocal results regarding the effects of HYP on static balance. Two investigations (Derave et al. 1998; Gauchard et al. 2002) demonstrated that exercise without fluid replacement, as compared to exercise with fluid replacement, decreased static balance ability. However, Patel et al. (2007) demonstrated that HYP −2.5 ± 0.6 % (range −1.7 to −4.2 %) did not affect static or dynamic balance ability when tested 25 min after HYP procedures. In accordance with the results of Patel et al. (2007), we did not observe any impact of hydration state on measures of dynamic balance, despite a greater average level of HYP (−4 %) and a longer recovery period (90 min) following exercise–heat exposure to minimize the effect of recent exercise, which may have confounded previous work through local muscle fatigue (Derave et al. 1998; Gauchard et al. 2002; Patel et al. 2007). The single significant effect observed was a 6 % improvement in Time in A during HYP, but as this fell within the typical measurement noise (%CV) established during training, it was not considered to be a meaningful difference.
Postural balance did decline in 10 °C compared to the 20–40 °C conditions. These results are in agreement with other investigations that have reported that exposure to cold environments (10 °C) alone (Makinen et al. 2005), or in combination (Cymerman et al. 2002) with other harsh conditions (4 °C, mild hypoxia, hypercapnia), resulted in a decrease in postural stability. The decline in postural stability in the 10 °C environment may have been the result of low-level shivering observed by investigators and reported by volunteers during the balance tasks. These involuntary muscle contractions added a greater degree of difficulty to the balance challenge thus negatively impacting the ability to maintain postural stability. In addition, previous investigations (Magnusson et al. 1990a, b; Stal et al. 2003) have demonstrated that when feet are severely cooled, mechanoreceptors become negatively affected which can result in a decrease in postural balance in cold environments.
In conclusion, HYP significantly increased TMD in all environments, with increases in fatigue, confusion, anger, and depression. Despite thirst, thermal discomfort, alterations in skin temperature, and adverse mood, cognition and balance were unaffected by HYP or thermal stress (20–40 °C) suggesting that some degree of cognitive resilience compensated for these negative distractions.
References
Adam GE, Carter R III, Cheuvront SN et al (2008) Hydration effects on cognitive performance during military tasks in temperate and cold environments. Physiol Behav 93:748–756
Adolph EF and Associates (1947) Physiology of man in the desert. Intersciences, Inc, New York
Armstrong LE, Ganio MS, Casa DJ et al (2012) Mild dehydration affects mood in healthy young women. J Nutr 142:382–388
Baddeley AD (1968) A 3 minute reasoning test based on grammatical transformation. Psychon Sci 10:341–342
Benton D (2011) Dehydration influences mood and cognition: a plausible hypothesis? Nutrients 3:555–573
Carter R 3rd, Cheuvront SN, Vernieuw CR, Sawka MN (2006) Hypohydration and prior heat stress exacerbates decreases in cerebral blood flow velocity during standing. J Appl Physiol 101(6):1744–1750
Cheuvront SN, Carter R, Castellani JW et al (2005) Hypohydration impairs endurance exercise performance in temperate but not cold air. J Appl Physiol 99:1972–1976
Cian C, Barraud PA, Melin B et al (2001) Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol 42:243–251
Cymerman A, Young AJ, Francis TJ et al (2002) Subjective symptoms and postural control during a disabled submarine simulation. Undersea Hyperb Med 29:204–215
Derave W, De CD, Bouckaert J et al (1998) The influence of exercise and dehydration on postural stability. Ergonomics 41:782–789
Dinges DF, Powell JW (1985) Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput 17:652–655
Engell DB, Maller O, Sawka MN et al (1987) Thirst and fluid intake following graded hypohydration levels in humans. Physiol Behav 40:229–236
Gagge AP, Stolwijk JA, Saltin B (1969) Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res 2:209–229
Ganio MS, Armstrong LE, Casa DJ et al (2011) Mild dehydration impairs cognitive performance and mood of men. Br J Nutr 106:1535–1543
Gauchard GC, Gangloff P, Vouriot A et al (2002) Effects of exercise-induced fatigue with and without hydration on static postural control in adult human subjects. Int J Neurosci 112:1191–1206
Gopinathan PM, Pichan G, Sharma VM (1988) Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health 43:15–17
Grandjean AC, Grandjean NR (2007) Dehydration and cognitive performance. J Am Coll Nutr 26:549S–554S
Hancock PA, Vasmatzidis I (2003) Effects of heat stress on cognitive performance: the current state of knowledge. Int J Hyperthermia 19:355–372
Hinman M (2000) Factors affecting reliability of the Biodex Balance System: a summary of four studies. J Sport Rehabil 9:240–252
Hocking C, Silberstein RB, Lau WM et al (2001) Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol A Mol Integr Physiol 128:719–734
Kenefick RW, Sawka MN (2007) Hydration at the work site. J Am Coll Nutr 26:597S–603S
Kenefick RW, Ely BR, Cheuvront SN et al (2009) Prior heat stress: effect on subsequent 15-min time trial performance in the heat. Med Sci Sports Exerc 41:1311–1316
Lieberman HR (2007) Hydration and cognition: a critical review and recommendations for future research. J Am Coll Nutr 26:555S–561S
Lieberman HR, Tharion WJ, Shukitt-Hale B et al (2002) Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Sea-Air-Land. Psychopharmacology 164:250–261
Lieberman HR, Castellani JW, Young AJ (2009) Cognitive function and mood during acute cold stress after extended military training and recovery. Aviat Space Environ Med 80:629–636
Magnusson M, Enbom H, Johansson R et al (1990a) Significance of pressor input from the human feet in anterior-posterior postural control. The effect of hypothermia on vibration-induced body-sway. Acta Otolaryngol 110:182–188
Magnusson M, Enbom H, Johansson R et al (1990b) Significance of pressor input from the human feet in lateral postural control. The effect of hypothermia on galvanically induced body-sway. Acta Otolaryngol 110:321–327
Makinen TM, Rintamaki H, Korpelainen JT et al (2005) Postural sway during single and repeated cold exposures. Aviat Space Environ Med 76:947–953
McNair DM, Lorr M, Droppleman LF (1971) Profile of mood states manual. Educational and Industrial Testing Service, San Diego
Park I, Schutz RW (1999) “Quick and Easy” formulae for approximating statistical power in repeated measures ANOVA. Meas Phys Education Exerc Sci 4:249–270
Patel AV, Mihalik JP, Notebaert AJ et al (2007) Neuropsychological performance, postural stability, and symptoms after dehydration. J Athl Train 42:66–75
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Ramsey JD, Dayal D, Ghahramani B (1975) Heat stress limits for the sedentary worker. Am Ind Hyg Assoc J 36:259–265
Sawka MN, Burke LM, Eichner ER et al (2007) American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39:377–390
Sinclair RC, Mark MM (1995) The effects of mood state on judgemental accuracy: processing strategy as a mechanism. Cogn Emot 9:417–438
Stal F, Fransson PA, Magnusson M et al (2003) Effects of hypothermic anesthesia of the feet on vibration-induced body sway and adaptation. J Vestib Res 13:39–52
Szinnai G, Schachinger H, Arnaud MJ et al (2005) Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol 289:275–280
Vernon HM (1928) Industrial fatigue in relation to atmospheric conditions. Physiol Rev 8:130–150
Wilson TE, Cui J, Zhang R, Crandall CG (2006) Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291(5):R1443–R1448
Acknowledgments
The authors would like to thank Mary Pardee, Laura Palombo, and Michael Stanger for their technical assistance. We also thank the 32 research volunteers who participated in this study. The opinions or assertions contained herein are the private views of the authors and should not be construed as official or reflecting the views of the Army the Department of Defense. The experiments comply with the present laws of the country in which they were performed.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fausto Baldissera.
Rights and permissions
About this article
Cite this article
Ely, B.R., Sollanek, K.J., Cheuvront, S.N. et al. Hypohydration and acute thermal stress affect mood state but not cognition or dynamic postural balance. Eur J Appl Physiol 113, 1027–1034 (2013). https://doi.org/10.1007/s00421-012-2506-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2506-6