Abstract
To determine if there is a sex dimorphism in the skeletal muscle signaling response to sprint exercise, 17 men and ten women performed a 30-s Wingate test. Muscle biopsies were taken before, immediately after the exercise and at 30 and 120 min during the recovery period. Thr172-AMPKα, Ser221-ACCβ, Thy705-STAT3, Thr202/Thy204-ERK1/2 and Thr180/Thy182-p38MAPK phosphorylation responses to sprint exercise were not statistically different between men and women. AMPKα phosphorylation was enhanced fourfold 30 min after the sprint exercise in males and females (P < 0.01). ACCβ phosphorylation was enhanced by about threefold just after the sprint test exercise and 30 min into the recovery period in males and females (P < 0.01). STAT3 phosphorylation was increased 2 h after the Wingate test compared to the value observed right after the end of the exercise (P < 0.05), and 30 min after the Wingate test there was a 2.5-fold increase in ERK1/2 phosphorylation, compared to both the pre-exercise and to the value observed right after the Wingate test (both, P < 0.05). In conclusion, the skeletal muscle signaling response to a single bout of sprint exercise mediated by AMPK, ACC, STAT3, ERK and p38MAPK is not statistically different between men and women. Marked increases in AMPKα, ACCβ, STAT3 and ERK phosphorylation were observed after a single 30-s all-out sprint (Wingate test) in the vastus lateralis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sprint exercise alters the energy charge of cell (Chen et al. 2000), the redox state (Cuevas et al. 2005; Kang et al. 2009), intracellular Ca++ concentrations (Ortenblad et al. 2000), metabolites (Cheetham et al. 1986; Gaitanos et al. 1993; Greenhaff et al. 1994; McKenna et al. 1993) and electrolytes (Harmer et al. 2000). This leads to the activation of several signaling cascades, particularly those involved in the regulation of metabolism and the response to cellular stress in the skeletal muscle (Chen et al. 2000; Gibala et al. 2009; Guerra et al. 2010; Treebak et al. 2007). Despite sex differences in the metabolic response to endurance (Lamont et al. 2003; Tarnopolsky et al. 1990; Zehnder et al. 2005) and sprint exercise (Esbjornsson et al. 2009), and despite that a significant sexual dimorphism exists in neuroendocrine, metabolic and cardiovascular counterregulatory responses to exercise in humans (Davis et al. 2000; Esbjornsson-Liljedahl et al. 1999, 2002; Esbjornsson et al. 2009), no single study has determined whether a sex dimorphism in skeletal muscle signaling response to sprint exercise exists in humans.
One of the main signaling pathways activated by sprint exercise in human skeletal muscle is AMP-activated protein kinase (AMPK). We have recently shown that a single 30-s sprint elicits a fourfold increase in AMPK phosphorylation 30 min after the end of the sprint (Guerra et al. 2010). The level of AMPK phosphorylation does not seem to be increased with repeated 30-s sprints (Gibala et al. 2009; Guerra et al. 2010). Phosphorylation and activation of AMPK is mainly regulated by the AMP/ATP ratio (Hardie 2003). Since women experience a smaller ATP reduction with repeated sprint exercise than men (Esbjornsson-Liljedahl et al. 2002), we hypothesized that AMPK phosphorylation in response to a single sprint exercise could be also attenuated in women compared to men, as previously reported during submaximal prolonged exercise (Roepstorff et al. 2006).
Acetyl-coenzyme A carboxylase β (ACCβ) phosphorylation (a downstream target for AMPK) is also increased after a single 30-s sprint (Ruderman et al. 1999). The latter causes a reduction of malonyl-coenzyme A facilitating fatty acids flux into the mitochondria (Ruderman et al. 1999). In men, ACCβ phosphorylation has been reported immediately after a 30-s sprint (Birk and Wojtaszewski 2006; Gibala et al. 2009; Guerra et al. 2010). Acetyl-coenzyme A carboxylase β phosphorylation during sprint exercise may be caused by AMPK-dependent and -independent mechanisms (Guerra et al. 2010; Jorgensen et al. 2004; Sakamoto et al. 2005). It remains unknown if sex differences exist in the sprint exercise-induced ACCβ phosphorylation.
Since extracellular signal-regulated kinase (ERK1/2) and p38-mitogen activated protein kinase (MAPK) signaling pathways may be activated by changes in the redox state (Kang et al. 2009; Torres 2003), we also hypothesized that sprint exercise, which causes marked oxidative stress (Cuevas et al. 2005; Kang et al. 2009; Powers et al. 2010), could elicit activation of ERK1/2 and p38MAPK signaling pathways. In fact, ERK1/2 and p38MAPK are activated during submaximal exercise in men (Egan et al. 2010; Little et al. 2010; Richter et al. 2004; Widegren et al. 2000). However, little is known about the responses of ERK1/2 and p38MAPK to sprint exercise (Gibala et al. 2009).
Compared to men, women have higher serum leptin concentrations and increased leptin receptors in their skeletal muscles (Guerra et al. 2008). Leptin promotes fat oxidation (Galgani et al. 2010). Thus, women may respond more easily to exercise-induced changes in circulating leptin concentrations. The leptin response to sprint exercise has not been studied. We decided to determine if sex differences in the leptin response could explain differences in skeletal muscle signaling to sprint exercise through the janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) cascade, which is activated by the binding of leptin to the leptin receptor in skeletal muscle (Bjorbaek and Kahn 2004). In rodent skeletal muscle, leptin also activates the ERK1/2 and p38MAPK signaling pathways (Maroni et al. 2003, 2005) and induces AMPK phosphorylation (Minokoshi et al. 2002). Thus, we also examined whether a potentially different between-sex leptin response to sprint exercise was associated with differences in ERK1/2, p38MAPK and AMPK phosphorylation in response to sprint exercise.
Therefore, the main aim of this study was to determine if there was a sex difference in muscle signaling in response to a single sprint exercise and to determine whether this difference could be explained by sex-specific changes in circulating leptin concentrations.
Materials and methods
Materials
The complete protease inhibitor cocktail was obtained from Roche Diagnostics (Mannheim, Germany). All the primary antibodies used were from Cell Signaling Technology (Danvers, MA, USA) except for the polyclonal rabbit antiphospho-acetyl CoA carboxylase (Ser79) antibody that was obtained from Upstate Biotechnology (Lake Placid, NY, USA). The secondary HRP-conjugated goat anti-rabbit antibody was from Jackson Immuno Research (West Grove, PA, USA). The Hybond-P transfer membranes and the ECL plus Western Blotting Detection System were from Amersham Biosciences (Little Chalfont, Buckinghamshire, UK). The ChemiDoc XRS System and the image analysis software Quantity One© were obtained from Bio-Rad Laboratories (Hemel Hempstead, Hertfordshire, UK).
Subjects
A total of 17 healthy male (age 24.4 ± 4.0 years, height 176.5 ± 7.1 cm, body mass 79.5 ± 10.1 kg, body fat 18.0 ± 6.2%) and ten healthy female physical education students (age 25.2 ± 3.9 years, height 160.7 ± 5.5 cm, body mass 57.0 ± 6.7 kg, body fat 26.3 ± 3.5%) agreed to participate in this investigation (Table 1). All women were eumenorrheic, and four were taking contraceptive pills. Before volunteering, subjects were given full oral and written information about the course of the study and possible risks associated with participation. Written consent was obtained from each subject. The study was performed in accordance with the Helsinki Declaration and approved by the Ethical Committee of the University of Las Palmas de Gran Canaria.
General procedures
The body composition of each subject was determined by DXA (Hologic QDR-1500, Hologic Corp., software version 7.10, Waltham, MA) as described elsewhere (Perez-Gomez et al. 2008; Serrano-Sanchez et al. 2010). Then, subjects reported to the laboratory on two different days to perform familiarization Wingate tests (Monark 818E, Monark AB, Vargerg, Sweden). On the experimental day, subjects reported to the laboratory at 8.00 a.m. after an overnight fast and an antecubital vein was catheterized. After 10 min of rest in the supine position, a 20-ml blood sample was withdrawn and used to measure serum leptin. Then a muscle biopsy was obtained from the middle portion of the vastus lateralis muscle using the Bergstrom’s technique with suction, as described elsewhere (Guerra et al. 2011a). Three minutes after the resting muscle biopsy and blood sample, the subject performed a 30-s Wingate test (Calbet et al. 1997, 2003). No warmup was allowed prior to the start of the Wingate test. Stop start Wingate tests were performed with braking loads equivalent to 10 and 8% of body weight for men and women, respectively (Calbet et al. 1997, 2003). Previous studies have shown that these braking forces are optimal to allow maximal peak power output during the Wingate test in physical education students (Bar-Or 1987; Calbet et al. 1997). Peak power output was calculated as the highest work output performed during a 1-s interval and mean power output from the average work performed during the 30 s.
Right after the Wingate test, another muscle biopsy and a blood sample were obtained. The time needed to obtain and freeze the muscle biopsies immediately after the Wingate test was always below 30 s in all cases. To reduce skin damage, only two 5-mm skin scissions were performed, one in each thigh. To avoid injury-triggered activation of p38MAPK or ERK1/2, the muscle biopsies were obtained at least 3 cm apart by changing the direction of the needle right after crossing the fascia (Guerra et al. 2011a). During the recovery period, additional muscle biopsies and blood samples were obtained at 30 and 120 min, while the subjects remained seated. During this period, they were allowed to drink only water. The muscle specimens were cleaned to remove any visible blood, fat or connective tissue. Then the muscle tissue was immediately frozen in liquid nitrogen and stored at −80°C for later analysis.
Blood lactate
Blood lactate concentration was determined in capillary blood obtained from the earlobe hyperemized with Finalgon®, prior to the start of the sprint and at 3, 5, 7 and 10 min into the recovery period, using a Lactate Pro analyzer (Arkay, JA).
Western blot analysis
Muscle protein extracts were prepared as described previously (Guerra et al. 2007) and total protein content was quantified using the bicinchoninic acid assay (Smith et al. 1985). Equal amounts of protein (50 μg) from each sample were subjected to immunoblotting protocol as described previously (Guerra et al. 2007). To determine Thr172-AMPKα, Ser221-ACCβ, Tyr705-STAT3, Thr202/Tyr204-ERK1/2 and Thr180/Tyr182-p38MAPK phosphorylation levels, antibodies directed against the phosphorylated and total form of these kinases were used. All antibodies were diluted in 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 (TBS-T) (BSA-blocking buffer). Antibody-specific labeling was revealed by incubation with an HRP-conjugated goat anti-rabbit antibody (1:20,000) or an HRP-conjugated donkey anti-mouse (1:10,000) antibody, both diluted in TBS-T with 5% blotting grade blocker non-fat dry milk (blotto-blocking buffer). Specific bands were visualized with the ECL chemiluminescence kit (Amersham Biosciences) using the ChemiDoc XRS system (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed with the image analysis program Quantity one© (Bio-Rad laboratories, Hercules, CA, USA). The densitometry analysis was carried out immediately before saturation of the immunosignal. For immunosignal quantification, band densities were normalized to the values obtained from the biopsies taken immediately before the start of the sprint. Data was represented as a percentage of immunostaining values obtained for the phosphorylated form of each kinase relative to those obtained for, respectively, total form. Samples pertaining to the same subject were run together in the same gel.
Leptin assays
Serum leptin was determined by enzyme-linked immunosorbent assay (ELISA) (ELx800 Universal Microplate Reader, Bioteck Instruments Inc, Vermont, USA), using reagent kits from Linco Research (#EZHL-80SK, Linco Research St. Charles, Missouri, USA) and following the manufacturer’s instructions. The sensitivity of the total leptin assays was 0.05 ng/mL. The intra-assay coefficient variation was 3.8%, and the inter-assay coefficient of variation was 4.4%.
Statistical analysis
Variables were checked for normal distribution by using the Shapiro–Wilk’s test and for equality of variances with the Levene’s test. When necessary, the analysis was done on logarithmically transformed data. For between-groups comparisons, the individual responses were normalized to the level of phosphorylation observed just before the start of the Wingate test. A mixed-model ANOVA with repeated measures over time and one factor (sex) with two levels (males vs. females) was used to compare the responses with the value just before the start of the Wingate test, using values normalized to the level of phosphorylation observed just before the start of the Wingate test. When there was a significant sex by time interaction, intra-group effects were tested using one-way ANOVA separately in each group, and pairwise comparisons were carried out using the Holm–Bonferroni method. Between-group differences at specific time points were analyzed with unpaired t tests, which were adjusted for multiple comparisons with the Holm–Bonferroni method. The relationship between variables was determined using linear regression analysis. Values are reported as the mean ± standard error of the mean (unless otherwise stated). P < 0.05 was considered to be significant. Statistical analysis was performed using SPSS v.15.0 for Windows (SPSS Inc., Chicago, IL).
Results
Body composition, peak power output (P max) and mean power output (P mean) in the Wingate test are reported in Table 1. Both genders were comparable in age, but women were smaller and had lower body mass and a higher percentage of body fat compared to men (all, P < 0.01). Men had higher performance in the Wingate test. However, when P max was expressed relative to the lean mass of the lower extremities not significant between-sex differences were observed (Table 1). The blood lactate responses to the Wingate test were similar in males and females (time × sex interaction P = 0.74) and the area under the curve as well (110 ± 14 and 103 ± 8 mM min, in men and women, respectively, P = 0.22) (Table 2).
Serum leptin concentrations
Serum leptin concentrations were higher in women compared to men at all time points. Compared to pre-exercise values, 2 h after exercise, leptin concentration was decreased in men by 27% (P < 0.01) and women by 13% (P < 0.01) (time × sex interaction P = 0.81, for the relative changes) (Table 3). There was no relationship between the lactate area under the curve and the leptin area under the curve (r = −0.19, P = 0.33, n = 27). However, the leptin area under the curve tended to be inversely associated with the mean power output per kg of lean mass (r = –0.35, P = 0.07, n = 27).
Skeletal muscle signaling response to sprint exercise
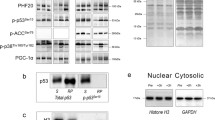
AMPKα, ACCβ, STAT3, ERK1/2 and p38MAPK phosphorylation responses to sprint exercise were not statistically different between men and women. Compared to pre-exercise values, AMPKα phosphorylation was enhanced fourfold 30 min after the sprint exercise in males and females (from 100 ± 11 to 437 ± 101%, P < 0.01; time × sex interaction, P = 0.49) (Fig. 1a). The ACCβ phosphorylation was enhanced by about fourfold just after the sprint test exercise and 30 min into the recovery period in males and females (from 100 ± 10 to 319 ± 53% and to 285 ± 41%, P < 0.01; time × sex interaction P = 0.25) (Fig. 1b). STAT3 phosphorylation was highly variable [as previously reported (Trenerry et al. 2007)], being significantly increased 2 h after the Wingate test compared to the value observed right after the end of the exercise (P < 0.05) (Fig. 2). Likewise 30 min after the Wingate test, there was a 2.5-fold increase in ERK1/2 phosphorylation, compared to both the pre-exercise and to the value observed right after the Wingate test (both, P < 0.05) (Fig. 3a).
Levels of Thr172-AMPKα (a) and Ser221-ACCβ (b) phosphorylation before and after a Wingate test in men (black bars) and women (gray bars). Values were normalized to the average obtained immediately before the sprint exercise (R), which were assigned a value of 100%. *P < 0.05 versus rest (R). a P = 0.49, time × sex interaction. b P = 0.14, time × sex interaction. N = 17 for the male group and n = 10 for the female group. The 30th min Ser221-ACCβ phosphorylation value would have been considered larger in men compared to women (P = 0.036) had the statistical comparison not been corrected for multiple comparisons. Thus, we cannot rule out a type II error at this time point
Levels of Thy705-STAT3 phosphorylation before and after a Wingate test in men (black bars) and women (gray bars). Values were normalized to the average obtained immediately before the sprint exercise (R), which were assigned a value of 100%. $ P < 0.05 versus immediately after exercise (0). P = 0.32, time × sex interaction. N = 17 for the male group and n = 10 for the female group. Statistical analysis was performed with logarithmically transformed data
Levels of Thr202/Thy204-ERK1/2 (a) and Thr180/Thy182-p38MAPK (b) phosphorylation before and after a Wingate test in men (black bars) and women (gray bars). Values were normalized to the average obtained immediately before the sprint exercise (R), which were assigned a value of 100%.*P < 0.05 versus rest (R). $ P < 0.05 versus immediately after exercise (0). a P = 0.12, time × sex interaction. b P = 0.42, time × sex interaction. N = 17 for the male group and n = 10 for the female group
No significant changes in p38MAPK phosphorylation were observed in response to the Wingate test in either group (all, time × sex interaction P = NS) (Fig. 3b).
The mean power developed per kg of lower extremities lean mass was linearly associated to the logarithm of the 30-min STAT3 phosphorylation response (r = 0.58, P < 0.01, n = 27, Fig. 4). A similar trend was observed for ERK1/2 phosphorylation (r = 0.31, P = 0.11, n = 27) (Table 4).
Discussion
In this investigation, we examined AMPK, MAPK/ERK and STAT3 muscle signaling pathways in response to a 30-s all-out sprint test (Wingate test) in men and women. The signaling response was not different between sexes. We have shown that sprint exercise increases AMPK phosphorylation at 30 min after the Wingate test and increases ACCβ phosphorylation immediately after and 30 min later, without significant differences between men and women. These results are in agreement with the study in men of Guerra et al. (2010) and support the idea that ACC phosphorylation in response to exercise is, at less in part, independent of AMPK activation (Dzamko et al. 2008; Jorgensen et al. 2004). In agreement with the studies performed in men by Gibala et al. (2009) and Guerra et al. (2010, 2011b), we did not observe AMPKα phosphorylation immediately after the 30-s sprint. We also showed that 2 h after a single sprint exercise, AMPKα phosphorylation returned to pre-exercise values.
The influence of gender on AMPKα phosphorylation in response to exercise has been only studied during endurance exercise. Roepstorff et al. (2006) reported lower increase of AMPKα phosphorylation in women compared to men after 90 min of bicycle exercise at 60% of VO2max. The sex difference in muscle AMPK activation with exercise was explained by an increase in muscle free AMP, AMP/ATP ratio and creatine in men but not in women. Although nucleotides were not measured in the present investigation, previous studies have failed to show between-sex significant differences in ATP use during a single sprint. The latter agrees with the similar peak power output developed during the Wingate test by men and women when normalized for the lean mass of the lower extremities (Perez-Gomez et al. 2008). However, Esbjornsson-Liljedahl et al. (2002) showed that women possessed a faster recovery of ATP via reamination of inosine monophosphate (IMP). Despite the latter, no between-sex differences in AMPKα phosphorylation or its downstream kinase ACCβ were observed 30 and 120 min after the Wingate test. In agreement, it has been reported that men and women experience similar changes in VO2max, peak and mean power output, and substrate oxidation in response to 6 days of low-volume high-intensity sprint training (four Wingate tests on days 1 and 2, five on days 3 and 4, and six on days 5 and 6) (Astorino et al. 2011).
The STAT3 phosphorylation response to exercise has been studied only in men (Boonsong et al. 2007; Trenerry et al. 2007). No significant changes in STAT3 phosphorylation were found after 90 min of leg cycling exercise (Boonsong et al. 2007). However, Trenerry et al. (2007) reported increased STAT3 phosphorylation 2 h after resistance exercise (leg extension: 3× 12RM). The latter agrees with the results obtained in the present investigation, where STAT3 phosphorylation occurred 2 h after the sprint. In this study, there was an association between the mean power developed per kg of lower extremities lean mass and STAT3 phosphorylation 30 min after the Wingate test. Combining the present results with those reported by Trenerry et al. (2007), it may be suggested that STAT3 phosphorylation is elicited by all-out sprint or weightlifting exercise, i.e., exercise modalities inducing fatigue and marked accumulation of metabolites (Gorostiaga et al. 2010; Jones et al. 1985). STAT3 phosphorylation after intense exercise is accompanied by translocation to the nucleus (Trenerry et al. 2007) and increased expression of the STAT3-regulated genes [interleukin-6 (IL-6), JUNB, c-MYC, c-FOS and suppressor of cytokine signaling (SOCS) 3], which likely play a role in the adaptation to high-intensity exercise (Trenerry et al. 2007, 2008).
In agreement with our hypothesis, ERK1/2 phosphorylation was increased 30 min after the sprint without significant differences between men and women. Similar increases in ERK1/2 phosphorylation have been reported by others in men after endurance exercise (Creer et al. 2005; Deldicque et al. 2008a; Goodyear et al. 1996; Widegren et al. 1998, 2000; Yu et al. 2001) and after resistance exercise in men (Deldicque et al. 2008a; Richter et al. 2004; Williamson et al. 2003) and overweight women (Harber et al. 2008). On the other hand, Richter et al. (2004) found that ERK1/2 phosphorylation was more marked as exercise intensity increased. In agreement, a trend for a liner relationship between the mean power developed per kg of lower extremities lean mass and the 30-min ERK1/2 phosphorylation response was observed in the present study. Nevertheless, increased ERK1/2 phosphorylation does imply necessarily more enzymatic activity (Richter et al. 2004).
p38MAPK phosphorylation is increased after endurance exercise in men (Aronson et al. 1997; Yu et al. 2001), resistance exercise in men (Deldicque et al. 2008b) and overweight women (Harber et al. 2008), and high-intensity intermittent exercise in men (Cochran et al. 2010).
Gibala et al. (2009) did not observe changes in p38MAPK phosphorylation immediately after a single Wingate test. The present investigation confirms these findings and shows that the level of p38MAPK phosphorylation remains unchanged during the next 2 h after a single sprint. However, a 30% increase in p38MAPK phosphorylation was reported by Gibala et al. (2009) after four repeated Wingate tests interspaced with 4-min rest periods. p38MAPK phosphorylation in response to high-intensity exercise may be modulated by energy availability (Cochran et al. 2010), and greater perturbation of the cellular energy status than that elicited by a single sprint may be necessary to elicit p38MAPK phosphorylation.
This study presents the first measurements of the serum leptin concentration changes in response to a single 30-s all-test (Wingate test) in men and women. The present investigation reveals that serum leptin concentration is not altered immediately after a 30-s sprint exercise, but decreases during the recovery period; this effect in relative terms is essentially similar in men and women. Although women had higher leptin concentrations than men during the recovery period and despite the fact that women have higher protein expression of leptin receptors (Guerra et al. 2008), no sex differences in the STAT3 phosphorylation after the Wingate test were observed. Moreover, despite the reduction of circulating leptin concentrations 2 h after the completion of the Wingate test, no reduction in STAT3 phosphorylation was observed, implying that other factors should have contributed to maintain skeletal muscle STAT3 phosphorylation when leptin was reduced. This reduction in serum leptin concentration could be explained by the exercise effect (Kraemer et al. 2002), via a β-adrenergic mediated stimulation (Couillard et al. 2002) through posttranscriptional mechanisms (Ricci et al. 2005). However, we cannot rule out some influence of fasting (Boden et al. 1996; Zhang et al. 2002).
Performance level and muscle signaling
Men achieved higher performance in the Wingate test. However, when P max was expressed relative to the lean mass of the lower extremities, men and women attained similar values as previously shown (Perez-Gomez et al. 2008). However, after normalization for the lean mass of the lower extremities, P mean was 6% higher in men compared to women. The sex difference in mean power output after normalization for the lean mass of the lower extremities could be due in part to higher anaerobic capacity of men, likely due to their greater glycolytic capacity (Jaworowski et al. 2002). As expected, the Wingate test was accompanied by a marked increase of the blood lactate concentration (Calbet et al. 2003). However, with this small between-sex difference in normalized mean power, the blood lactate response was also rather similar in both sexes. No relationship was observed between peak or mean power output normalized per kg of lower extremities lean mass and the sprint-induced AMPK phosphorylation. This finding is also compatible with a similar perturbation of the cellular energy status in men and women after a single Wingate test.
Cell culture studies with adipocytes indicate that inhibition of glycolysis reduces leptin gene expression and leptin release (Mueller et al. 1998). Lactate suppresses lipolysis (Liu et al. 2009), and free fatty acids decrease circulating leptin levels, therefore, the increase in circulating lactate should have had, if any, a positive influence on leptin release (Vestergaard et al. 2005). However, the blood lactate response to exercise was not related to the leptin response, implying that other factors should explain the reduction in circulating leptin levels after the Wingate test in both groups.
Limitations
Despite the large number of subjects included in this investigation, we cannot rule out a type II error for some comparisons, due to the high variability of some phosphorylations, such as STAT3. The same applies to the 30th min Ser221-ACCβ phosphorylation, which would have been considered larger in men compared to women (P = 0.036) had the statistical comparison not been corrected for multiple comparisons (Fig. 1b).
In conclusion, marked increases in AMPK, ACC, STAT3 and ERK phosphorylation were observed after a single 30-s all-out sprint (Wingate test) in the musculus vastus lateralis. We have shown that the muscle signaling response to a single bout of sprint exercise mediated by AMPK, ACC, STAT3, ERK and p38MAPK is comparable in men and women. Finally, this study reveals that serum leptin concentrations are reduced after a sprint exercise, with this reduction being similar in men and women when expressed as a relative change from the pre-exercise value.
References
Aronson D, Violan MA, Dufresne SD, Zangen D, Fielding RA, Goodyear LJ (1997) Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest 99:1251–1257
Astorino TA, Allen RP, Roberson DW, Jurancich M, Lewis R, McCarthy K, Trost E (2011) Adaptations to high-intensity training are independent of gender. Eur J Appl Physiol 111:1279–1286
Bar-Or O (1987) The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med 4:381–394
Birk JB, Wojtaszewski JF (2006) Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol 577:1021–1032
Bjorbaek C, Kahn BB (2004) Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res 59:305–331
Boden G, Chen X, Mozzoli M, Ryan I (1996) Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81:3419–3423
Boonsong T, Norton L, Chokkalingam K, Jewell K, Macdonald I, Bennett A, Tsintzas K (2007) Effect of exercise and insulin on SREBP-1c expression in human skeletal muscle: potential roles for the ERK1/2 and Akt signalling pathways. Biochem Soc Trans 35:1310–1311
Calbet JA, Chavarren J, Dorado C (1997) Fractional use of anaerobic capacity during a 30- and a 45-s Wingate test. Eur J Appl Physiol 76:308–313
Calbet JA, De Paz JA, Garatachea N, Cabeza de Vaca S, Chavarren J (2003) Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J Appl Physiol 94:668–676
Cochran AJ, Little JP, Tarnopolsky MA, Gibala MJ (2010) Carbohydrate feeding during recovery alters the skeletal muscle metabolic response to repeated sessions of high-intensity interval exercise in humans. J Appl Physiol 108:628–636
Couillard C, Mauriege P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Despres JP (2002) Plasma leptin response to an epinephrine infusion in lean and obese women. Obes Res 10:6–13
Creer A, Gallagher P, Slivka D, Jemiolo B, Fink W, Trappe S (2005) Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol 99:950–956
Cuevas MJ, Almar M, Garcia-Glez JC, Garcia-Lopez D, De Paz JA, Alvear-Ordenes I, Gonzalez-Gallego J (2005) Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radic Res 39:431–439
Cheetham ME, Boobis LH, Brooks S, Williams C (1986) Human muscle metabolism during sprint running. J Appl Physiol 61:54–60
Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE (2000) AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab 279:E1202–E1206
Davis SN, Galassetti P, Wasserman DH, Tate D (2000) Effects of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J Clin Endocrinol Metab 85:224–230
Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M (2008a) Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol 104:57–65
Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M (2008b) Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol 104:371–378
Dzamko N, Schertzer JD, Ryall JG, Steel R, Macaulay SL, Wee S, Chen ZP, Michell BJ, Oakhill JS, Watt MJ, Jorgensen SB, Lynch GS, Kemp BE, Steinberg GR (2008) AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol 586:5819–5831
Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ (2010) Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol 588:1779–1790
Esbjornsson-Liljedahl M, Bodin K, Jansson E (2002) Smaller muscle ATP reduction in women than in men by repeated bouts of sprint exercise. J Appl Physiol 93:1075–1083
Esbjornsson-Liljedahl M, Sundberg CJ, Norman B, Jansson E (1999) Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J Appl Physiol 87:1326–1332
Esbjornsson M, Norman B, Suchdev S, Viru M, Lindhgren A, Jansson E (2009) Greater growth hormone and insulin response in women than in men during repeated bouts of sprint exercise. Acta Physiol (Oxf) 197:107–115
Gaitanos GC, Williams C, Boobis LH, Brooks S (1993) Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 75:712–719
Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E (2010) Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab 95:851–855
Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M (2009) Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol 106:929–934
Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE (1996) Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol 271:E403–E408
Gorostiaga EM, Navarro-Amezqueta I, Cusso R, Hellsten Y, Calbet JA, Guerrero M, Granados C, Gonzalez-Izal M, Ibanez J, Izquierdo M (2010) Anaerobic energy expenditure and mechanical efficiency during exhaustive leg press exercise. PLoS One 5:e13486
Greenhaff PL, Nevill ME, Soderlund K, Bodin K, Boobis LH, Williams C, Hultman E (1994) The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol 478:149–155
Guerra B, Fuentes T, Delgado-Guerra S, Guadalupe-Grau A, Olmedillas H, Santana A, Ponce-Gonzalez JG, Dorado C, Calbet JA (2008) Gender dimorphism in skeletal muscle leptin receptors, serum leptin and insulin sensitivity. PLoS One 3:e3466
Guerra B, Gomez-Cabrera MC, Ponce-Gonzalez JG, Martinez-Bello VE, Guadalupe-Grau A, Santana A, Sebastia V, Vina J, Calbet JA (2011a) Repeated muscle biopsies through a single skin incision do not elicit muscle signaling, but IL-6 mRNA and STAT3 phosphorylation increase in injured muscle. J Appl Physiol 110:1708–1715
Guerra B, Guadalupe-Grau A, Fuentes T, Ponce-Gonzalez JG, Morales-Alamo D, Olmedillas H, Guillen-Salgado J, Santana A, Calbet JA (2010) SIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: influence of glucose ingestion. Eur J Appl Physiol 109:731–743
Guerra B, Olmedillas H, Guadalupe-Grau A, Ponce-Gonzalez JG, Morales-Alamo D, Fuentes T, Chapinal E, Fernandez-Perez L, De Pablos-Velasco P, Santana A, Calbet JA (2011b) Is sprint exercise a leptin signaling mimetic in human skeletal muscle? J Appl Physiol 111:715–725
Guerra B, Santana A, Fuentes T, Delgado-Guerra S, Cabrera-Socorro A, Dorado C, Lopez Calbet JA (2007) Leptin receptors in human skeletal muscle. J Appl Physiol 102:1786–1792
Harber MP, Crane JD, Douglass MD, Weindel KD, Trappe TA, Trappe SW, Fink WF (2008) Resistance exercise reduces muscular substrates in women. Int J Sports Med 29:719–725
Hardie DG (2003) Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144:5179–5183
Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Eager DM (2000) Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol 89:1793–1803
Jaworowski A, Porter MM, Holmback AM, Downham D, Lexell J (2002) Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand 176:215–225
Jones NL, McCartney N, Graham T, Spriet LL, Kowalchuk JM, Heigenhauser GJ, Sutton JR (1985) Muscle performance and metabolism in maximal isokinetic cycling at slow and fast speeds. J Appl Physiol 59:132–136
Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF (2004) Knockout of the alpha2 but not alpha1 5’-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279:1070–1079
Kang C, O’Moore KM, Dickman JR, Ji LL (2009) Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med 47:1394–1400
Kraemer RR, Chu H, Castracane VD (2002) Leptin and exercise. Exp Biol Med (Maywood) 227:701–708
Lamont LS, McCullough AJ, Kalhan SC (2003) Gender differences in the regulation of amino acid metabolism. J Appl Physiol 95:1259–1265
Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ (2010) Acute endurance exercise increases the nuclear abundance of PGC-1alpha in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 298:R912–R917
Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, Mazur C, Kamme F, Lovenberg TW (2009) Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem 284:2811–2822
Maroni P, Bendinelli P, Piccoletti R (2003) Early intracellular events induced by in vivo leptin treatment in mouse skeletal muscle. Mol Cell Endocrinol 201:109–121
Maroni P, Bendinelli P, Piccoletti R (2005) Intracellular signal transduction pathways induced by leptin in C2C12 cells. Cell Biol Int 29:542–550
McKenna MJ, Schmidt TA, Hargreaves M, Cameron L, Skinner SL, Kjeldsen K (1993) Sprint training increases human skeletal muscle Na(+)-K(+)-ATPase concentration and improves K+ regulation. J Appl Physiol 75:173–180
Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB (2002) Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415:339–343
Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ (1998) Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology 139:551–558
Ortenblad N, Lunde PK, Levin K, Andersen JL, Pedersen PK (2000) Enhanced sarcoplasmic reticulum Ca(2+) release following intermittent sprint training. Am J Physiol Regul Integr Comp Physiol 279:R152–R160
Perez-Gomez J, Rodriguez GV, Ara I, Olmedillas H, Chavarren J, Gonzalez-Henriquez JJ, Dorado C, Calbet JA (2008) Role of muscle mass on sprint performance: gender differences? Eur J Appl Physiol 102:685–694
Powers SK, Nelson WB, Hudson MB (2010) Exercise-induced oxidative stress in humans: Cause and consequences. Free radic Biol Med 51:942–950
Ricci MR, Lee MJ, Russell CD, Wang Y, Sullivan S, Schneider SH, Brolin RE, Fried SK (2005) Isoproterenol decreases leptin release from rat and human adipose tissue through posttranscriptional mechanisms. Am J Physiol Endocrinol Metab 288:E798–E804
Richter EA, Vistisen B, Maarbjerg SJ, Sajan M, Farese RV, Kiens B (2004) Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle. J Physiol 560:909–918
Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B (2006) Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol 574:125–138
Ruderman NB, Saha AK, Vavvas D, Witters LA (1999) Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol 276:E1–E18
Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR (2005) Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24:1810–1820
Serrano-Sanchez JA, Delgado-Guerra S, Olmedillas H, Guadalupe-Grau A, Arteaga-Ortiz R, Sanchis-Moysi J, Dorado C, Calbet JA (2010) Adiposity and age explain most of the association between physical activity and fitness in physically active men. PLoS One 5:e13435
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR (1990) Gender differences in substrate for endurance exercise. J Appl Physiol 68:302–308
Torres M (2003) Mitogen-activated protein kinase pathways in redox signaling. Front Biosci 8:d369–d391
Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF (2007) AS160 phosphorylation is associated with activation of alpha2beta2gamma1—but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292:E715–E722
Trenerry MK, Carey KA, Ward AC, Cameron-Smith D (2007) STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol 102:1483–1489
Trenerry MK, Carey KA, Ward AC, Farnfield MM, Cameron-Smith D (2008) Exercise-induced activation of STAT3 signaling is increased with age. Rejuvenation Res 11:717–724
Vestergaard ET, Hansen TK, Nielsen S, Moller N, Christiansen JS, Jorgensen JO (2005) Effects of GH replacement therapy in adults on serum levels of leptin and ghrelin: the role of lipolysis. Eur J Endocrinol 153:545–549
Widegren U, Jiang XJ, Krook A, Chibalin AV, Bjornholm M, Tally M, Roth RA, Henriksson J, Wallberg-henriksson H, Zierath JR (1998) Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB J 12:1379–1389
Widegren U, Wretman C, Lionikas A, Hedin G, Henriksson J (2000) Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflugers Arch 441:317–322
Williamson D, Gallagher P, Harber M, Hollon C, Trappe S (2003) Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 547:977–987
Yu M, Blomstrand E, Chibalin AV, Krook A, Zierath JR (2001) Marathon running increases ERK1/2 and p38 MAP kinase signalling to downstream targets in human skeletal muscle. J Physiol 536:273–282
Zehnder M, Ith M, Kreis R, Saris W, Boutellier U, Boesch C (2005) Gender-specific usage of intramyocellular lipids and glycogen during exercise. Med Sci Sports Exerc 37:1517–1524
Zhang Y, Matheny M, Zolotukhin S, Tumer N, Scarpace PJ (2002) Regulation of adiponectin and leptin gene expression in white and brown adipose tissues: influence of beta3-adrenergic agonists, retinoic acid, leptin and fasting. Biochim Biophys Acta 1584:115–122
Acknowledgments
This study was supported by grants from the Ministerio de Educación y Ciencia (BFU2006-13784 and FEDER) and FUNCIS (PI/10/07), Proyecto Estructurante de la ULPGC: ULPAPD-08/01-4, Proyecto del Programa Propio de la ULPGC (ULPGC 2009-07), III Convocatoria de Ayudas a la Investigación Cátedra Real Madrid- UEM (2010/01RM, Universidad Europea de Madrid) and Ministerio de Ciencia e Innovación (DEP2010-21866). Our special thanks are given to José Navarro de Tuero and María Carmen García Chicano for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Håkan Westerblad.
Rights and permissions
About this article
Cite this article
Fuentes, T., Guerra, B., Ponce-González, J.G. et al. Skeletal muscle signaling response to sprint exercise in men and women. Eur J Appl Physiol 112, 1917–1927 (2012). https://doi.org/10.1007/s00421-011-2164-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2164-0